Plasma Rich in Growth Factors Enhances Cell Survival after in Situ Retinal Degeneration
Abstract
1. Introduction
2. Results
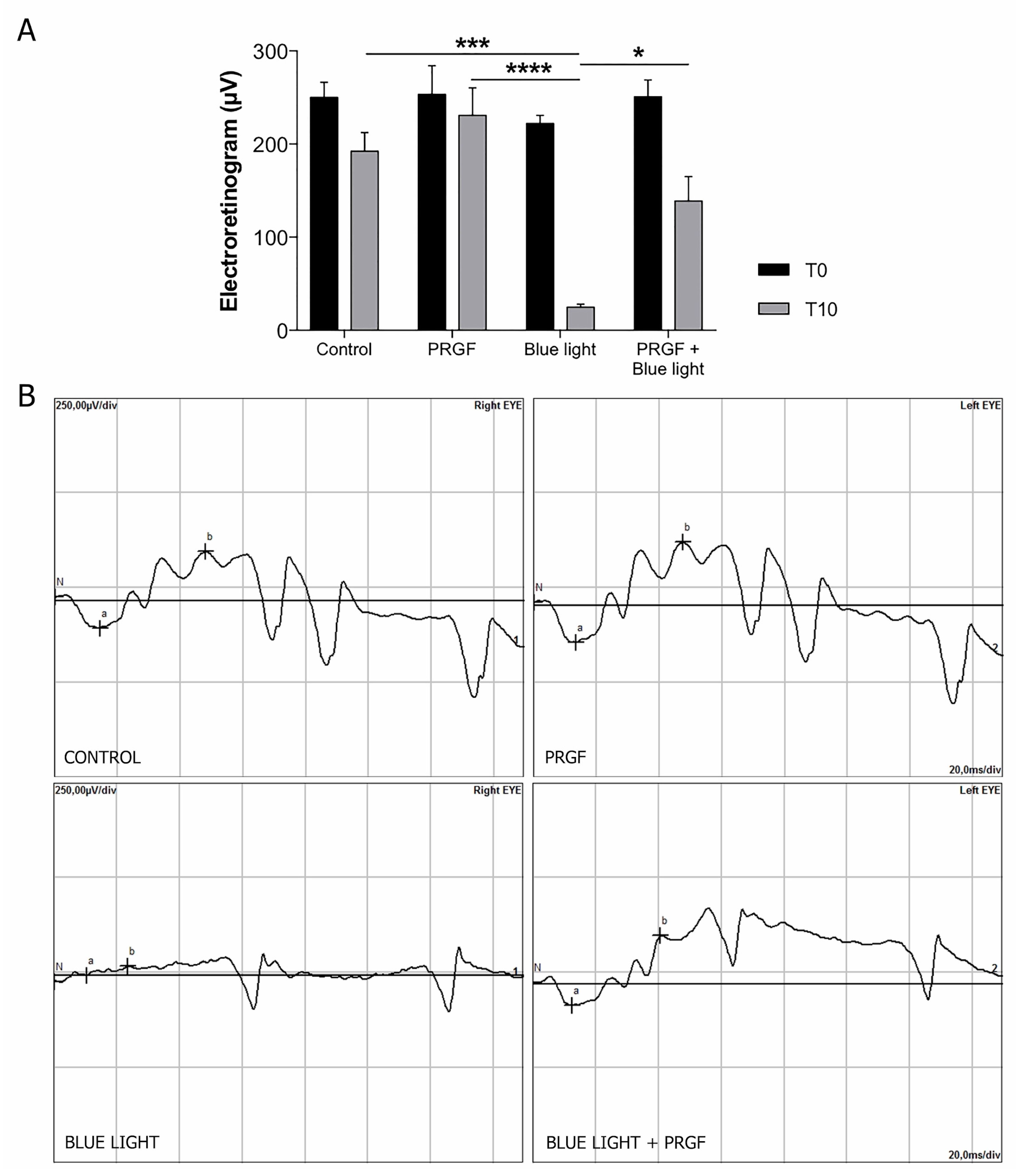
2.1. Electroretinogram and Intraocular Pressure (IoP)
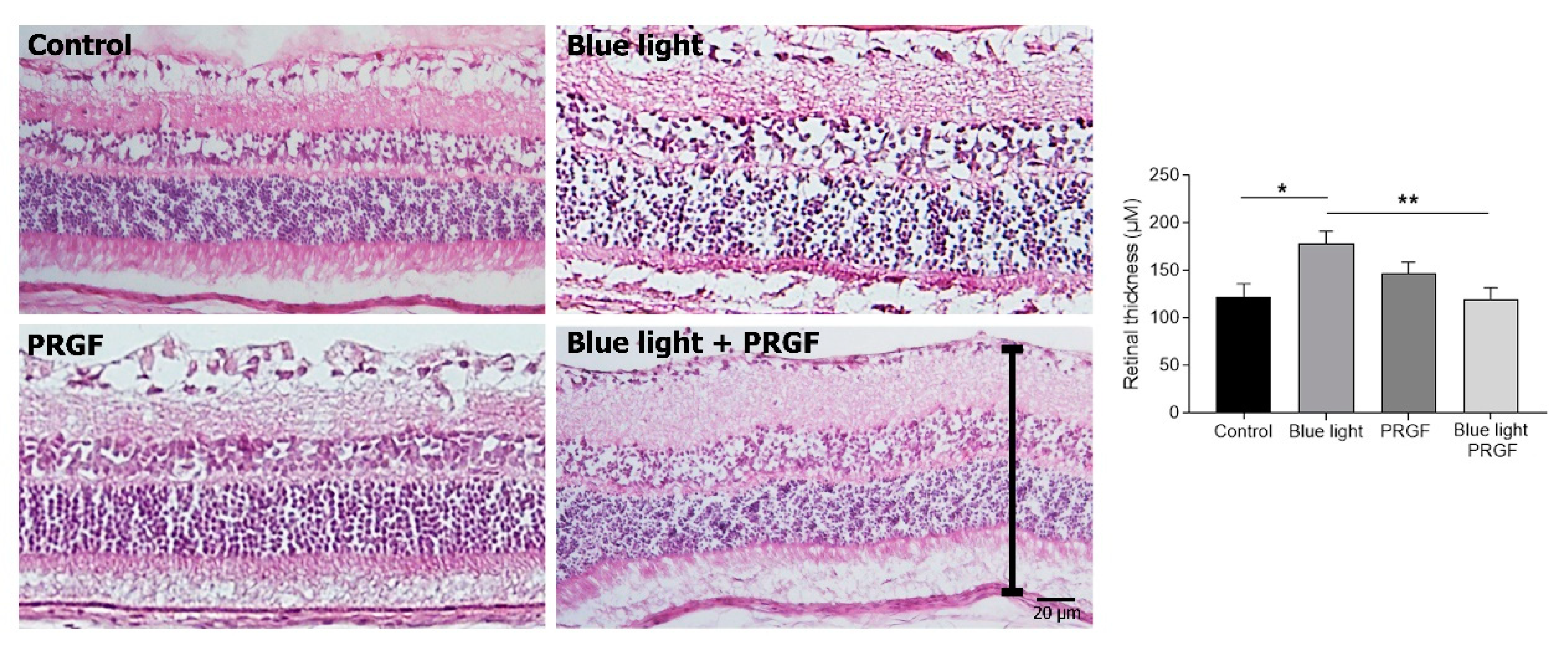
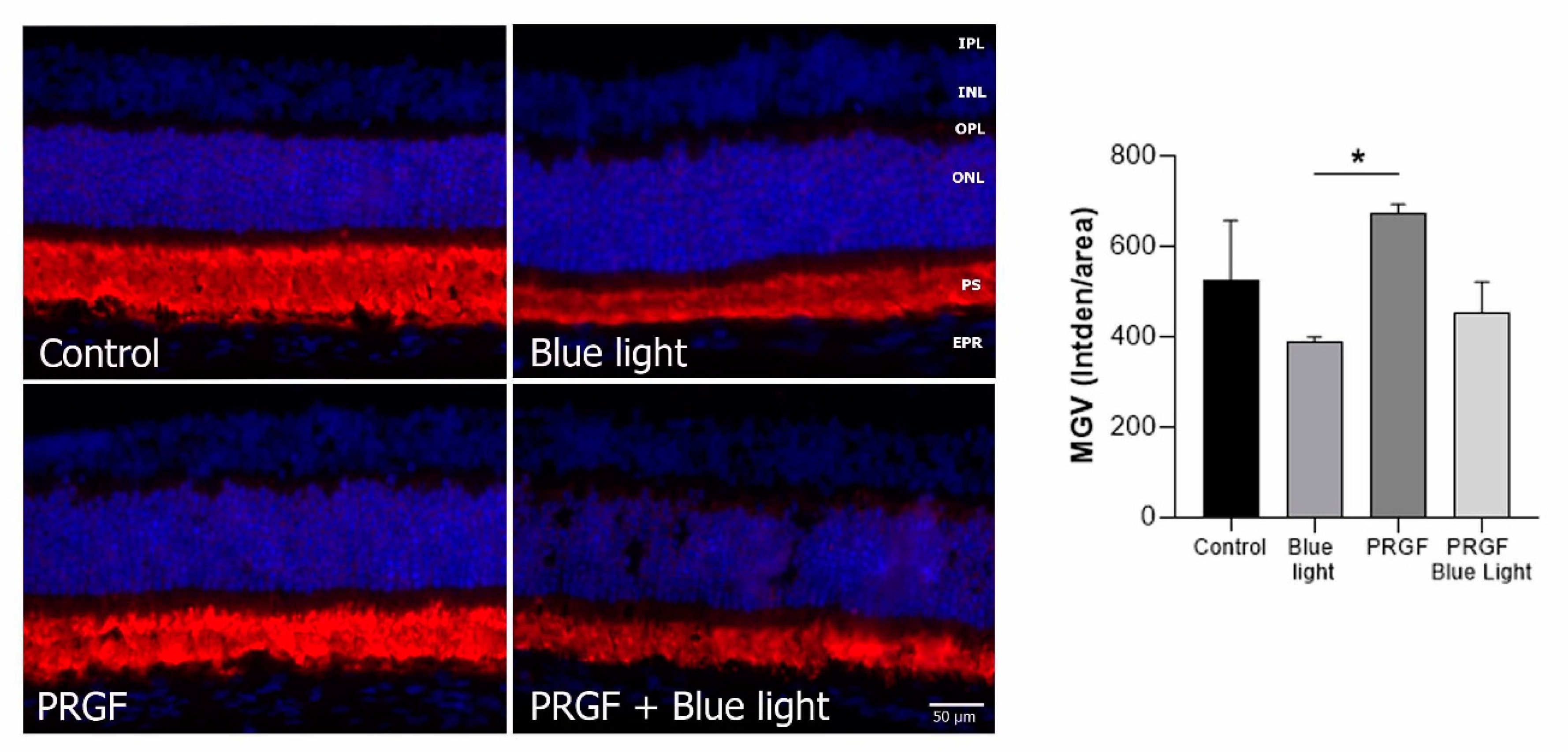
2.2. Retinal Degeneration Can Involve Changes in Retinal Thickness
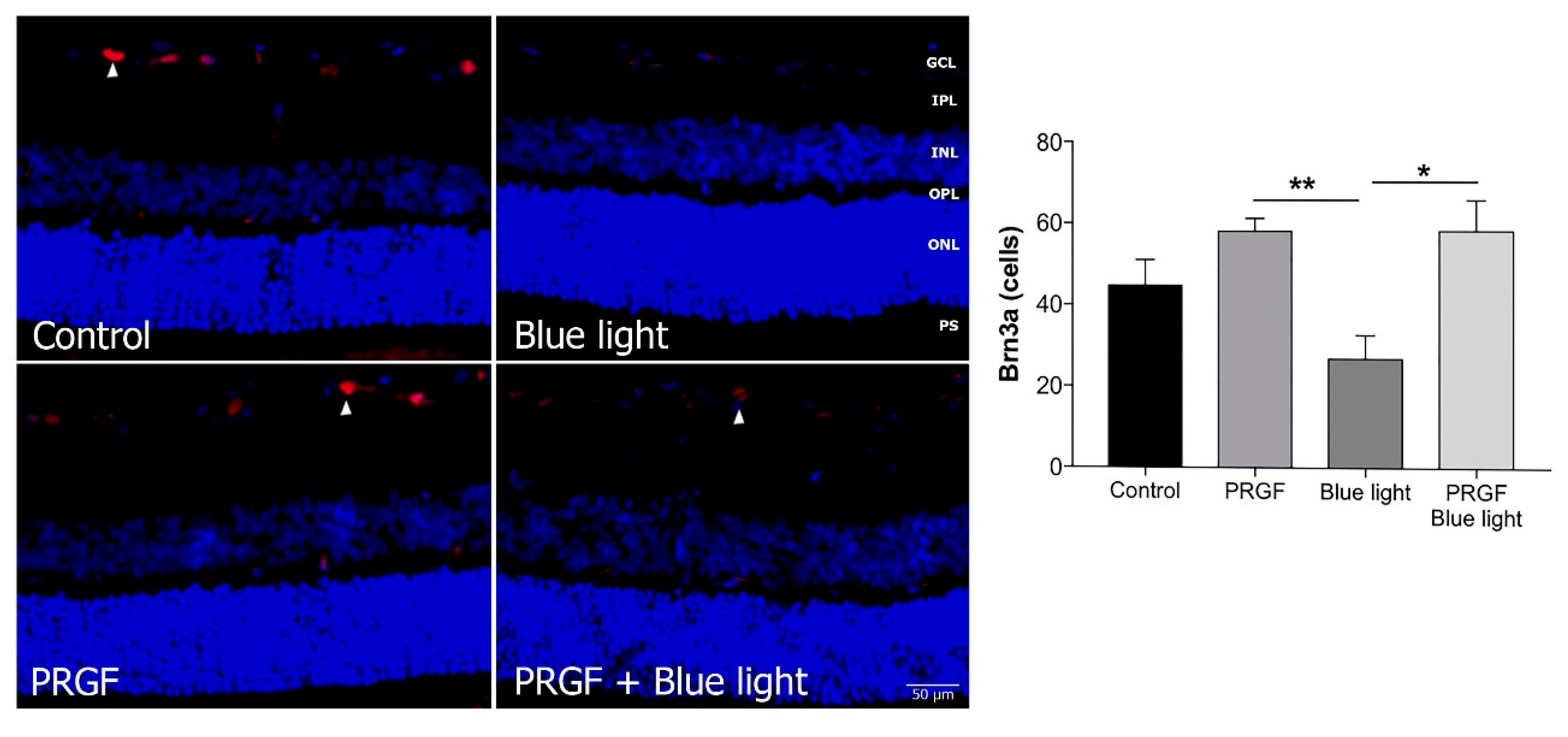
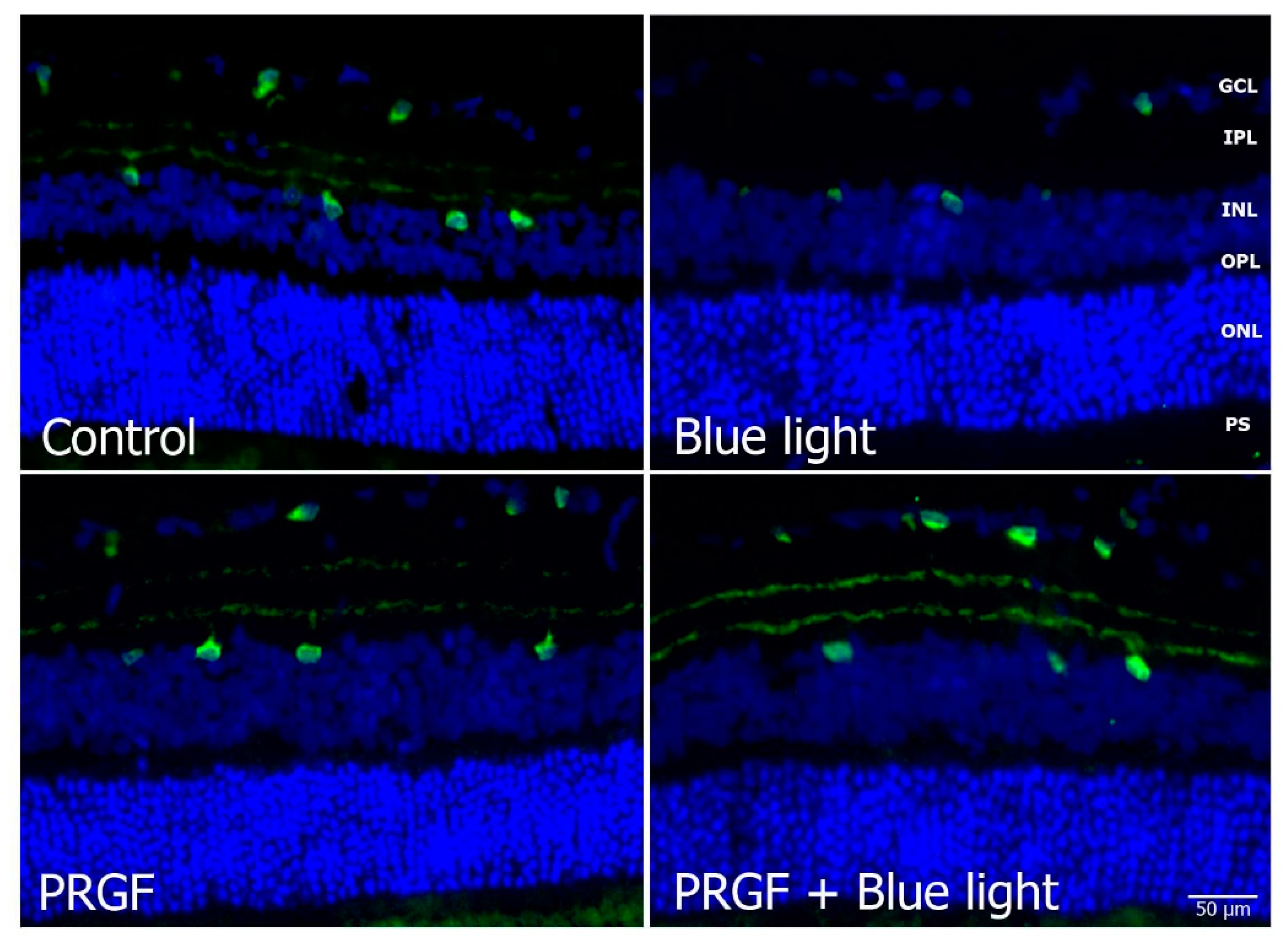
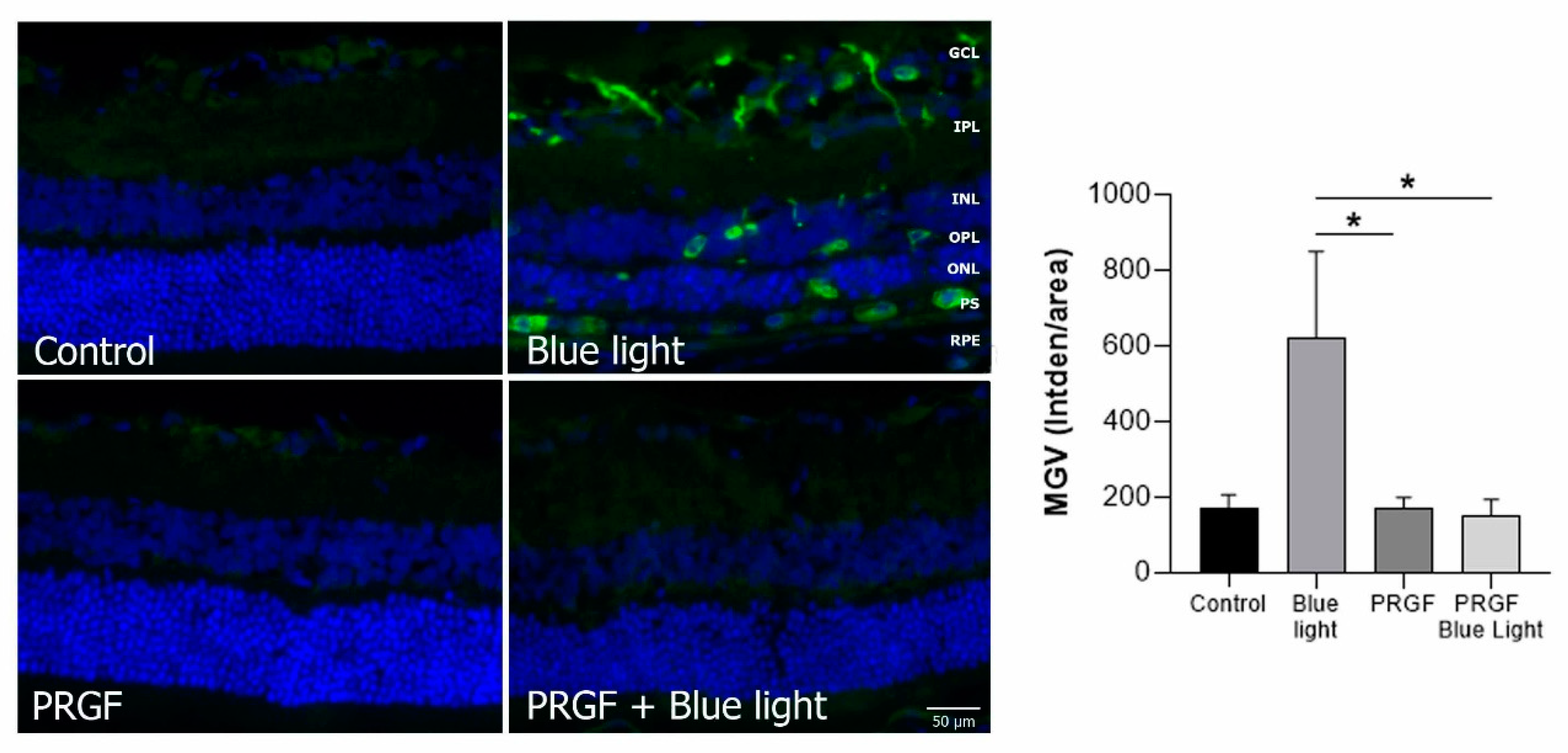
2.3. Immunofluorescence Study
3. Discussion
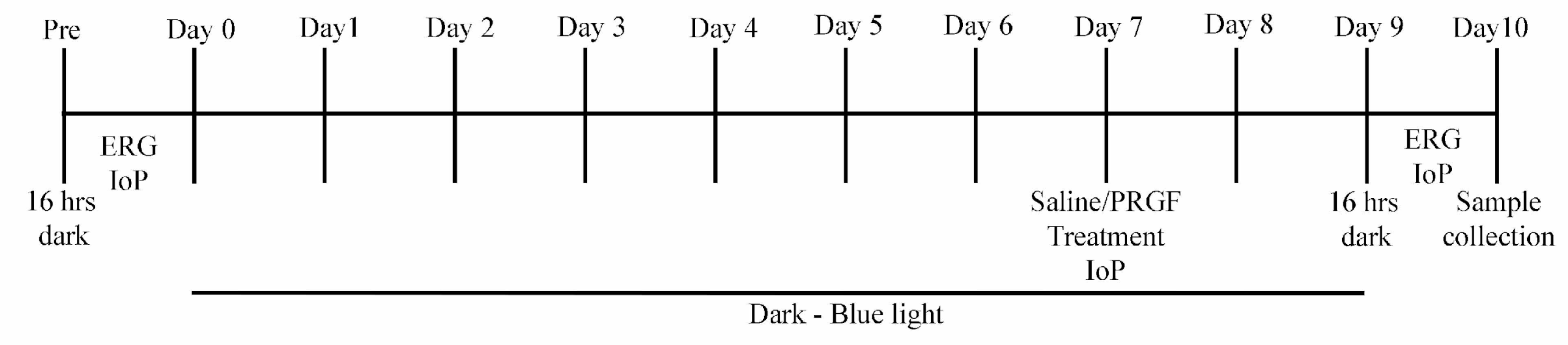
4. Animals, Materials and Methods
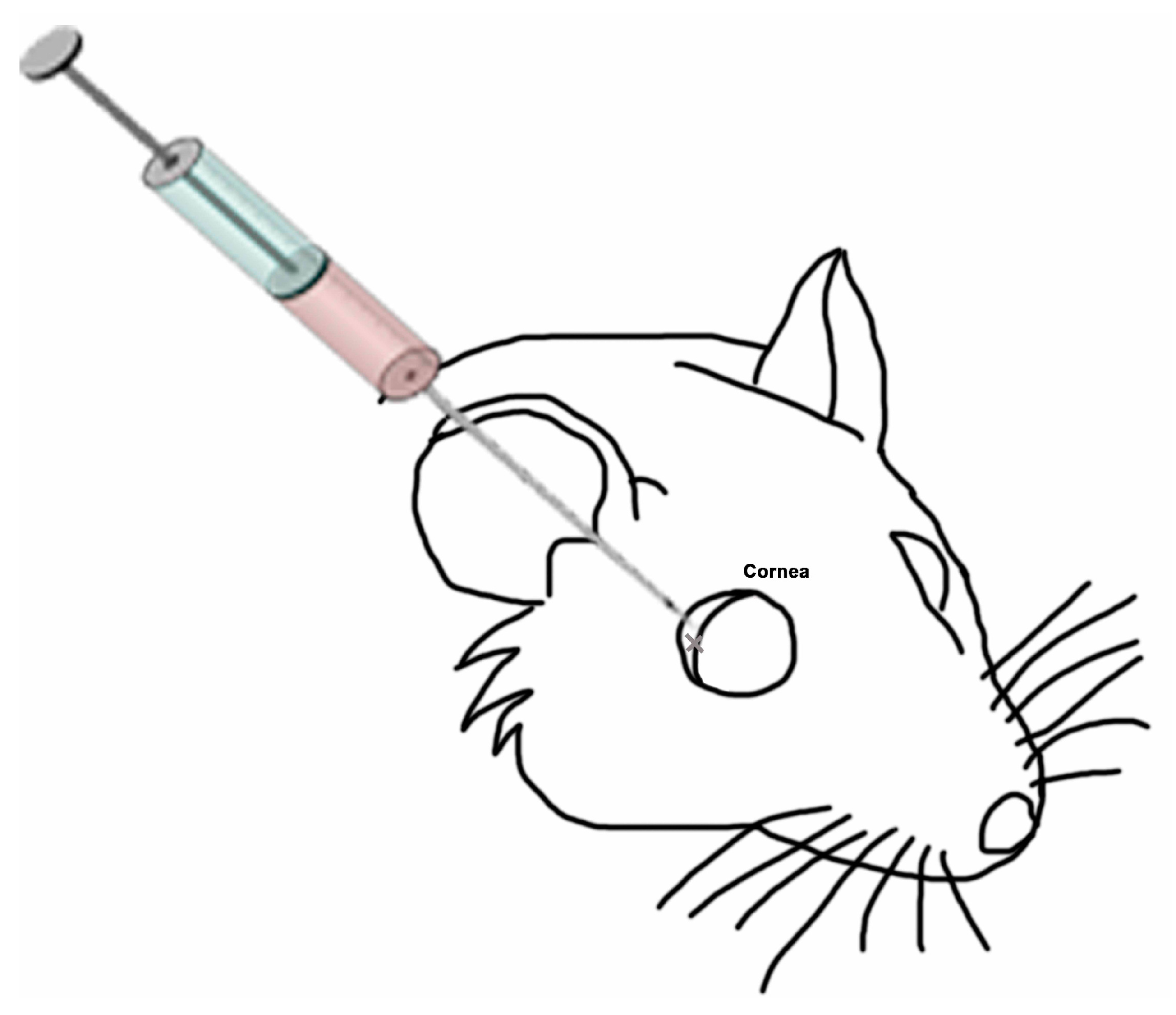
4.1. PRGF
4.2. Animals
4.3. Electroretinogram and Intraocular Pressure
4.4. Immunofluorescence
4.5. Histological Studies and Retinal Thickness Quantification
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Albarracin, R.; Valter, K. 670 nm red light preconditioning supports Müller cell function: Evidence from the white light-induced damage model in the rat retina. Photochem. Photobiol. 2012, 88, 1418–1427. [Google Scholar] [CrossRef]
- Behar-Cohen, F.; Martinsons, C.; Viénot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef]
- Iandiev, I.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Grimm, C.; Remé, C.E.; Reichenbach, A.; Pannicke, T.; Bringmann, A. Müller Cell Response to Blue Light Injury of the Rat Retina. Investig. Opthalmol. Vis. Sci. 2008, 49, 3559. [Google Scholar] [CrossRef]
- Jaadane, I.; Villalpando Rodriguez, G.E.; Boulenguez, P.; Chahory, S.; Carré, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo. J. Cell. Mol. Med. 2017, 21, 3453–3466. [Google Scholar] [CrossRef]
- Osborne, N.N.; Li, G.-Y.; Ji, D.; Mortiboys, H.J.; Jackson, S. Light affects mitochondria to cause apoptosis to cultured cells: Possible relevance to ganglion cell death in certain optic neuropathies. J. Neurochem. 2008, 105, 2013–2028. [Google Scholar] [CrossRef]
- Shang, Y.M.; Wang, G.S.; Sliney, D.; Yang, C.H.; Lee, L.L. White light-emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environ. Health Perspect. 2014, 122, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Zhou, Y.; Bai, S. Light-induced photoreceptor and RPE degeneration involve zinc toxicity and are attenuated by pyruvate, nicotinamide, or cyclic light. Mol. Vis. 2010, 16, 2639–2652. [Google Scholar]
