Current Progress in Cross-Linked Peptide Self-Assemblies
Abstract
1. Introduction
2. Peptide-Based Self-Assemblies
3. Effects of Cross-Linking
4. Cross-Linked Self-Assembling Peptides
4.1. Covalent Approach
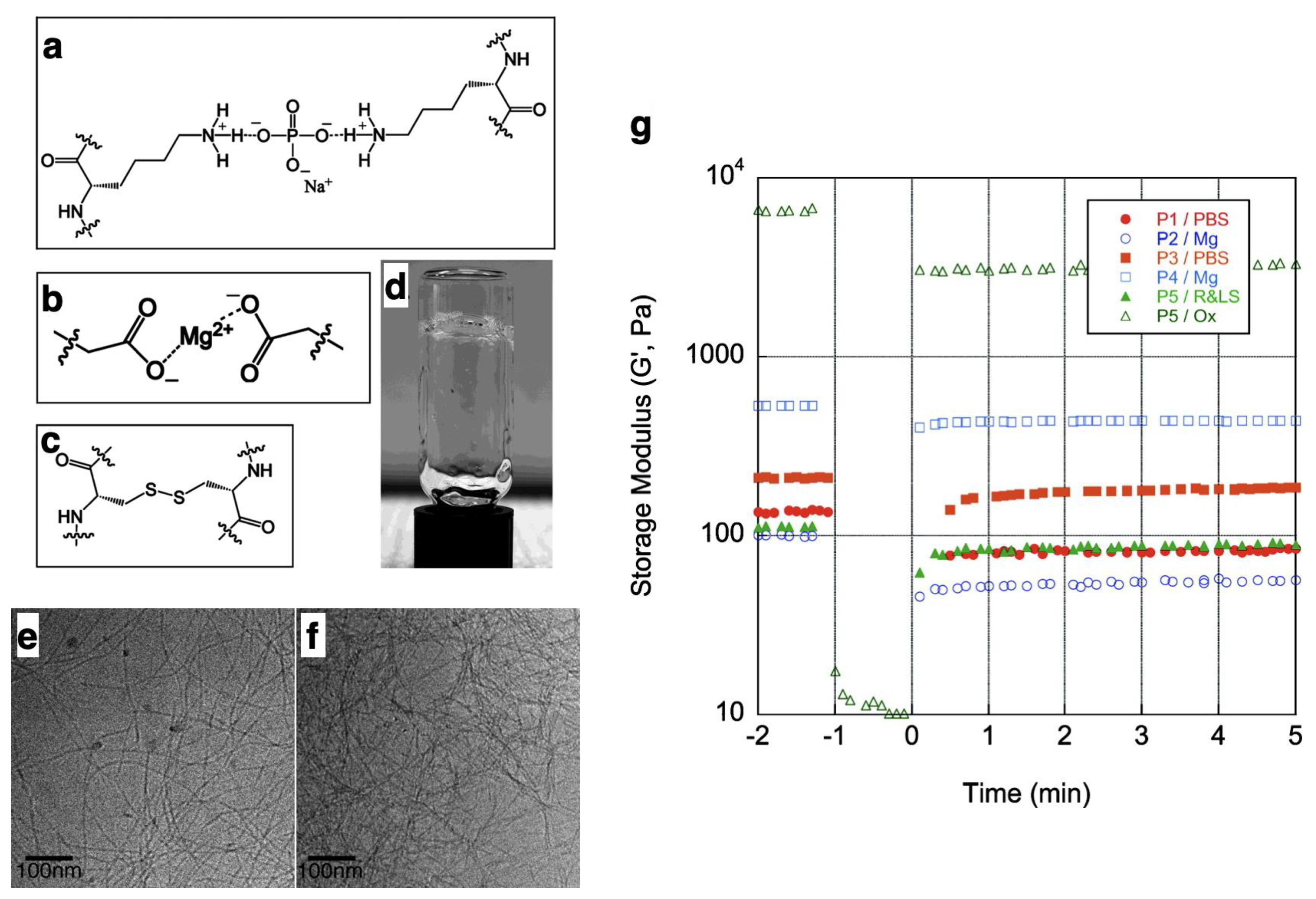
4.2. Non-Covalent Approach
5. Applications
5.1. Cell Scaffold Materials
5.2. Drug Delivery System
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newcomb, C.J.; Sur, S.; Ortony, J.H.; Lee, O.; Matson, J.B.; Boekhoven, J.; Yu, J.M.; Schatz, G.C.; Stupp, S.I. Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat. Commun. 2014, 5, 3321. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ji, W.; Palmer, L.C.; Weber, B.; Barz, M.; Stupp, S.I. Programmable assembly of peptide amphiphile via noncovalent-to-covalent bond conversion. J. Am. Chem. Soc. 2017, 139, 8995–9000. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Cui, H.; Stupp, S.I. Quadruple helix formation of a photoresponsive peptide amphiphile and its light-triggered dissociation into single fibers. J. Am. Chem. Soc. 2008, 130, 2946–2947. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Koh, C.; Cui, H.; Stupp, S.I. Light-triggered bioactivity in three dimensions. Angew. Chem. Int. Ed. 2009, 48, 5946–5949. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Iscen, A.; Sai, H.; Sato, K.; Sather, N.A.; Chin, S.M.; Álvarez, Z.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Supramolecular–covalent hybrid polymers for light-activated mechanical actuation. Nat. Mater. 2020, 19, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Iscen, A.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Light-driven expansion of spiropyran hydrogels. J. Am. Chem. Soc. 2020, 142, 8447–8453. [Google Scholar] [CrossRef]
- Berns, E.J.; Sur, S.; Pan, L.; Goldberger, J.E.; Suresh, S.; Zhang, S.; Kessler, J.A.; Stupp, S.I. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials 2014, 35, 185–195. [Google Scholar] [CrossRef]
- Ortony, J.H.; Newcomb, C.J.; Matson, J.B.; Palmer, L.C.; Doan, P.E.; Ho, B.M.; Stupp, S.I. Internal dynamics of a supramolecular nanofibre. Nat. Mater. 2014, 13, 812–816. [Google Scholar] [CrossRef]
- Schatz, G.C.; Stupp, S.I. Simultaneous covalent and noncovalent hybrid polymerizations. Science 2016, 351, 497–502. [Google Scholar] [CrossRef]
- Hsu, L.; Cvetanovich, G.L.; Stupp, S.I. Peptide amphiphile nanofibers with conjugated polydiacetylene backbones in their core. J. Am. Chem. Soc. 2008, 130, 3892–3899. [Google Scholar] [CrossRef]
