Development of Silica-Based Biodegradable Submicrometric Carriers and Investigating Their Characteristics as in Vitro Delivery Vehicles
Abstract
1. Introduction
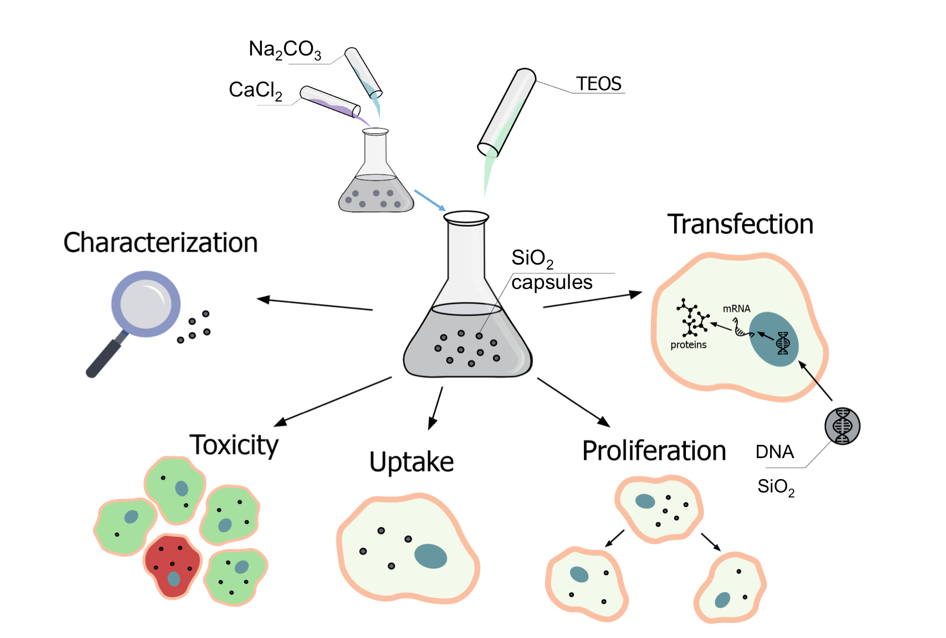
2. Results and Discussion
2.1. Synthesis, Characterization, and Loading of Submicrometric Carriers Based on SiO2 and Polyelectrolytes
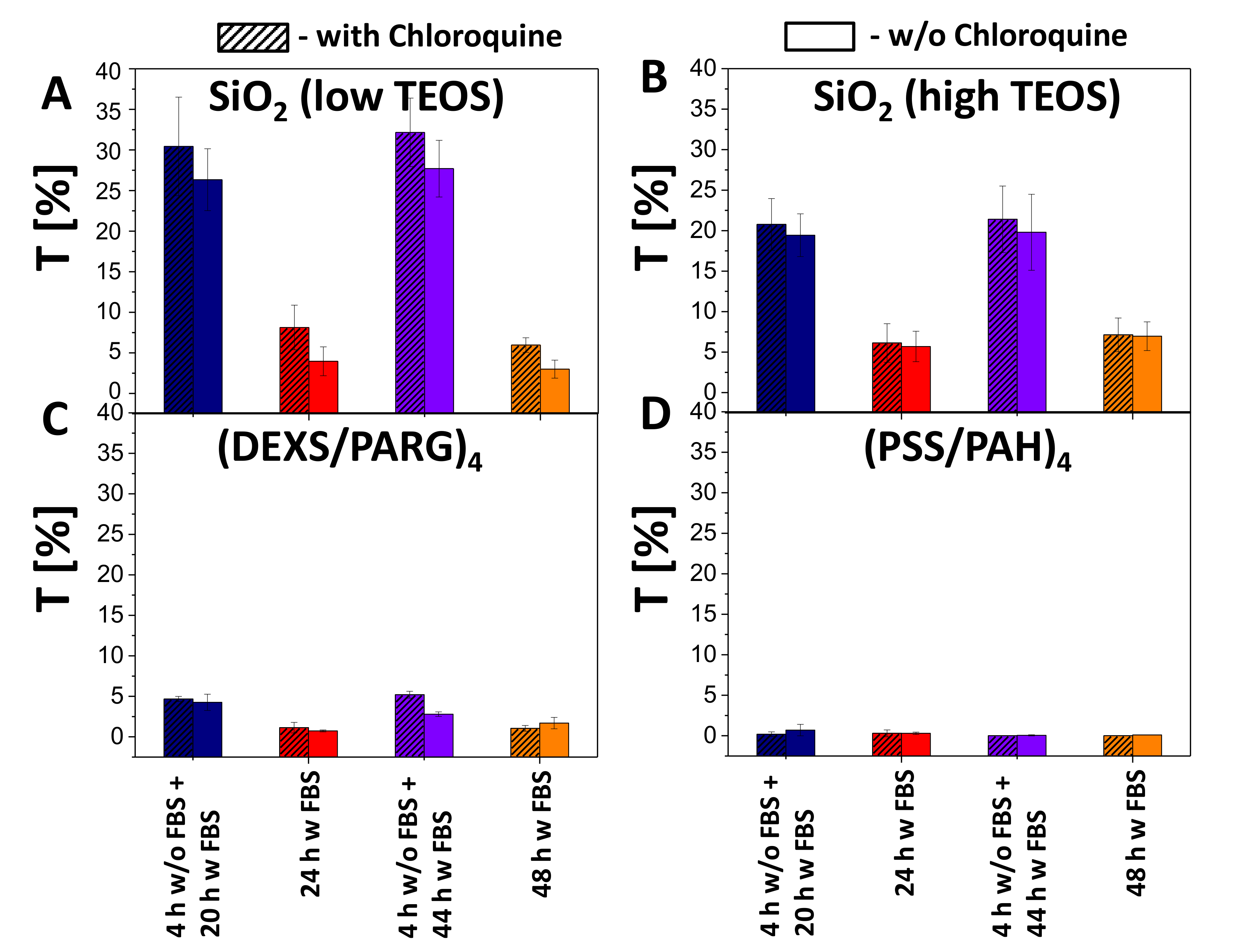
2.2. Biological Characterization of Submicrometric SiO2, (PSS-PAH)4, (DEXS-PARG)4 Capsules
2.2.1. Cell Viability Studies
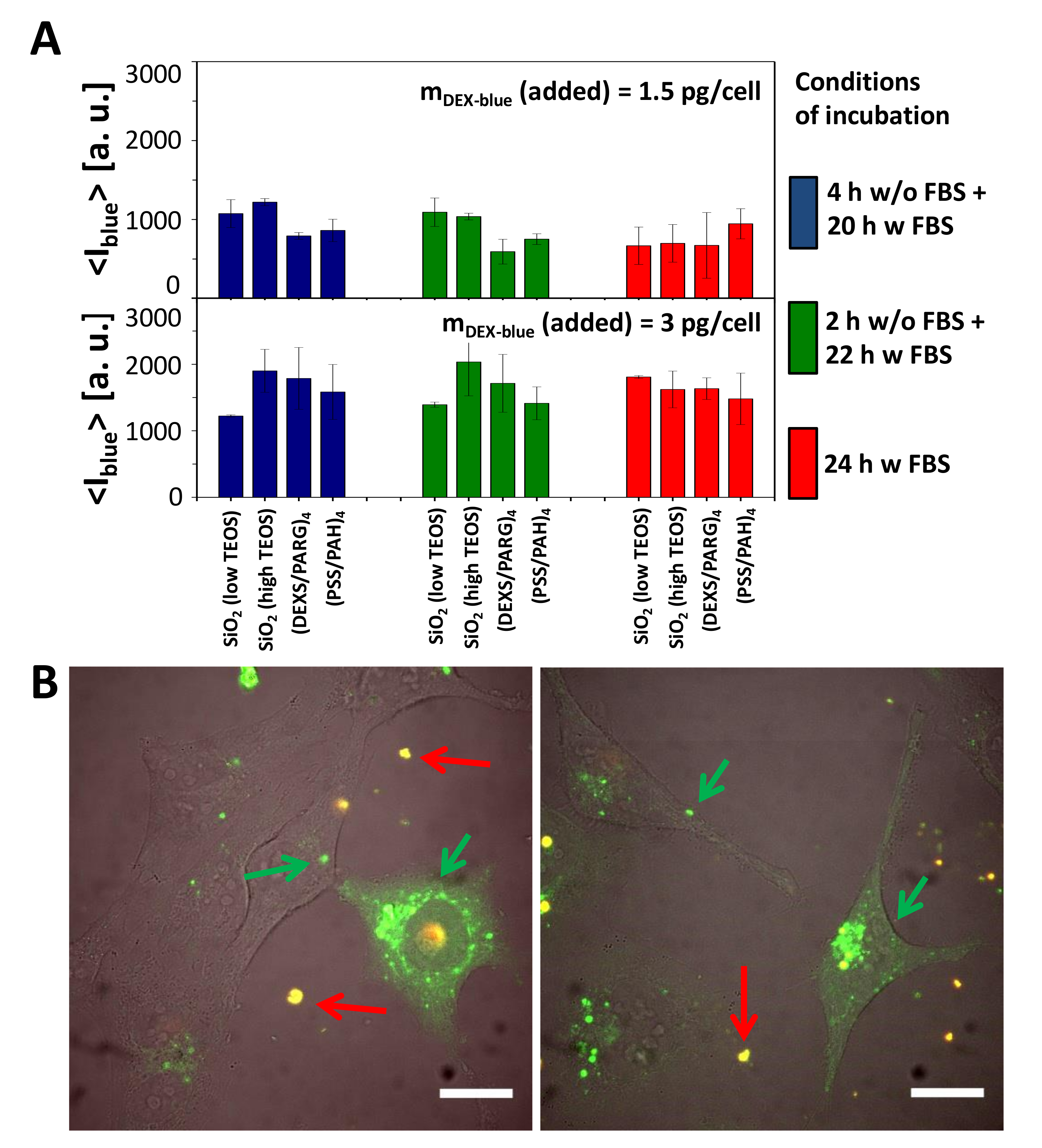
2.2.2. Uptake Studies
2.2.3. Intracellular Degradation of SiO2 Capsules
2.2.4. Stability of DNA in Biological Fluids
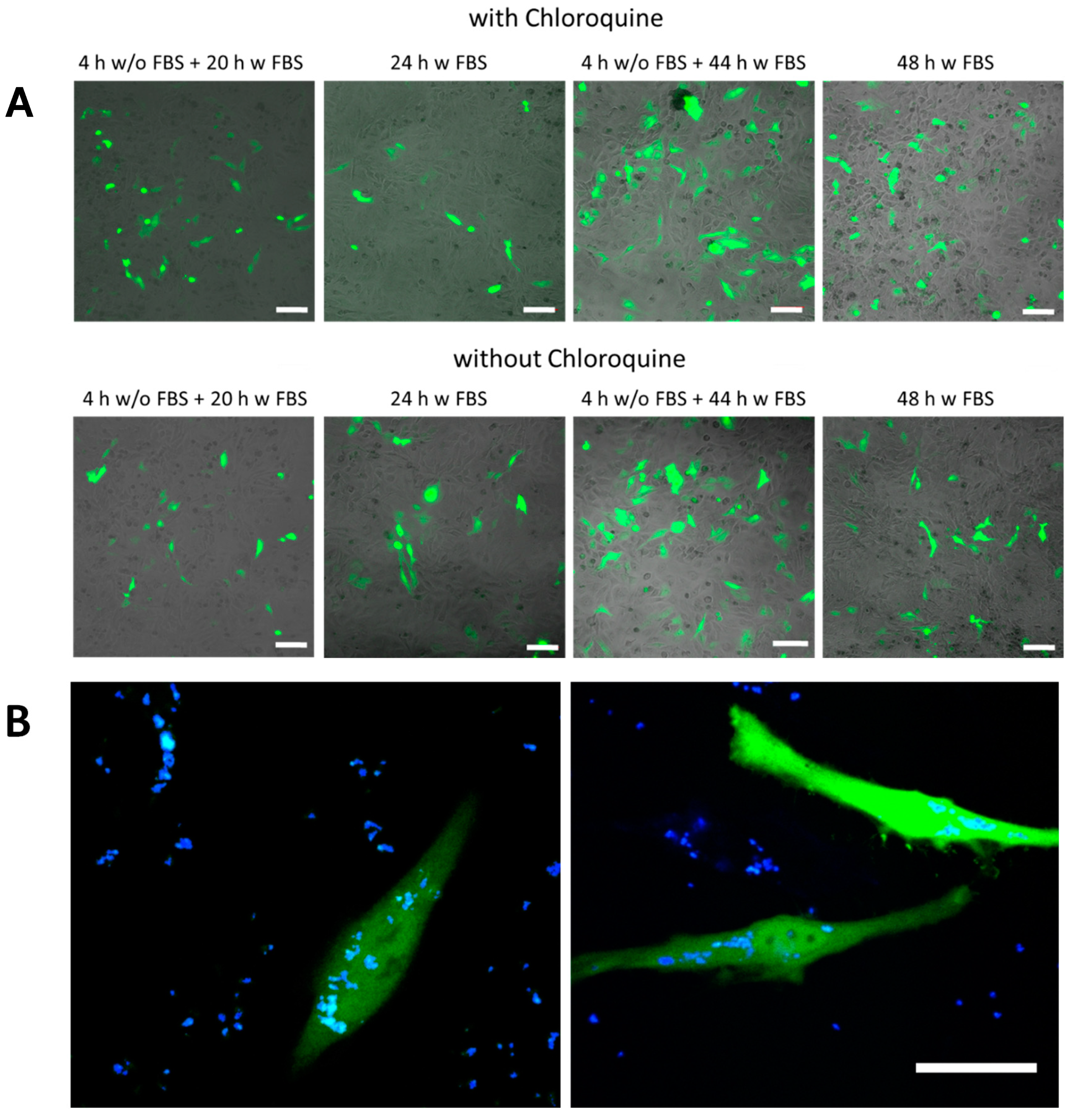
2.2.5. Delivery of DNA Using SiO2-Based Capsules
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Koryakina, I.; Kuznetsova Daria, S.; Zuev Dmitry, A.; Milichko Valentin, A.; Timin Alexander, S.; Zyuzin Mikhail, V. Optically responsive delivery platforms: From the design considerations to biomedical applications. Nanophotonics 2020, 9, 39–74. [Google Scholar] [CrossRef]
- Tarakanchikova, Y.; Alzubi, J.; Pennucci, V.; Follo, M.; Kochergin, B.; Muslimov, A.; Skovorodkin, I.; Vainio, S.; Antipina, M.N.; Atkin, V.; et al. Biodegradable nanocarriers resembling extracellular vesicles deliver genetic material with the highest efficiency to various cell types. Small 2020, 16, 1904880. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Del. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Timin, A.S.; Sukhorukov, G.B. Multilayer capsules inside biological systems: State-of-the-art and open challenges. Langmuir 2019, 35, 4747–4762. [Google Scholar] [CrossRef]
- Kim, C.-K.; Ghosh, P.; Rotello, V.M. Multimodal drug delivery using gold nanoparticles. Nanoscale 2009, 1, 61–67. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311-312, 170–189. [Google Scholar] [CrossRef]
- Timin, A.S.; Peltek, O.O.; Zyuzin, M.V.; Muslimov, A.R.; Karpov, T.E.; Epifanovskaya, O.S.; Shakirova, A.I.; Zhukov, M.V.; Tarakanchikova, Y.V.; Lepik, K.V.; et al. Safe and effective delivery of antitumor drug using mesenchymal stem cells impregnated with submicron carriers. ACS Appl. Mater. Inter. 2019, 11, 13091–13104. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional scaffolds with improved antimicrobial properties and osteogenicity based on piezoelectric electrospun fibers decorated with bioactive composite microcapsules. ACS Appl. Mater. Inter. 2018, 10, 34849–34868. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Díez, P.; Goldsmith, M.; Carregal-Romero, S.; Teodosio, C.; Rejman, J.; Feliu, N.; Escudero, A.; Almendral, M.J.s.; Linne, U. , et al. Comprehensive and systematic analysis of the immunocompatibility of polyelectrolyte capsules. Bioconjugate Chem. 2017, 28, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Mohwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous caco3 particles on their uptake by cells. J. Nanobiotechnol. 2015, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Nazarenus, M.; Zhang, Q.; Soliman, M.G.; del Pino, P.; Pelaz, B.; Carregal_Romero, S.; Rejman, J.; Rothen-Ruthishauser, B.; Clift, M.J.D.; Zellner, R.; et al. In vitro interaction of colloidal nanoparticles with mammalian cells: What have we learned thus far? Beilstein J. Nanotechnol. 2014, 5, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, M.; Mason, D.; Haimovich, G.; Puntes, V.F.; Bergese, P.; Bereta, J.; Lévy, R. Re-evaluating the spherical-nucleic acid theory. ChemRxiv 2018. [Google Scholar] [CrossRef]
- Ganas, C.; Weiß, A.; Nazarenus, M.; Rösler, S.; Kissel, T.; Rivera Gil, P.; Parak, W.J. Biodegradable capsules as non-viral vectors for in vitro delivery of pei/sirna polyplexes for efficient gene silencing. J. Control. Release 2014, 196, 132–138. [Google Scholar] [CrossRef]
- Ochs, M.; Carregal Romero, S.; Rejman, J.; Braeckmans, K.; De Smedt, S.C.; Parak, W.J. Light-addressable capsules as caged compound matrix for controlled in vitro release. Angew. Chem. Int. Ed. 2013, 52, 695–699. [Google Scholar] [CrossRef]
- Ahmed, M.; Narain, R. Cell line dependent uptake and transfection efficiencies of pei–anionic glycopolymer systems. Biomaterials 2013, 34, 4368–4376. [Google Scholar] [CrossRef]
- Dinçer, S.; Türk, M.; Pişkin, E. Intelligent polymers as nonviral vectors. Gene Ther. 2005, 12, S139–S145. [Google Scholar] [CrossRef]