- Geiger, P.; Barben, M.; Grimm, C.; Samardzija, M. Blue light-induced retinal lesions, intraretinal vascular leakage and edema formation in the all-cone mouse retina. Cell Death Dis. 2015, 6, e1985. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.-Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue Light Induces Mitochondrial DNA Damage and Free Radical Production in Epithelial Cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef]
- Núñez-Álvarez, C.; Suárez-Barrio, C.; del Olmo Aguado, S.; Osborne, N.N. Blue light negatively affects the survival of ARPE19 cells through an action on their mitochondria and blunted by red light. Acta Ophthalmol. 2019, 97, e103–e115. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Moutray, T.; Chakravarthy, U. Age-related macular degeneration: Current treatment and future options. Ther. Adv. Chronic Dis. 2011, 2, 325–331. [Google Scholar] [CrossRef]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Remé, C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M.; Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef]
- El-Esawi, M.; Arthaut, L.D.; Jourdan, N.; D’Harlingue, A.; Link, J.; Martino, C.F.; Ahmad, M. Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 2017, 7, 13875. [Google Scholar] [CrossRef]
- Lockwood, D.B.; Wataha, J.C.; Lewis, J.B.; Tseng, W.Y.; Messer, R.L.W.; Hsu, S.D. Blue light generates reactive oxygen species (ROS) differentially in tumor vs. normal epithelial cells. Dent. Mater. 2005, 21, 683–688. [Google Scholar] [CrossRef]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.-A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef]
- Osborne, N.N.; Núñez-Álvarez, C.; Del Olmo-Aguado, S. The effect of visual blue light on mitochondrial function associated with retinal ganglions cells. Exp. Eye Res. 2014, 128, 8–14. [Google Scholar] [CrossRef]