- Restuccia, A.; Tian, Y.F.; Collier, J.H.; Hudalla, G.A. Self-assembled glycopeptide nanofibers as modulators of galectin-1 bioactivity. Cell. Mol. Bioeng. 2015, 8, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, M.P.; Sato, K.; Palmer, L.C.; Stupp, S.I. Supramolecular assembly of peptide amphiphiles. Acc. Chem. Res. 2017, 50, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Toft, D.J.; Moyer, T.J.; Standley, S.M.; Ruff, Y.; Ugolkov, A.; Stupp, S.I.; Cryns, V.L. Coassembled cytotoxic and pegylated peptide amphiphiles form filamentous nanostructures with potent antitumor activity in models of breast cancer. ACS Nano 2012, 6, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective differentiation of neural progenitor cells by high–epitope density nanofibers. Science 2004, 303, 1352–1356. [Google Scholar] [CrossRef]
- Ustun Yaylaci, S.; Sardan Ekiz, M.; Arslan, E.; Can, N.; Kilic, E.; Ozkan, H.; Orujalipoor, I.; Ide, S.; Tekinay, A.B.; Guler, M.O. Supramolecular GAG-like self-assembled glycopeptide nanofibers induce chondrogenesis and cartilage regeneration. Biomacromolecules 2016, 17, 679–689. [Google Scholar] [CrossRef]
- Shah, R.N.; Shah, N.A.; Lim, M.M.D.R.; Hsieh, C.; Nuber, G.; Stupp, S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 3293–3298. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1689. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Benlash, E.; Stupp, S.I. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133–5138. [Google Scholar] [CrossRef]
- Silva, R.M.P.; van der Zwaag, D.; Albertazzi, L.; Lee, S.S.; Meijer, E.W.; Stupp, S.I. Super-resolution microscopy reveals structural diversity in molecular exchange among peptide amphiphile nanofibres. Nat. Commun. 2016, 7, 11561. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Hao, R.; Wang, Z.; Hou, Z.; Zhao, Y.; Zong, C.; Xu, H. Transglutaminase-triggered gelation and functionalization of designed self-assembling peptides for guiding cell migration. ACS Appl. Bio Mater. 2018, 1, 2110–2119. [Google Scholar] [CrossRef]
- Mata, A.; Hsu, L.; Capito, R.; Aparicio, C.; Henrikson, K.; Stupp, S.I. Micropatterning of bioactive self-assembling gels. Soft Matter 2009, 5, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.E.; Dombrowski, A.W.; Rubert, C.M.; Bahnson, E.S.M.; Tsihlis, N.D.; Jiang, W.; Jiang, Q.; Vercammen, J.M.; Prakash, V.S.; Pritts, T.A.; et al. Tissue-factor targeted peptide amphiphile nanofibers as an injectable therapy to control hemorrhage. ACS Nano 2016, 12, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Tongers, J.; Newcomb, C.J.; Marquardt, K.; Bauersachs, J.; Losordo, D.W.; Stupp, S.I. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc. Natl. Acad. Sci. USA 2011, 109, 13438–13443. [Google Scholar] [CrossRef] [PubMed]
- So, M.M.; Mansukhani, N.A.; Peters, E.B.; Albaghdadi, M.S.; Wang, Z.; Pérez, C.M.R.; Kibbe, M.R.; Stupp, S.I. Peptide amphiphile nanostructures for targeting of atherosclerotic plaque and drug delivery. Adv. Biosyst. 2018, 2, 1700123. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Pashuck, E.T.; Velichko, Y.S.; Weigand, S.J.; Cheetham, A.G.; Newcomb, C.J.; Stupp, S.I. Spontaneous and X-ray-triggered crystallization at long range in self-assembling filament networks. Science 2010, 327, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.M.; Synatschke, C.V.; Liu, S.; Nap, R.J.; Sather, N.A.; Wang, Q.; Álvarez, Z.; Edelbrock, A.N.; Fyrner, T.; Palmer, L.C.; et al. Covalent-supramolecular hybrid polymers as muscle-inspired anisotropic actuators. Nat. Commun. 2018, 9, 2395. [Google Scholar] [CrossRef] [PubMed]