- Lee, C.-S.; Park, W.; Park, S.-J.; Na, K. Endolysosomal environment-responsive photodynamic nanocarrier to enhance cytosolic drug delivery via photosensitizer-mediated membrane disruption. Biomaterials 2013, 34, 9227–9236. [Google Scholar] [CrossRef]
- Ott, A.; Yu, X.; Hartmann, R.; Rejman, J.; Schütz, A.; Ochs, M.; Parak, W.J.; Carregal Romero, S. Light-addressable and degradable silica capsules for delivery of molecular cargo to the cytosol of cells. Chem. Mater. 2015, 27, 1929–1942. [Google Scholar] [CrossRef]
- Knopp, D.; Tang, D.; Niessner, R. Review: Bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal. Chim. Acta 2009, 647, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Linden, M.; et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of notch signaling in cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martinez, A.; Perez-Juste, J.; Liz-Marzan, L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2012, 22, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, R.; Xue, Y.; Chen, L.; Wan, Q.-H. Templated synthesis of monodisperse mesoporous maghemite/silica microspheres for magnetic separation of genomic DNA. J. Magn. Magn. Mater. 2010, 322, 2439–2445. [Google Scholar] [CrossRef]
- Chen, J.-F.; Ding, H.-M.; Wang, J.-X.; Shao, L. Preparation and characterization of porous hollow silica nanoparticles for drug delivery application. Biomaterials 2004, 25, 723–727. [Google Scholar] [CrossRef]
- Delle Piane, M.; Corno, M.; Ugliengo, P. Does dispersion dominate over h-bonds in drug-surface interactions? The case of silica-based materials as excipients and drug-delivery agents. J. Chem. Theory Comput. 2013, 9, 2404–2415. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Epifanovskaya, O.S.; Shakirova, A.I.; Mock, U.; Riecken, K.; Okilova, M.V.; Sergeev, V.S.; Afanasyev, B.V.; et al. Efficient gene editing via non-viral delivery of crispr–cas9 system using polymeric and hybrid microcarriers. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 97–108. [Google Scholar] [CrossRef]
- Sripanyakorn, S.; Jugdaohsingh, R.; Dissayabutr, W.; Anderson, S.H.C.; Thompson, R.P.H.; Powell, J.J. The comparative absorption of silicon from different foods and food supplements. Br. J. Nutr. 2009, 102, 825–834. [Google Scholar] [CrossRef]