- Lascaratos, G.; Ji, D.; Wood, J.P.M.; Osborne, N.N. Visible light affects mitochondrial function and induces neuronal death in retinal cell cultures. Vision Res. 2007, 47, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; De la Fuente, M.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sánchez, M.; Orive, G.; Andía, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Paknejad, M.; Shayesteh, Y.S.; Yaghobee, S.; Shariat, S.; Dehghan, M.; Motahari, P. Evaluation of the Effect of Plasma Rich in Growth Factors (PRGF) on Bone Regeneration. J. Dent. 2012, 9, 59–67. [Google Scholar]
- Etxebarria, J.; Sanz-Lázaro, S.; Hernáez-Moya, R.; Freire, V.; Durán, J.A.; Morales, M.C.; Andollo, N. Serum from plasma rich in growth factors regenerates rabbit corneas by promoting cell proliferation, migration, differentiation, adhesion and limbal stemness. Acta Ophthalmol. 2017, 95, e693–e705. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Hernáez-Moya, R.; Durán, J.A.; Morales, M.C. Corneal wound healing promoted by 3 blood derivatives: An in vitro and in vivo comparative study. Cornea 2014, 33, 614–620. [Google Scholar] [CrossRef] [PubMed]
- López-Plandolit, S.; Morales, M.-C.; Freire, V.; Etxebarría, J.; Durán, J.A. Plasma Rich in Growth Factors as a Therapeutic Agent for Persistent Corneal Epithelial Defects. Cornea 2010, 29, 843–848. [Google Scholar] [CrossRef]
- López-Plandolit, S.; Morales, M.-C.; Freire, V.; Grau, A.E.; Durán, J.A. Efficacy of Plasma Rich in Growth Factors for the Treatment of Dry Eye. Cornea 2011, 30, 1312–1317. [Google Scholar] [CrossRef]
- Suárez-Barrio, C.; Etxebarria, J.; Hernáez-Moya, R.; Del Val-Alonso, M.; Rodriguez-Astigarraga, M.; Urkaregi, A.; Freire, V.; Morales, M.C.; Durán, J.A.; Vicario, M.; et al. Hyaluronic acid combined with serum rich in growth factors in corneal epithelial defects. Int. J. Mol. Sci. 2019, 20, 1655. [Google Scholar] [CrossRef]
- De la Sen-Corcuera, B.; Montero-Iruzubieta, J.; Sánchez-Ávila, R.M.; Orive, G.; Anitua, E.; Caro-Magdaleno, M.; Merayo-Lloves, J. Plasma rich in growth factors for the treatment of cicatrizing conjunctivitis. Clin. Ophthalmol. 2020, 14, 1619–1627. [Google Scholar] [CrossRef]
- Sánchez-Avila, R.M.; Merayo-Lloves, J.; Fernández, M.L.; Rodríguez-Gutiérrez, L.A.; Rodríguez-Calvo, P.P.; Fernández-Vega Cueto, A.; Muruzabal, F.; Orive, G.; Anitua, E. Plasma rich in growth factors eye drops to treat secondary ocular surface disorders in patients with glaucoma. Int. Med. Case Rep. J. 2018, 11, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.D.; Hoyos, A.T.; Alcántara, B.; Sanchez-Avila, R.M.; Arango, F.J.; Galvis, V. Plasma Rich in Growth Factors for Persistent Macular Hole. Retin. Cases Brief Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-ávila, R.M.; González, Á.F.V.; Sanz, Á.F.V.; Merayo-Lloves, J. Treatment of recurrent myopic macular hole using membrane of plasma rich in growth factors. Int. Med. Case Rep. J. 2019, 12, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Barrio, C.; Del Olmo-Aguado, S.; García-Pérez, E.; De la Fuente, M.; Muruzabal, F.; Anitua, E.; Baamonde-Arbaiza, B.; Fernández-Vega-Cueto, L.; Fernández-Vega, L.; Merayo-Lloves, J. Antioxidant role of PRGF on RPE cells after blue light insult as a therapy for neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 1021. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; De la Fuente, M.; Del Olmo-Aguado, S.; Suarez-Barrio, C.; Merayo-Lloves, J.; Muruzabal, F. Plasma rich in growth factors reduces blue light-induced oxidative damage on retinal pigment epithelial cells and restores their homeostasis by modulating vascular endothelial growth factor and pigment epithelium-derived factor expression. Clin. Exp. Ophthalmol. 2020, 48, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Sernagor, E.; Eglen, S.J.; Wong, R.O.L. Development of retinal ganglion cell structure and function. Prog. Retin. Eye Res. 2001, 20, 139–174. [Google Scholar] [CrossRef]
- VanderWall, K.B.; Vij, R.; Ohlemacher, S.K.; Sridhar, A.; Fligor, C.M.; Feder, E.M.; Edler, M.C.; Baucum, A.J.; Cummins, T.R.; Meyer, J.S. Astrocytes Regulate the Development and Maturation of Retinal Ganglion Cells Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2019, 12, 201–212. [Google Scholar] [CrossRef]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15, 384–389. [Google Scholar] [CrossRef]
- Nirenberg, S.; Pandarinath, C. Retinal prosthetic strategy with the capacity to restore normal vision. Proc. Natl. Acad. Sci. USA 2012, 109, 15012–15017. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Qiu, S.; Chen, X.-C.; Dai, Z.-H.; Huang, Y.-C.; Li, Y.-N.; Cai, R.-H.; Lei, H.-T.; Gu, H.-Y. Neuroglobin—A potential biological marker of retinal damage induced by LED light. Neuroscience 2014, 270, 158–167. [Google Scholar] [CrossRef]