- Wester, J.R.; Lewis, J.A.; Freeman, R.; Sai, H.; Palmer, L.C.; Henrich, S.E.; Stupp, S.I. Supramolecular exchange among assemblies of opposite charge leads to hierarchical structures. J. Am. Chem. Soc. 2020, 142, 12216–12225. [Google Scholar] [CrossRef]
- Capito, R.M.; Azevedo, H.S.; Velichko, Y.S.; Mata, A.; Stupp, S.I. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 2008, 319, 1812–1817. [Google Scholar] [CrossRef]
- Freeman, R.; Han, M.; Álvarez, Z.; Lewis, J.A.; Wester, J.R.; Stephanopoulos, N.; McClendon, M.T.; Lynsky, C.; Godbe, J.M.; Sangji, H.; et al. Reversible self-assembly of superstructured networks. Science 2018, 362, 808–813. [Google Scholar] [CrossRef]
- Sato, K.; Hendricks, M.P.; Palmer, L.C.; Stupp, S.I. Peptide supramolecular materials for therapeutics. Chem. Soc. Rev. 2018, 47, 7539–7551. [Google Scholar] [CrossRef]
- Chen, C.H.; Palmer, L.C.; Stupp, S.I. Self-repair of structure and bioactivity in a supramolecular nanostructure. Nano Lett. 2018, 18, 6832–6841. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hsu, E.L.; Stupp, S.I. Supramolecular self-assembling peptides to deliver bone morphogenetic proteins for skeletal regeneration. Bone 2020, 141, 115565. [Google Scholar] [CrossRef]
- Stupp, S.I. On supramolecular self-assembly: Interview with Samuel Stupp. Adv. Mater. 2020, 32, 1906741. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Kim, S.H.; Sun, Y.; Kaplan, J.A.; Grinstaff, M.W.; Parquette, J.R. Photo-crosslinking of a self-assembled coumarin-dipeptide hydrogel. New J. Chem. 2015, 39, 3225–3228. [Google Scholar] [CrossRef]
- Fan, T.; Yu, X.; Shen, B.; Sun, L. Peptide self-assembled nanostructures for drug delivery applications. J. Nanomater. 2017, 2017, 4562474. [Google Scholar] [CrossRef]
- Pugliese, R.; Marchini, A.; Saracino, G.A.A.; Zuckermann, R.N.; Gelain, F. Cross-linked self-assembling peptide scaffolds. Nano Res. 2018, 11, 586–602. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, H.; Roy, S. Inducing differential self-assembling behavior in ultrashort peptide hydrogelators using simple metal salts. Biomacromolecules 2019, 20, 2610–2624. [Google Scholar] [CrossRef]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef]
- Li, I.C.; Hartgerink, J.D. Covalent capture of aligned self-assembling nanofibers. J. Am. Chem. Soc. 2017, 139, 8044–8050. [Google Scholar] [CrossRef] [PubMed]
- Bakota, E.L.; Sensoy, O.; Ozgur, B.; Sayar, M.; Hartgerink, J.D. Self-assembling multidomain peptide fibers with aromatic cores. Biomacromolecules 2013, 14, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Liu, Q.; Wickremasinghe, N.C.; Shi, S.; Cornwright, T.T.; Deng, Y.; Azares, A.; Moore, A.N.; Acevedo-jake, A.M.; Agudo, N.R.; et al. Biomaterials treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials 2016, 98, 113–119. [Google Scholar] [CrossRef]
