Advances, Perspectives and Potential Engineering Strategies of Light-Gated Phosphodiesterases for Optogenetic Applications
Abstract
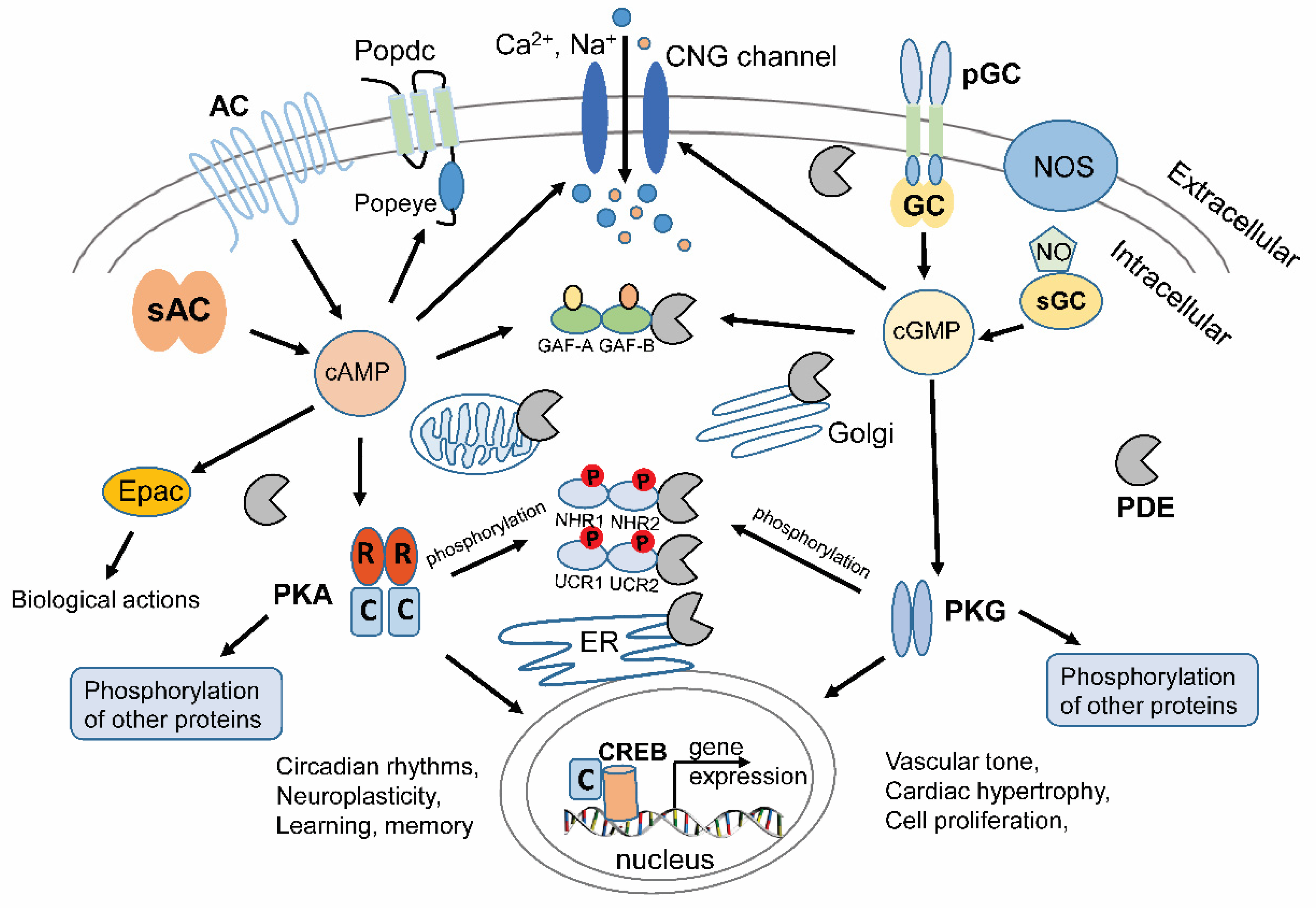
:1. Introduction to Cyclic Nucleotides and Signal Transduction
2. Therapeutic Targeting of PDEs
3. Light-Regulated PDEs
3.1. Indirect Light Regulation of PDE Activity in Visual Phototransduction
3.2. Artificial Light-Activated PDE (LAPD)
3.3. Direct Light-Gated PDEs (RhoPDEs) from Nature
4. Light-Regulated Nucleotide Cyclases
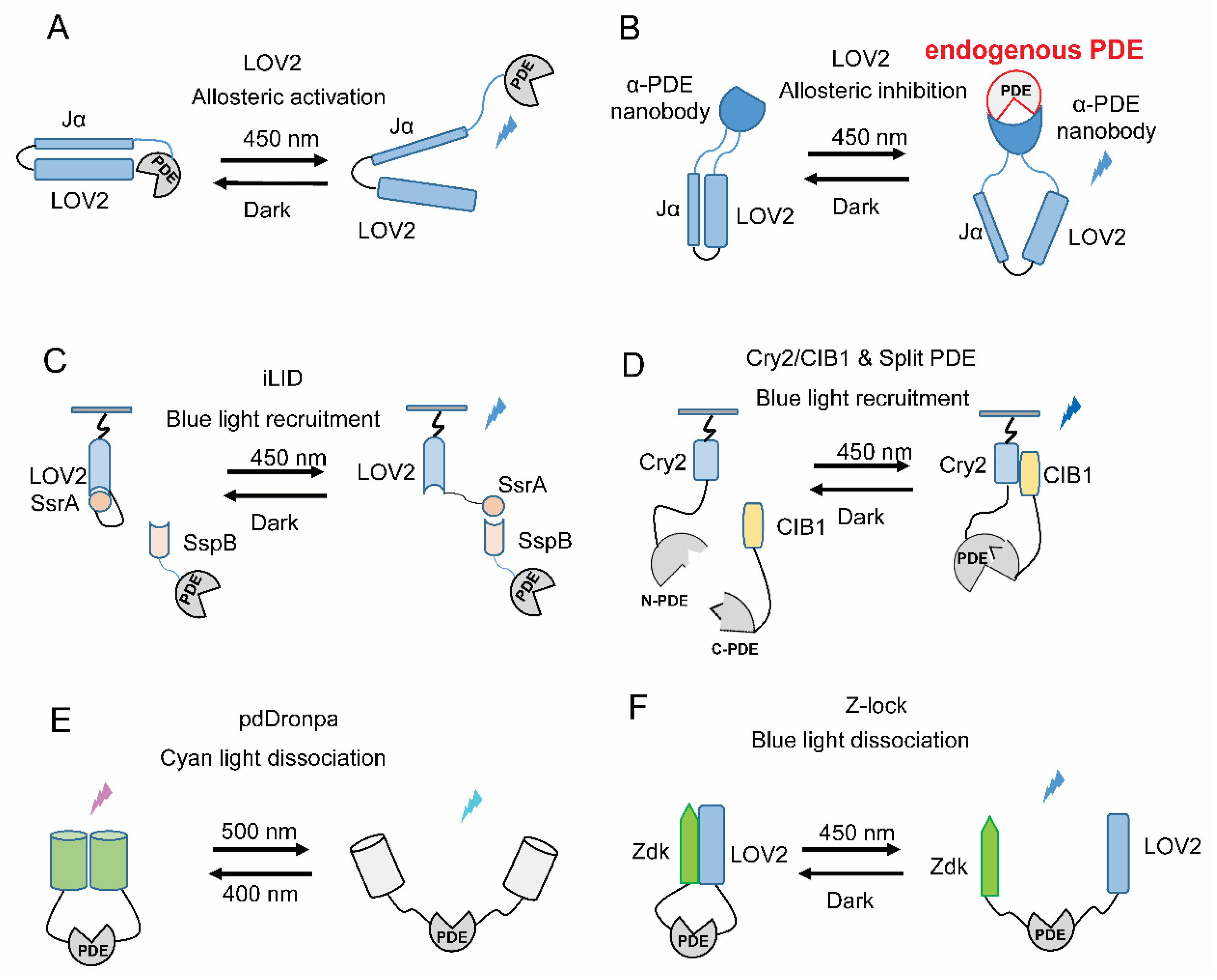
5. Strategies for Engineering New Light-Regulated PDEs
5.1. Allosteric Light Regulation
5.2. Light-Induced Translocation
5.3. Light-Gated Recovery of Split PDEs
5.4. Light-Gated Uncaging of PDEs
6. Applications of Light-Gated PDEs
7. Conclusions
Funding
Conflicts of Interest
References
- Vuong, T.M.; Chabre, M.; Stryer, L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature 1984, 311, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Yan, C.; Bornfeldt, K.E.; Beavo, J.A. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ. Res. 2003, 93, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Dodge-Kafka, K.L.; Langeberg, L.; Scott, J.D. Compartmentation of cyclic nucleotide signaling in the heart: The role of A-kinase anchoring proteins. Circ. Res. 2006, 98, 993–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, Y.; Sandoz, J.C.; Devaud, J.M.; Lormant, F.; Mizunami, M.; Giurfa, M. Cyclic nucleotide-gated channels, calmodulin, adenylyl cyclase, and calcium/calmodulin-dependent protein kinase II are required for late, but not early, long-term memory formation in the honeybee. Learn. Mem. 2014, 21, 272–286. [Google Scholar] [CrossRef] [Green Version]
- Bassler, J.; Schultz, J.E.; Lupas, A.N. Adenylate cyclases: Receivers, transducers, and generators of signals. Cell Signal. 2018, 46, 135–144. [Google Scholar] [CrossRef]
- Khannpnavar, B.; Mehta, V.; Qi, C.; Korkhov, V. Structure and function of adenylyl cyclases, key enzymes in cellular signaling. Curr. Opin. Struct. Biol. 2020, 63, 34–41. [Google Scholar] [CrossRef]
- Linder, J.U.; Schultz, J.E. The class III adenylyl cyclases: Multi-purpose signalling modules. Cell. Signal. 2003, 15, 1081–1089. [Google Scholar] [CrossRef]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol. 2006, 296, 353–362. [Google Scholar] [CrossRef]
- Steegborn, C. Structure, mechanism, and regulation of soluble adenylyl cyclases—Similarities and differences to transmembrane adenylyl cyclases. Biochim. Biophys. Acta 2014, 1842, 2535–2547. [Google Scholar] [CrossRef] [Green Version]
- Halls, M.L.; Cooper, D.M.F. Regulation by Ca2+-Signaling Pathways of Adenylyl Cyclases. Cold Spring Harb. Perspect. Biol. 2011, 3, a004143. [Google Scholar] [CrossRef]