- Misra, V.; Rahman, Q.; Viswanathan, P.N. Binding of silicic acid by proteins and its relation to toxicity of silicate dusts. J. Appl. Toxicol. 1983, 3, 135–138. [Google Scholar] [CrossRef]
- Engelbrecht, F.M.; Burger, F.J. The toxicity of silicic acid. S. Afr. J. Lab. Clin. Med. 1961, 7, 16–21. [Google Scholar]
- Soenen, S.J.H.; Rivera Gil, P.; Montenegro, J.M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Cattoën, X.; Wong Chi Man, M.; Durand, J.-O.; Khashab, N.M. Syntheses and applications of periodic mesoporous organosilica nanoparticles. Nanoscale 2015, 7, 20318–20334. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: Fresh outlook and future perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Karpov, T.E.; Peltek, O.O.; Muslimov, A.R.; Tarakanchikova, Y.V.; Grunina, T.M.; Poponova, M.S.; Karyagina, A.S.; Chernozem, R.V.; Pariy, I.O.; Mukhortova, Y.R.; et al. Development of optimized strategies for growth factor incorporation onto electrospun fibrous scaffolds to promote prolonged release. ACS Appl. Mater. Inter. 2020, 12, 5578–5592. [Google Scholar] [CrossRef]
- Muslimov, A.R.; Timin, A.S.; Bichaykina, V.R.; Peltek, O.O.; Karpov, T.E.; Dubavik, A.; Nominé, A.; Ghanbaja, J.; Sukhorukov, G.B.; Zyuzin, M.V. Biomimetic drug delivery platforms based on mesenchymal stem cells impregnated with light-responsive submicron sized carriers. Biomaterials Science 2020, 8, 1137–1147. [Google Scholar] [CrossRef]
- Yeh, Y.-Q.; Chen, B.-C.; Lin, H.-P.; Tang, C.-Y. Synthesis of hollow silica spheres with mesostructured shell using cationic−anionic-neutral block copolymer ternary surfactants. Langmuir 2006, 22, 6–9. [Google Scholar] [CrossRef]
- Alkilany, A.; Murphy, C. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Sung, C.T.; Aljuffali, I.A.; Chang, Y.-T.; Fang, J.-Y. Cationic surfactants in the form of nanoparticles and micelles elicit different human neutrophil responses: A toxicological study. Colloids Surf. B. Biointerfaces 2014, 114, 334–341. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Ruiz-Hernández, E.; Hennink, W.E.; Vallet-Regí, M. Magnetic mesoporous silica-based core/shell nanoparticles for biomedical applications. RSC Advances 2013, 3, 9584–9593. [Google Scholar] [CrossRef]