- Leach, D.G.; Dharmaraj, N.; Piotrowski, S.L.; Lopez-silva, T.L.; Lei, Y.L.; Sikora, A.G.; Young, S.; Hartgerink, J.D. STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 2018, 163, 67–75. [Google Scholar] [CrossRef]
- Lopez-silva, T.L.; Leach, D.G.; Azares, A.; Li, I.; Woodside, D.G.; Hartgerink, D. Chemical functionality of multidomain peptide hydrogels governs early host immune response. Biomaterials 2020, 231, 119667. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Tsutsumi, H.; Mihara, H. Cell differentiation on disk- and string-shaped hydrogels fabricated from Ca2+-responsive self-assembling peptides. Biopolymers 2016, 106, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Li, I.; Moore, A.N.; Hartgerink, D. “Missing tooth” multidomain peptide nanofibers for delivery of small molecule drugs. Biomacromolecules 2016, 17, 2087–2095. [Google Scholar] [CrossRef]
- Moore, A.N.; Lopez, T.L.; Carrejo, N.C.; Origel, C.A.; Li, I.; Hartgerink, J.D. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials 2018, 161, 154–163. [Google Scholar] [CrossRef]
- Kumar, V.A.; Shi, S.; Wang, B.K.; Li, I.C.; Jalan, A.A.; Sarkar, B.; Wickremasinghe, N.C.; Hartgerink, J.D. Drug-triggered and cross-linked self-assembling nanofibrous hydrogels. J. Am. Chem. Soc. 2015, 137, 4823–4830. [Google Scholar] [CrossRef]
- Moore, A.N.; Hartgerink, J.D. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Newton, J.M.; Florez, M.A.; Lopez-Silva, T.L.; Jones, A.A.; Young, S.; Sikora, A.G.; Hartgerink, J.D. Drug-mimicking nanofibrous peptide hydrogel for inhibition of inducible nitric oxide synthase. ACS Biomater. Sci. Eng. 2019, 5, 6755–6765. [Google Scholar] [CrossRef]
- Aulisa, L.; Dong, H.; Hartgerink, J.D. Self-assembly of multidomain peptides: Sequence variation allows control over cross-linking and viscoelasticity. Biomacromolecules 2009, 10, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Bakota, E.L.; Aulisa, L.; Galler, K.M.; Hartgerink, J.D. Enzymatic cross-linking of a nanofibrous peptide hydrogel. Biomacromolecules 2011, 12, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Kinoshita, T.; Zhang, S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Watanabe, G.; Oshikawa, M.; Ajioka, I.; Muraoka, T. Glycine substitution effects on the supramolecular morphology and rigidity of cell-adhesive amphiphilic peptides. Chem. Eur. J. 2019, 25, 13523–13530. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Oshikawa, M.; Ajioka, I.; Muraoka, T. Sequence-dependent bioactivity and self-assembling properties of RGD-containing amphiphilic peptides as extracellular scaffolds. ACS Appl. Bio Mater. 2020, 3, 3605–3611. [Google Scholar] [CrossRef]