- Wang, H.; Storm, D.R. Calmodulin-Regulated Adenylyl Cyclases: Cross-Talk and Plasticity in the Central Nervous System. Mol. Pharmacol. 2003, 63, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanoune, J.; Defer, N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 145–174. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.J.; Kass, D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009, 122, 216–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, I.N. Receptor Guanylyl Cyclases in Sensory Processing. Front. Endocrinol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Chen, C.-C.; Lin, Y.-C.; Breer, H.; Fleischer, J.; Yang, R.-B. Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures. EMBO J. 2015, 34, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Zabel, U.; Kleinschnitz, C.; Oh, P.; Nedvetsky, P.; Smolenski, A.; Müller, H.; Kronich, P.; Kugler, P.; Walter, U.; Schnitzer, J.E.; et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat. Cell Biol. 2002, 4, 307–311. [Google Scholar] [CrossRef]
- Sharin, V.G.; Mujoo, K.; Kots, A.Y.; Martin, E.; Murad, F.; Sharina, I.G. Nitric oxide receptor soluble guanylyl cyclase undergoes splicing regulation in differentiating human embryonic cells. Stem Cells Dev. 2011, 20, 1287–1293. [Google Scholar] [CrossRef] [Green Version]
- Krumenacker, J.S.; Hanafy, K.A.; Murad, F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res. Bull. 2004, 62, 505–515. [Google Scholar] [CrossRef]
- Gomelsky, M.; Galperin, M.Y. Bacterial second messengers, cGMP and c-di-GMP, in a quest for regulatory dominance. EMBO J. 2013, 32, 2421–2423. [Google Scholar] [CrossRef] [Green Version]
- Wehbe, N.; Nasser, S.A.; Al-Dhaheri, Y.; Iratni, R.; Bitto, A.; El-Yazbi, A.F.; Badran, A.; Kobeissy, F.; Baydoun, E.; Eid, A.H. EPAC in Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2020, 21, 5160. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Schindler, R. New kids on the block: The Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle. Cell. Signal. 2017, 40, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Poon, K.L.; Simrick, S.; Schindler, R.F.R. The Popeye Domain Containing Genes and cAMP Signaling. J. Cardiovasc. Dev. Dis. 2014, 1, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Brand, T. The Popeye Domain Containing Genes and Their Function as cAMP Effector Proteins in Striated Muscle. J. Cardiovasc. Dev. Dis. 2018, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teich, A.F.; Nicholls, R.E.; Puzzo, D.; Fiorito, J.; Purgatorio, R.; Fa’, M.; Arancio, O. Synaptic Therapy in Alzheimer’s Disease: A CREB-centric Approach. Neurotherapeutics 2015, 12, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Pilz, R.B.; Casteel, D.E. Regulation of gene expression by cyclic GMP. Circ. Res. 2003, 93, 1034–1046. [Google Scholar] [CrossRef] [Green Version]
- Rainer, P.P.; Kass, D.A. Old dog, new tricks: Novel cardiac targets and stress regulation by protein kinase G. Cardiovasc. Res. 2016, 111, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Oeing, C.U.; Mishra, S.; Dunkerly-Eyring, B.L.; Ranek, M.J. Targeting Protein Kinase G to Treat Cardiac Proteotoxicity. Front. Physiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Buxton, I.L.; Brunton, L.L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem. 1983, 258, 10233–10239. [Google Scholar] [PubMed]