- Timin, A.S.; Solomonov, A.V.; Kumagai, A.; Miyawaki, A.; Khashirova, S.Y.; Zhansitov, A.; Rumyantsev, E.V. Magnetic polymer-silica composites as bioluminescent sensors for bilirubin detection. Mater. Chem. Phys. 2016, 183, 422–429. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Cassani, M.; Barthel, M.J.; Escudero, A.; Gavilan, H.; Silvestri, N.; Escudero, A.; Scarpellini, A.; Lucchesi, F.; Teran, F.J.; et al. Confining iron oxide nanocubes inside submicrometric cavities as a key strategy to preserve magnetic heat losses in an intracellular environment. ACS Appl. Mat. Interfaces 2019, 11, 41957–41971. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small 2010, 6, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; Geest, B.G.D.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The future of layer-by-layer assembly: A tribute to acs nano associate editor helmuth möhwald. ACS Nano 2019, 13, 66151–66169. [Google Scholar] [CrossRef]
- Kastl, L.; Sasse, D.; Wulf, V.; Hartmann, R.; Mircheski, J.; Ranke, C.; Carregal-Romero, S.; Martínez-López, J.A.; Fernández-Chacón, R.; Parak, W.J.; et al. Multiple internalization pathways of polyelectrolyte multilayer capsules into mammalian cells. ACS Nano 2013, 7, 6605–6618. [Google Scholar] [CrossRef]
- Ashraf, S.; Said, A.H.; Hartmann, R.; Assmann, M.-A.; Feliu, N.; Lenz, P.; Parak, W.J. Quantitative particle uptake by cells as analyzed by different methods. Angew. Chem. 2020, 59, 5438–5453. [Google Scholar] [CrossRef]
- Kirchner, C.; Javier, A.M.; Susha, A.S.; Rogach, A.L.; Kreft, O.; Sukhorukov, G.B.; Parak, W.J. Cytotoxicity of nanoparticle-loaded polymer capsules. Talanta 2005, 67, 486–491. [Google Scholar] [CrossRef]
- Rivera-Gil, P.; Koker, S.D.; Geest, B.G.D.; Parak, W.J. Intracellular processing of proteins mediated by biodegradable polyelectrolyte capsules. Nano Lett. 2009, 9, 4398–4402. [Google Scholar] [CrossRef]
- Roy, S.; Elbaz, N.M.; Parak, W.J.; Feliu, N. Biodegradable alginate polyelectrolyte capsules as plausible biocompatible delivery carriers. ACS Applied Bio Materials 2019, 2, 3245–3256. [Google Scholar] [CrossRef]
- Nazarenus, M.; Abasolo, I.; García-Aranda, N.; Voccoli, V.; Rejman, J.; Cecchini, M.; Jr, S.S.; Rivera_Gil, P.; Parak, W.J. Polymer capsules as a theranostic tool for a universal in vitro screening assay—the case of lysosomal storage diseases. Part. Part. Syst. Charact. 2015, 32, 991–998. [Google Scholar] [CrossRef]