- King, A.; Gottlieb, E.; Brooks, D.G.; Murphy, M.P.; Dunaief, J.L. Mitochondria-derived reactive oxygen species mediate blue light-induced death of retinal pigment epithelial cells. Photochem. Photobiol. 2004, 79, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, P.; Wang, W.; Xu, Y.; Wang, M.; Chen, X.; Dong, X. Long-term blue light exposure induces RGC-5 cell death in vitro: Involvement of mitochondria-dependent apoptosis, oxidative stress, and MAPK signaling pathways. Apoptosis 2014, 19, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Krigel, A.; Berdugo, M.; Picard, E.; Levy-Boukris, R.; Jaadane, I.; Jonet, L.; Dernigoghossian, M.; Andrieu-Soler, C.; Torriglia, A.; Behar-Cohen, F. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience 2016, 339, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Roehlecke, C.; Schaller, A.; Knels, L.; Funk, R.H.W. The influence of sublethal blue light exposure on human RPE cells. Mol. Vis. 2009, 15, 1929–1938. [Google Scholar] [PubMed]
- Wataha, J.C.; Lockwood, P.E.; Lewis, J.B.; Rueggeberg, F.A.; Messer, R.L.W. Biological effects of blue light from dental curing units. Dent. Mater. 2004, 20, 150–157. [Google Scholar] [CrossRef]
- Del Olmo-Aguado, S.; Manso, A.G.; Osborne, N.N. Light Might Directly Affect Retinal Ganglion Cell Mitochondria to Potentially Influence Function. Photochem. Photobiol. 2012, 88, 1346–1355. [Google Scholar] [CrossRef]
- Anitua, E.; De la Fuente, M.; Muruzabal, F.; Riestra, A.; Merayo-Lloves, J.; Orive, G. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp. Eye Res. 2015, 135, 118–126. [Google Scholar] [CrossRef]
- Molina-Miñano, F.; López-Jornet, P.; Camacho-Alonso, F.; Vicente-Ortega, V. The use of plasma rich in growth factors on wound healing in the skin: Experimental study in rabbits. Int. Wound J. 2009, 6, 145–148. [Google Scholar] [CrossRef]
- Freire, V.; Andollo, N.; Etxebarria, J.; Durán, J.A.; Morales, M.-C. In Vitro Effects of Three Blood Derivatives on Human Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 5571. [Google Scholar] [CrossRef]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye 2011, 25, 1–14. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Zhou, Y.; Tan, G.; Li, J. Research progress about the effect and prevention of blue light on eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Alburquerque-Béjar, J.J.; Vidal-Sanz, M.; Agudo-Barriuso, M. Whole Number, Distribution and Co-Expression of Brn3 Transcription Factors in Retinal Ganglion Cells of Adult Albino and Pigmented Rats. PLoS ONE 2012, 7, e49830. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Sobrado-Calvo, P.; Nieto-López, L.; Cánovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naïve and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Stankowska, D.L.; Minton, A.Z.; Rutledge, M.A.; Mueller, B.H.; Phatak, N.R.; He, S.; Ma, H.-Y.; Forster, M.J.; Yorio, T.; Krishnamoorthy, R.R. Neuroprotective Effects of Transcription Factor Brn3b in an Ocular Hypertension Rat Model of Glaucoma. Invest. Ophthalmol. Vis. Sci. 2015, 56, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo-Aguado, S.; Núñez-Álvarez, C.; Osborne, N.N. Blue Light Action on Mitochondria Leads to Cell Death by Necroptosis. Neurochem. Res. 2016, 41, 2324–2335. [Google Scholar] [CrossRef]
- Kim, I.B.; Lee, E.J.; Kim, M.K.; Park, D.K.; Chun, M.H. Choline acetyltransferase-immunoreactive neurons in the developing rat retina. J. Comp. Neurol. 2000, 427, 604–616. [Google Scholar] [CrossRef]
- Gonzalez-Cordero, A.; West, E.L.; Pearson, R.A.; Duran, Y.; Carvalho, L.S.; Chu, C.J.; Naeem, A.; Blackford, S.J.I.; Georgiadis, A.; Lakowski, J.; et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 2013, 31, 741–747. [Google Scholar] [CrossRef]
- Grimm, C.; Wenzel, A.; Williams, T.P.; Rol, P.O.; Hafezi, F.; Remé, C.E. Rhodopsin-mediated blue-light damage to the rat retina: Effect of photoreversal of bleaching. Investig. Ophthalmol. Vis. Sci. 2001, 42, 497–505. [Google Scholar]
- Reuter, T.E.; White, R.H.; Wald, G. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J. Gen. Physiol. 1971, 58, 351–371. [Google Scholar] [CrossRef]
- Begum, R.; Powner, M.B.; Hudson, N.; Hogg, C.; Jeffery, G. Treatment with 670 nm Light Up Regulates Cytochrome C Oxidase Expression and Reduces Inflammation in an Age-Related Macular Degeneration Model. PLoS ONE 2013, 8, e57828. [Google Scholar] [CrossRef]