- Kritzer, M.D.; Li, J.; Dodge-Kafka, K.; Kapiloff, M.S. AKAPs: The architectural underpinnings of local cAMP signaling. J. Mol. Cell. Cardiol. 2012, 52, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Quesada, O.; Mayrhofer, J.E.; Stefan, E. The many faces of compartmentalized PKA signalosomes. Cell. Signal. 2017, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ercu, M.; Klussmann, E. Roles of A-Kinase Anchoring Proteins and Phosphodiesterases in the Cardiovascular System. J. Cardiovasc. Dev. Dis. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Ye, F.; Bastidas, A.C.; Kornev, A.P.; Wu, J.; Ginsberg, M.H.; Taylor, S.S. An Isoform-Specific Myristylation Switch Targets Type II PKA Holoenzymes to Membranes. Structure 2015, 23, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Guinzberg, R.; Díaz-Cruz, A.; Acosta-Trujillo, C.; Vilchis-Landeros, M.M.; Vázquez-Meza, H.; Lozano-Flores, C.; Chiquete-Felix, N.; Varela-Echavarría, A.; Uribe-Carvajal, S.; Riveros-Rosas, H.; et al. Newly synthesized cAMP is integrated at a membrane protein complex signalosome to ensure receptor response specificity. FEBS J. 2017, 284, 258–276. [Google Scholar] [CrossRef] [Green Version]
- Monterisi, S.; Zaccolo, M. Components of the mitochondrial cAMP signalosome. Biochem. Soc. Trans. 2017, 45, 269–274. [Google Scholar] [CrossRef]
- Nikolaev, V.O.; Bunemann, M.; Hein, L.; Hannawacker, A.; Lohse, M.J. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 2004, 279, 37215–37218. [Google Scholar] [CrossRef] [Green Version]
- Ponsioen, B.; Zhao, J.; Riedl, J.; Zwartkruis, F.; van der Krogt, G.; Zaccolo, M.; Moolenaar, W.H.; Bos, J.L.; Jalink, K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004, 5, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Saulnier, J.L.; Yellen, G.; Sabatini, B.L. A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging. Front. Pharmacol. 2014, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Surdo, N.C.; Berrera, M.; Koschinski, A.; Brescia, M.; Machado, M.R.; Carr, C.; Wright, P.; Gorelik, J.; Morotti, S.; Grandi, E.; et al. FRET biosensor uncovers cAMP nano-domains at beta-adrenergic targets that dictate precise tuning of cardiac contractility. Nat. Commun. 2017, 8, 15031. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jongbloets, B.C.; Xiong, W.H.; Melander, J.B.; Qin, M.; Lameyer, T.J.; Harrison, M.F.; Zemelman, B.V.; Mao, T.; Zhong, H. A Highly Sensitive A-Kinase Activity Reporter for Imaging Neuromodulatory Events in Awake Mice. Neuron 2018, 99, 665–679 e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, A.R.; Tesmer, A.L.; Tantama, M. Dual-Mode FRET and BRET Sensors for Detecting cAMP Dynamics. ACS Omega 2019, 4, 15504–15511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaev, V.O.; Gambaryan, S.; Lohse, M.J. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat. Methods 2006, 3, 23–25. [Google Scholar] [CrossRef]
- Wen, L.; Feil, S.; Wolters, M.; Thunemann, M.; Regler, F.; Schmidt, K.; Friebe, A.; Olbrich, M.; Langer, H.; Gawaz, M.; et al. A shear-dependent NO-cGMP-cGKI cascade in platelets acts as an auto-regulatory brake of thrombosis. Nat. Commun. 2018, 9, 4301. [Google Scholar] [CrossRef]