- O'Brien, J.; Wilson, I.; Orton, T.; Pognan, F.O. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, E.J.; Barminko, J.; Schloss, R.S.; Yarmush, M.L. Serum starvation improves transient transfection efficiency in differentiating embryonic stem cells. Biotechnol. Progr. 2010, 26, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.-U.; Coombs, K.M. Serum-reduced media impacts on cell viability and protein expression in human lung epithelial cells. J. Cell. Physiol. 2019, 234, 7718–7724. [Google Scholar] [CrossRef]
- Ferruzza, S.; Rossi, C.S.; Scarino, M.Y. Serum-reduced and serum-free media for differentiation of caco-2 cells. ALTEX - Alternatives to animal experimentation 2013, 30, 159–168. [Google Scholar]
- Ma, X.; Hartmann, R.; Aberasturi, D.J.D.; Yang, F.; Soenen, S.J.H.; Manshian, B.B.; Franz, J.; Valdeperez, D.; Pelaz, B.; Feliu, N.; et al. Colloidal gold nanoparticles induce changes in cellular and subcellular morphology. ACS Nano 2017, 11, 7807–7820. [Google Scholar] [CrossRef]
- Kai, L.; Lee, L.A.; Xiaobing, L.; Qian, W. Fluorogenic “click” reaction for labeling and detection of DNA in proliferating cells. BioTechniques 2010, 49, 525–527. [Google Scholar]
- Semmling, M.; Kreft, O.; Muñoz Javier, A.; Sukhorukov, G.B.; Käs, J.; Parak, W.J. A novel flow-cytometry-based assay for cellular uptake studies of polyelectrolyte microcapsules. Small 2008, 4, 1763–1768. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Yan, Y.; Hartmann, R.; Gause, K.T.; Nazarenus, M.; Cui, J.; Caruso, F.; Parak, W.J. Role of the protein corona derived from human plasma in cellular interactions between nanoporous human serum albumin particles and endothelial cells. Bioconjugate Chem. 2017, 28, 2062–2068. [Google Scholar] [CrossRef]
- James Kain, C.; Pooja, R.; Nandhini, M.; Sankha, B.; Jonathan, S.F. A role for ampk in increased insulin action after serum starvation. Am. J. Physiol.-Cell Physiol. 2010, 299, C1171–C1179. [Google Scholar]