- Casson, R.J.; Wood, J.P.M.; Melena, J.; Chidlow, G.; Osborne, N.N. The effect of ischemic preconditioning on light-induced photoreceptor injury. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Hsu, J.R.; Hsu, P.C.; Chuang, J.I. The retina as a novel in Vivo model for studying the role of molecules of the Bcl-2 family in relation to MPTP neurotoxicity. Neurochem. Res. 2003, 28, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.V.; Martin, K.R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.R.; Agarwal, P.; Prasanna, G.; Vopat, K.; Lambert, W.; Sheedlo, H.J.; Pang, I.H.; Shade, D.; Wordinger, R.J.; Yorio, T.; et al. Characterization of a transformed rat retinal ganglion cell line. Mol. Brain Res. 2001, 86, 1–12. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.-C.; So, K.-F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 Antioxidant Pathway Contributes to the Protective Effects of Lycium Barbarum Polysaccharides in the Rodent Retina after Ischemia-Reperfusion-Induced Damage. PLoS ONE 2014, 9, e84800. [Google Scholar] [CrossRef]
- Ji, D.; Kamalden, T.A.; del Olmo-Aguado, S.; Osborne, N.N. Light- and sodium azide-induced death of RGC-5 cells in culture occurs via different mechanisms. Apoptosis 2011, 16, 425–437. [Google Scholar] [CrossRef]
- Kutty, R.K.; Nagineni, C.N.; Kutty, G.; Hooks, J.J.; Chader, G.J.; Wiggert, B. Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J. Cell Physiol. 1994, 159, 371–378. [Google Scholar] [CrossRef]
- Ursu, O.N.; Sauter, M.; Ettischer, N.; Kandolf, R.; Klingel, K. Heme oxygenase-1 mediates oxidative stress and apoptosis in coxsackievirus B3-induced myocarditis. Cell. Physiol. Biochem. 2014, 33, 52–66. [Google Scholar] [CrossRef]


| Treatment | Medium | Dark/Blue Light |
|---|---|---|
| Control | 10 µL saline solution | Dark |
| Blue light | 10 µL Saline solution | Blue light |
| PRGF | 10 µL PRGF 100% | Dark |
| Blue light + PRGF | 10 µL PRGF 100% | Blue light |
| Antibody | Reference (RRID) | Species | Dilution | Company |
|---|---|---|---|---|
| Primary Antibodies | ||||
| HO-1 | Enzo Life Sciences Cat# SPA-894F, RRID:AB_991588 | Rabbit | 1:100 | Enzo LS, Farmingdale, NY, USA |
| GFAP | Agilent Cat# Z0334, RRID:AB_10013382 | Rabbit | 1:500 | Dako, Santa Clara, CA, USA |