- Woldemariam, S.; Nagpal, J.; Hill, T.; Li, J.; Schneider, M.W.; Shankar, R.; Futey, M.; Varshney, A.; Ali, N.; Mitchell, J.; et al. Using a Robust and Sensitive GFP-Based cGMP Sensor for Real-Time Imaging in Intact Caenorhabditis elegans. Genetics 2019, 213, 59–77. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef] [Green Version]
- Heikaus, C.C.; Pandit, J.; Klevit, R.E. Cyclic Nucleotide Binding GAF Domains from Phosphodiesterases: Structural and Mechanistic Insights. Structure 2009, 17, 1551–1557. [Google Scholar] [CrossRef] [Green Version]
- Kokkonen, K.; Kass, D.A. Nanodomain Regulation of Cardiac Cyclic Nucleotide Signaling by Phosphodiesterases. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 455–479. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef] [Green Version]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic Nucleotide Phosphodiesterases: Molecular Regulation to Clinical Use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Baliga, R.S.; Zhao, L.; Madhani, M.; Lopez-Torondel, B.; Visintin, C.; Selwood, D.; Wilkins, M.R.; MacAllister, R.J.; Hobbs, A.J. Synergy between Natriuretic Peptides and Phosphodiesterase 5 Inhibitors Ameliorates Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 861–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H. Advances in diagnosis and treatment in patients with pulmonary arterial hypertension. Catheter. Cardiovasc. Interv. 2008, 71, 205–213. [Google Scholar] [CrossRef]
- Anantharaman, V.; Koonin, E.V.; Aravind, L. Regulatory Potential, Phyletic Distribution and Evolution of Ancient, Intracellular Small-Molecule-Binding Domains. J. Mol. Biol. 2001, 307, 1271–1292. [Google Scholar] [CrossRef]
- Zoraghi, R.; Corbin, J.D.; Francis, S.H. Properties and Functions of GAF Domains in Cyclic Nucleotide Phosphodiesterases and Other Proteins. Mol. Pharmacol. 2004, 65, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Omar, F.; Findlay, J.E.; Carfray, G.; Allcock, R.W.; Jiang, Z.; Moore, C.; Muir, A.L.; Lannoy, M.; Fertig, B.A.; Mai, D.; et al. Small-molecule allosteric activators of PDE4 long form cyclic AMP phosphodiesterases. Proc. Natl. Acad. Sci. USA 2019, 116, 13320–13329. [Google Scholar] [CrossRef] [Green Version]
- Arshavsky, V.Y.; Lamb, T.D.; Pugh, E.N. G Proteins and Phototransduction. Annu. Rev. Physiol. 2002, 64, 153–187. [Google Scholar] [CrossRef] [Green Version]
- Shichida, Y.; Matsuyama, T. Evolution of opsins and phototransduction. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2881–2895. [Google Scholar] [CrossRef]
- Palczewski, K. Chemistry and Biology of Vision. J. Biol. Chem. 2012, 287, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veleri, S.; Lazar, C.H.; Chang, B.; Sieving, P.A.; Banin, E.; Swaroop, A. Biology and therapy of inherited retinal degenerative disease: Insights from mouse models. Dis. Models Mech. 2015, 8, 109–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, C.; Taiber, S.; Yeh, C.-M.; Wittig, C.H.; Hegemann, P.; Ryu, S.; Wunder, F.; Möglich, A. Engineering of a red-light–activated human cAMP/cGMP-specific phosphodiesterase. Proc. Natl. Acad. Sci. USA 2014, 111, 8803–8808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, J.; Forman, M.D.; Fennell, K.F.; Dillman, K.S.; Menniti, F.S. Mechanism for the allosteric regulation of phosphodiesterase 2A deduced from the X-ray structure of a near full-length construct. Proc. Natl. Acad. Sci. USA 2009, 106, 18225–18230. [Google Scholar] [CrossRef] [Green Version]