- Wang, J.T.H.; Teasdale, R.D.; Liebl, D. Macropinosome quantitation assay. MethodsX 2014, 1, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Weidenbach, M.; Neubauer, M.; Fery, A.; Parak, W.J. Stiffness-dependent in vitro uptake and lysosomal acidification of colloidal particles. Angew. Chem. Int. Ed. 2015, 54, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Chanana, M.; Rivera_Gil, P.; Correa-Duarte, M.A.; Parak, W.J.; Liz-Marzán, L.M. Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem. Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
- Boonacker, E.; Van Noorden, C.J.F. Enzyme cytochemical techniques for metabolic mapping in living cells, with special reference to proteolysis. J. Histochem. Cytochem. 2001, 49, 1473–1486. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Fujiwara, Y.; Kikuchi, H.; Aizawa, S.; Furuta, A.; Hatanaka, Y.; Konya, C.; Uchida, K.; Wada, K.; Kabuta, T. Direct uptake and degradation of DNA by lysosomes. Autophagy 2013, 9, 1167–1171. [Google Scholar] [CrossRef]
- Rivera Gil, P.; Nazarenus, M.; Ashraf, S.; Parak, W.J. Ph sensitive capsules as intracellular optical reporters for monitoring lysosomal ph changes upon stimulation. Small 2012, 8, 943–948. [Google Scholar] [CrossRef]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef]
- Kantner, K.; Rejman, J.; Kraf, K.V.L.; Soliman, M.G.; Zyuzin, M.V.; Escudero, A.; Pino, P.D.; Parak, W.J. Laterally and temporally controlled intracellular staining by light-triggered release of encapsulated fluorescent markers. Chem. Eur. J 2018, 24, 2098–2102. [Google Scholar] [CrossRef]
- Ditto, A.J.; Shah, P.N.; Yun, Y.H. Non-viral gene delivery using nanoparticles. Expert Opinion on Drug Delivery 2009, 6, 1149–1160. [Google Scholar] [CrossRef]
- Durymanov, M.; Reineke, J. Non-viral delivery of nucleic acids: Insight into mechanisms of overcoming intracellular barriers. Front. Pharmacol. 2018, 9, 971. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Bahrom, H.; Goncharenko, A.A.; Fatkhutdinova, L.I.; Peltek, O.O.; Muslimov, A.R.; Koval, O.Y.; Eliseev, I.E.; Manchev, A.; Gorin, D.; Shishkin, I.I.; et al. Controllable synthesis of calcium carbonate with different geometry: Comprehensive analysis of particle formation, cellular uptake, and biocompatibility. ACS Sustainable Chemistry & Engineering 2019, 7, 19142–19156. [Google Scholar]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2202–2205. [Google Scholar] [CrossRef]