| ChAT | Millipore Cat# AB144P, RRID:AB_2079751 | Goat | 1:250 | Millipore, Burlington, MA, USA |
| Brn3a | Santa Cruz Biotechnology Cat# sc-31984, RRID:AB_2167511 | Mouse | 1:200 | Santa Cruz, Dallas, TX, USA |
| Rhodopsin | Millipore Cat# MABN15, RRID:AB_10807045 | Mouse | 1:200 | Millipore, Burlington, MA, USA |
| Secondary Antibodies | ||||
| Anti-rabbit Alexa Fluor 488 | Thermo Fisher Scientific Cat# A32731TR, RRID:AB_2866491 | Goat | 1:300 | ThermoFisher, Waltham, MA, USA |
| Anti-goat Alexa Fluor 488 | Thermo Fisher Scientific Cat# A32814TR, RRID:AB_2866497 | Donkey | 1:300 | ThermoFisher, Waltham, MA, USA |
| Anti-mouse Alexa Fluor 594 | Thermo Fisher Scientific Cat# A32742, RRID:AB_2762825 | Goat | 1:300 | ThermoFisher, Waltham, MA, USA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Barrio, C.; del Olmo-Aguado, S.; García-Pérez, E.; Artime, E.; de la Fuente, M.; Muruzabal, F.; Anitua, E.; Baamonde-Arbaiza, B.; Fernández-Vega, L.; Merayo-Lloves, J. Plasma Rich in Growth Factors Enhances Cell Survival after in Situ Retinal Degeneration. Int. J. Mol. Sci. 2020, 21, 7442. https://doi.org/10.3390/ijms21207442
Suárez-Barrio C, del Olmo-Aguado S, García-Pérez E, Artime E, de la Fuente M, Muruzabal F, Anitua E, Baamonde-Arbaiza B, Fernández-Vega L, Merayo-Lloves J. Plasma Rich in Growth Factors Enhances Cell Survival after in Situ Retinal Degeneration. International Journal of Molecular Sciences. 2020; 21(20):7442. https://doi.org/10.3390/ijms21207442
Chicago/Turabian StyleSuárez-Barrio, Carlota, Susana del Olmo-Aguado, Eva García-Pérez, Enol Artime, María de la Fuente, Francisco Muruzabal, Eduardo Anitua, Begoña Baamonde-Arbaiza, Luis Fernández-Vega, and Jesús Merayo-Lloves. 2020. "Plasma Rich in Growth Factors Enhances Cell Survival after in Situ Retinal Degeneration" International Journal of Molecular Sciences 21, no. 20: 7442. https://doi.org/10.3390/ijms21207442
APA StyleSuárez-Barrio, C., del Olmo-Aguado, S., García-Pérez, E., Artime, E., de la Fuente, M., Muruzabal, F., Anitua, E., Baamonde-Arbaiza, B., Fernández-Vega, L., & Merayo-Lloves, J. (2020). Plasma Rich in Growth Factors Enhances Cell Survival after in Situ Retinal Degeneration. International Journal of Molecular Sciences, 21(20), 7442. https://doi.org/10.3390/ijms21207442