- Brunet, T.; Larson, B.T.; Linden, T.A.; Vermeij, M.J.A.; McDonald, K.; King, N. Light-regulated collective contractility in a multicellular choanoflagellate. Science 2019, 366, 326–334. [Google Scholar] [CrossRef]
- Clement, M.; Daniel, G.; Trelles, M. Optimising the design of a broad-band light source for the treatment of skin. J. Cosmet. Laser Ther. 2005, 7, 177–189. [Google Scholar] [CrossRef]
- Stabel, R.; Stüven, B.; Hansen, J.N.; Körschen, H.G.; Wachten, D.; Möglich, A. Revisiting and Redesigning Light-Activated Cyclic-Mononucleotide Phosphodiesterases. J. Mol. Biol. 2019, 431, 3029–3045. [Google Scholar] [CrossRef]
- Avelar, G.M.; Schumacher, R.I.; Zaini, P.A.; Leonard, G.; Richards, T.A.; Gomes, S.L. A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr. Biol. 2014, 24, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Tsunoda, S.P.; Brown, L.S.; Kandori, H. A unique choanoflagellate enzyme rhodopsin exhibits light-dependent cyclic nucleotide phosphodiesterase activity. J. Biol. Chem. 2017, 292, 7531–7541. [Google Scholar] [CrossRef] [Green Version]
- Lamarche, L.B.; Kumar, R.P.; Trieu, M.M.; Devine, E.L.; Cohen-Abeles, L.E.; Theobald, D.L.; Oprian, D.D. Purification and Characterization of RhoPDE, a Retinylidene/Phosphodiesterase Fusion Protein and Potential Optogenetic Tool from the Choanoflagellate Salpingoeca rosetta. Biochemistry 2017, 56, 5812–5822. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.H.; Gao, S.Q.; Yang, S.; Nagel, G. A novel rhodopsin phosphodiesterase from Salpingoeca rosetta shows light-enhanced substrate affinity. Biochem. J. 2018, 475, 1121–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuta, T.; Shihoya, W.; Sugiura, M.; Yoshida, K.; Watari, M.; Tokano, T.; Yamashita, K.; Katayama, K.; Tsunoda, S.P.; Uchihashi, T.; et al. Structural insights into the mechanism of rhodopsin phosphodiesterase. bioRxiv 2020, 2020.04.14.040642. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.J.; Fozouni, P.; Eisen, M.B.; King, N. Gene family innovation, conservation and loss on the animal stem lineage. eLife 2018, 7, e34226. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Tsunoda, S.P.; Hibi, M.; Kandori, H. Molecular Properties of New Enzyme Rhodopsins with Phosphodiesterase Activity. ACS Omega 2020, 5, 10602–10609. [Google Scholar] [CrossRef]
- Schroder-Lang, S.; Schwarzel, M.; Seifert, R.; Strunker, T.; Kateriya, S.; Looser, J.; Watanabe, M.; Kaupp, U.B.; Hegemann, P.; Nagel, G. Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 2007, 4, 39–42. [Google Scholar] [CrossRef]