- De-Geest, B.G.; Vandenbroucke, R.E.; Guenther, A.M.; Sukhorukov, G.B.; Hennink, W.E.; Sanders, N.N.; Demeester, J.; Smedt, S.C.D. Intracellularly degradable polyelectrolyte microcapsules. Adv. Mater. 2006, 18, 1005–1009. [Google Scholar] [CrossRef]
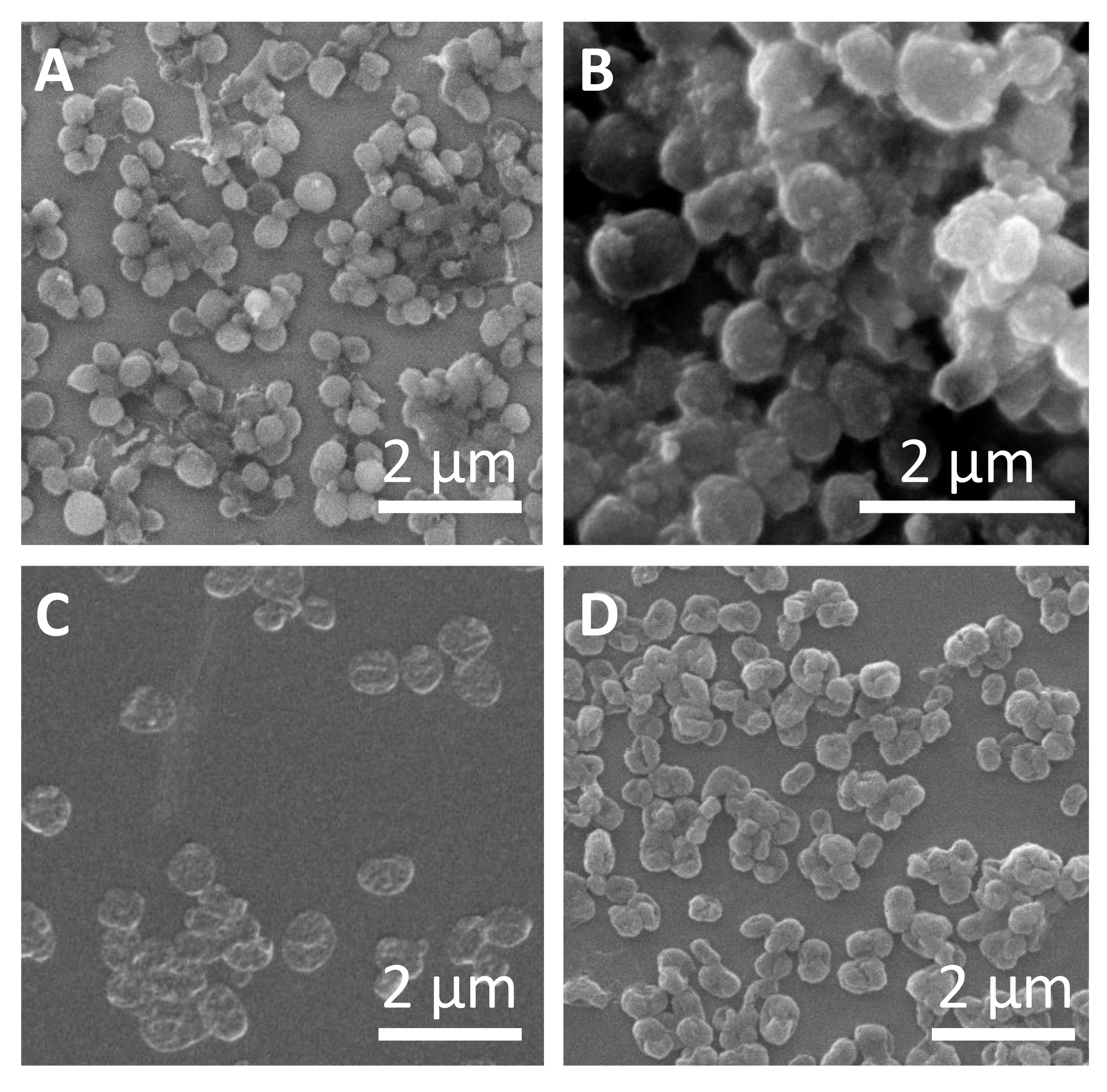

| Capsule Architecture | dc [nm] | dh [nm] | ζ [mV] |
|---|---|---|---|
| SiO2 (low TEOS) | 602 ± 124 | 744 ± 25 | 29 ± 2 |
| SiO2 (high TEOS) | 686 ± 195 | 753 ± 58 | 27± 1 |
| (DEXS/PARG)4 | 625 ± 71 | 762 ± 81 | 20 ± 1 |
| (PSS/PAH)4 | 694 ± 95 | 690 ± 20 | 16 ± 1 |
| Vbio [µL] | Cbio [mg/mL] | |
|---|---|---|
| Dextran labelled with Cascade Blue (DEX-blue) in water | 25 | 6.5 |
| Dextran labelled with Alexa Fluor 647 (DEX-AF647) in water | 25 | 6.5 |
| DQ-Ovalbumin (DQ-OVA) in water | 200 | 2 |
| GFP plasmids in Tris-EDTA buffer together with | 200 | 1.8 |
| Dextran Cascade Blue (DEX-blue) in water | 25 | 6.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyuzin, M.V.; Zhu, D.; Parak, W.J.; Feliu, N.; Escudero, A. Development of Silica-Based Biodegradable Submicrometric Carriers and Investigating Their Characteristics as in Vitro Delivery Vehicles. Int. J. Mol. Sci. 2020, 21, 7563. https://doi.org/10.3390/ijms21207563
Zyuzin MV, Zhu D, Parak WJ, Feliu N, Escudero A. Development of Silica-Based Biodegradable Submicrometric Carriers and Investigating Their Characteristics as in Vitro Delivery Vehicles. International Journal of Molecular Sciences. 2020; 21(20):7563. https://doi.org/10.3390/ijms21207563
Chicago/Turabian StyleZyuzin, Mikhail V., Dingcheng Zhu, Wolfgang J. Parak, Neus Feliu, and Alberto Escudero. 2020. "Development of Silica-Based Biodegradable Submicrometric Carriers and Investigating Their Characteristics as in Vitro Delivery Vehicles" International Journal of Molecular Sciences 21, no. 20: 7563. https://doi.org/10.3390/ijms21207563
APA StyleZyuzin, M. V., Zhu, D., Parak, W. J., Feliu, N., & Escudero, A. (2020). Development of Silica-Based Biodegradable Submicrometric Carriers and Investigating Their Characteristics as in Vitro Delivery Vehicles. International Journal of Molecular Sciences, 21(20), 7563. https://doi.org/10.3390/ijms21207563