- Stierl, M.; Stumpf, P.; Udwari, D.; Gueta, R.; Hagedorn, R.; Losi, A.; Gartner, W.; Petereit, L.; Efetova, M.; Schwarzel, M.; et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 2011, 286, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Nagpal, J.; Schneider, M.W.; Kozjak-Pavlovic, V.; Nagel, G.; Gottschalk, A. Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp. Nat. Commun. 2015, 6, 8046. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Gao, S.; von der Heyde, E.L.; Hallmann, A.; Nagel, G. Two-component cyclase opsins of green algae are ATP-dependent and light-inhibited guanylyl cyclases. BMC Biol. 2018, 16, 144. [Google Scholar] [CrossRef]
- Scheib, U.; Broser, M.; Constantin, O.M.; Yang, S.; Gao, S.; Mukherjee, S.; Stehfest, K.; Nagel, G.; Gee, C.E.; Hegemann, P. Rhodopsin-cyclases for photocontrol of cGMP/cAMP and 2.3 A structure of the adenylyl cyclase domain. Nat. Commun. 2018, 9, 2046. [Google Scholar] [CrossRef]
- Scheib, U.; Stehfest, K.; Gee, C.E.; Körschen, H.G.; Fudim, R.; Oertner, T.G.; Hegemann, P. The rhodopsin–guanylyl cyclase of the aquatic fungus Blastocladiella emersonii enables fast optical control of cGMP signaling. Sci. Signal. 2015, 8, rs8. [Google Scholar] [CrossRef] [Green Version]
- Weissenberger, S.; Schultheis, C.; Liewald, J.F.; Erbguth, K.; Nagel, G.; Gottschalk, A. PACalpha—An optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J. Neurochem. 2011, 116, 616–625. [Google Scholar] [CrossRef]
- Ryu, M.H.; Moskvin, O.V.; Siltberg-Liberles, J.; Gomelsky, M. Natural and Engineered Photoactivated Nucleotidyl Cyclases for Optogenetic Applications. J. Biol. Chem. 2010, 285, 41501–41508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, J.M.; Reymond, P.; Powell, G.K.; Bernasconi, P.; Raibekas, A.A.; Liscum, E.; Briggs, W.R. NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 1998, 282, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.M.; Neil, L.C.; Gardner, K.H. Structural Basis of a Phototropin Light Switch. Science 2003, 301, 1541–1544. [Google Scholar] [CrossRef]
- Halavaty, A.S.; Moffat, K. N- and C-Terminal Flanking Regions Modulate Light-Induced Signal Transduction in the LOV2 Domain of the Blue Light Sensor Phototropin 1 from Avena sativa. Biochemistry 2007, 46, 14001–14009. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Rosen, M.K.; Gardner, K.H. Estimation of the available free energy in a LOV2-Jα photoswitch. Nat. Chem. Biol. 2008, 4, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.I.; Frey, D.; Lungu, O.I.; Jaehrig, A.; Schlichting, I.; Kuhlman, B.; Hahn, K.M. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 2009, 461, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagliyan, O.; Tarnawski, M.; Chu, P.H.; Shirvanyants, D.; Schlichting, I.; Dokholyan, N.V.; Hahn, K.M. Engineering extrinsic disorder to control protein activity in living cells. Science 2016, 354, 1441–1444. [Google Scholar] [CrossRef] [Green Version]
- Gil, A.A.; Carrasco-Lopez, C.; Zhu, L.; Zhao, E.M.; Ravindran, P.T.; Wilson, M.Z.; Goglia, A.G.; Avalos, J.L.; Toettcher, J.E. Optogenetic control of protein binding using light-switchable nanobodies. Nat. Commun. 2020, 11, 4044. [Google Scholar] [CrossRef]
- Strickland, D.; Lin, Y.; Wagner, E.; Hope, C.M.; Zayner, J.; Antoniou, C.; Sosnick, T.R.; Weiss, E.L.; Glotzer, M. TULIPs: Tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 2012, 9, 379–384. [Google Scholar] [CrossRef]
- Guntas, G.; Hallett, R.A.; Zimmerman, S.P.; Williams, T.; Yumerefendi, H.; Bear, J.E.; Kuhlman, B. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc. Natl. Acad. Sci. USA 2015, 112, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, S.P.; Hallett, R.A.; Bourke, A.M.; Bear, J.E.; Kennedy, M.J.; Kuhlman, B. Tuning the Binding Affinities and Reversion Kinetics of a Light Inducible Dimer Allows Control of Transmembrane Protein Localization. Biochemistry 2016, 55, 5264–5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, M.J.; Hughes, R.M.; Peteya, L.A.; Schwartz, J.W.; Ehlers, M.D.; Tucker, C.L. Rapid blue-light–mediated induction of protein interactions in living cells. Nat. Methods 2010, 7, 973–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, M.; Tepperman, J.M.; Quail, P.H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 1999, 400, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Redchuk, T.A.; Omelina, E.S.; Chernov, K.G.; Verkhusha, V.V. Near-infrared optogenetic pair for protein regulation and spectral multiplexing. Nat. Chem. Biol. 2017, 13, 633–639. [Google Scholar] [CrossRef]
- Zhou, X.X.; Chung, H.K.; Lam, A.J.; Lin, M.Z. Optical Control of Protein Activity by Fluorescent Protein Domains. Science 2012, 338, 810–814. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.X.; Fan, L.Z.; Li, P.; Shen, K.; Lin, M.Z. Optical control of cell signaling by single-chain photoswitchable kinases. Science 2017, 355, 836–842. [Google Scholar] [CrossRef] [Green Version]
- Stone, O.J.; Pankow, N.; Liu, B.; Sharma, V.P.; Eddy, R.J.; Wang, H.; Putz, A.T.; Teets, F.D.; Kuhlman, B.; Condeelis, J.S.; et al. Optogenetic control of cofilin and alphaTAT in living cells using Z-lock. Nat. Chem. Biol. 2019, 15, 1183–1190. [Google Scholar] [CrossRef]
- Mongillo, M.; Tocchetti Carlo, G.; Terrin, A.; Lissandron, V.; Cheung, Y.-F.; Dostmann Wolfgang, R.; Pozzan, T.; Kass David, A.; Paolocci, N.; Houslay Miles, D.; et al. Compartmentalized Phosphodiesterase-2 Activity Blunts β-Adrenergic Cardiac Inotropy via an NO/cGMP-Dependent Pathway. Circ. Res. 2006, 98, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Houslay, M.D.; Baillie, G.S.; Maurice, D.H. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: A molecular toolbox for generating compartmentalized cAMP signaling. Circ. Res. 2007, 100, 950–966. [Google Scholar] [CrossRef]
- Stangherlin, A.; Gesellchen, F.; Zoccarato, A.; Terrin, A.; Fields, L.A.; Berrera, M.; Surdo, N.C.; Craig, M.A.; Smith, G.; Hamilton, G.; et al. cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes. Circ. Res. 2011, 108, 929–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levskaya, A.; Weiner, O.D.; Lim, W.A.; Voigt, C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 2009, 461, 997–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, D.N.; Hansen, J.N.; Rassmann, S.; Stuven, B.; Jikeli, J.E.; Strunker, T.; Korschen, H.G.; Moglich, A.; Wachten, D. Cyclic Nucleotide-Specific Optogenetics Highlights Compartmentalization of the Sperm Flagellum into cAMP Microdomains. Cells 2019, 8, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.N.; Kaiser, F.; Klausen, C.; Stuven, B.; Chong, R.; Bonigk, W.; Mick, D.U.; Moglich, A.; Jurisch-Yaksi, N.; Schmidt, F.I.; et al. Nanobody-directed targeting of optogenetic tools to study signaling in the primary cilium. eLife 2020, 9, e57907. [Google Scholar] [CrossRef] [PubMed]
- Rost, B.R.; Schneider-Warme, F.; Schmitz, D.; Hegemann, P. Optogenetic Tools for Subcellular Applications in Neuroscience. Neuron 2017, 96, 572–603. [Google Scholar] [CrossRef] [Green Version]
- Rich, T.C.; Webb, K.J.; Leavesley, S.J. Can we decipher the information content contained within cyclic nucleotide signals? J. Gen. Physiol. 2014, 143, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Gorshkov, K.; Zhang, J. Visualization of cyclic nucleotide dynamics in neurons. Front. Cell. Neurosci. 2014, 8, 395. [Google Scholar] [CrossRef] [Green Version]
- Klausen, C.; Kaiser, F.; Stuven, B.; Hansen, J.N.; Wachten, D. Elucidating cyclic AMP signaling in subcellular domains with optogenetic tools and fluorescent biosensors. Biochem. Soc. Trans. 2019, 47, 1733–1747. [Google Scholar] [CrossRef]


| Light-Gated PDEs | Km | Kcat, Vmax or Turnover | L/D Ratio | References |
|---|---|---|---|---|
| LAPD | Dark (cGMP): ~440 μM | Dark turnover (cGMP): ~ 42 s−1 | ~6 (cGMP) | [63] |
| 690 nm (cGMP): ~340 μM | Red 690 nm turnover (cGMP): ~252 s−1 | ~3.6 (cAMP) | ||
| Dark (cAMP): ~470 μM | Dark turnover (cAMP): ~30 s−1 | - | ||
| 690 nm (cAMP): ~180 μM | Red 690 nm turnover (cAMP): ~108 s−1 | - | ||
| Dr-BtPDE2A | - | Red 670 nm s−1 turnover (cGMP): ~225 s−1 | Red/far-red | [67] |
| Far-red 780 nm turnover (cGMP): ~38 s−1 | ~6 (cGMP) | |||
| SrRhoPDE | Dark (cGMP): ~80 μM | Dark turnover (cGMP): ~12 s−1 | 2–6 * (cGMP) | [71] |
| 473 nm (cGMP): ~13 μM | Blue 473 nm turnover (cGMP): ~28 s−1 | ~5 (cAMP) | ||
| MrRh-PDE | - | Dark (cGMP): ~600 pmol·min−1 # | ~1.1 (cGMP) | [74] |
| 520 nm (cGMP): ~672 pmol·min−1 # | ~1.7 (cAMP) | |||
| Dark (cAMP): ~145 pmol·min−1 # | - | |||
| 520 nm (cAMP): ~250 pmol·min−1 # | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Yang, S.; Gao, S. Advances, Perspectives and Potential Engineering Strategies of Light-Gated Phosphodiesterases for Optogenetic Applications. Int. J. Mol. Sci. 2020, 21, 7544. https://doi.org/10.3390/ijms21207544
Tian Y, Yang S, Gao S. Advances, Perspectives and Potential Engineering Strategies of Light-Gated Phosphodiesterases for Optogenetic Applications. International Journal of Molecular Sciences. 2020; 21(20):7544. https://doi.org/10.3390/ijms21207544
Chicago/Turabian StyleTian, Yuehui, Shang Yang, and Shiqiang Gao. 2020. "Advances, Perspectives and Potential Engineering Strategies of Light-Gated Phosphodiesterases for Optogenetic Applications" International Journal of Molecular Sciences 21, no. 20: 7544. https://doi.org/10.3390/ijms21207544
APA StyleTian, Y., Yang, S., & Gao, S. (2020). Advances, Perspectives and Potential Engineering Strategies of Light-Gated Phosphodiesterases for Optogenetic Applications. International Journal of Molecular Sciences, 21(20), 7544. https://doi.org/10.3390/ijms21207544