- Ramadan, D.; Cline, D.J.; Bai, S.; Thorpe, C.; Schneider, J.P. Effects of As (III) binding on hairpin structure. J. Am. Chem. Soc. 2007, 129, 2981–2988. [Google Scholar] [CrossRef]
- Sinthuvanich, C.; Nagy-smith, K.J.; Walsh, S.T.R.; Schneider, J.P. Triggered formation of anionic hydrogels from self-assembling acidic peptide amphiphiles. Macromolecules 2017, 50, 5643–5651. [Google Scholar] [CrossRef]
- Salick, D.A.; Kretsinger, J.K.; Pochan, D.J.; Schneider, J.P. Inherent antibacterial activity of a peptide-based-hairpin hydrogel. J. Am. Chem. Soc. 2007, 129, 14793–14799. [Google Scholar] [CrossRef]
- Veiga, S.A.; Sinthuvanich, C.; Gaspar, D.; Franquelim, H.G.; Castanho, M.A.R.B.; Schneider, J.P. Arginine-rich self-assembling peptides as potent antibacterial gels. Biomaterials 2012, 33, 8907–8916. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.; Rajagopal, K.; Palla, C.S.; Pochan, D.J.; Schneider, J.P.; Furst, E.M. Gelation kinetics of hairpin peptide hydrogel networks. Macromolecules 2006, 39, 6608–6614. [Google Scholar] [CrossRef]
- Larsen, T.H.; Branco, M.C.; Rajagopal, K.; Schneider, J.P.; Furst, E.M. Sequence-dependent gelation kinetics of β-hairpin peptide hydrogels. Macromolecules 2009, 42, 8443–8450. [Google Scholar] [CrossRef] [PubMed]
- Haines, L.A.; Rajagopal, K.; Ozbas, B.; Salick, D.A.; Pochan, D.J.; Schneider, J.P. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 2005, 127, 17025–17029. [Google Scholar] [CrossRef] [PubMed]
- Ozbas, B.; Rajagopal, K.; Schneider, J.P.; Pochan, D.J. Semiflexible chain networks formed via self-assembly of hairpin molecules. Phys. Rev. Lett. 2004, 93, 268106. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Patel, N.L.; Kalen, J.D.; Schneider, J.P. Design of a peptide-based electronegative hydrogel for the direct encapsulation, 3D culturing, in vivo syringe-based delivery, and long-term tissue engraftment of cells. ACS Appl. Mater. Interfaces 2019, 11, 34688–34697. [Google Scholar] [CrossRef]
- Rughani, R.V.; Branco, M.C.; Pochan, D.J.; Schneider, J.P. De novo design of a shear-thin recoverable peptide-based hydrogel capable of intrafibrillar photopolymerization. Macromolecules 2010, 43, 7924–7930. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Salehi, R.; Khalandi, B.; Davaran, S.; Akbarzadeh, A. Nanocomposite hydrogels for cartilage tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 465–471. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef]
- Sano, K.; Arazoe, Y.O.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hikima, T.; Aida, T. Extra-large mechanical anisotropy of a hydrogel with maximized electrostatic repulsion between cofacially aligned 2D electrolytes. Angew. Chem. Int. Ed. 2018, 57, 12508–12513. [Google Scholar] [CrossRef]
- Sun, Z.; Yamauchi, Y.; Araoka, F.; Kim, Y.S.; Bergueiro, J.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hikima, T.; Aida, T. An anisotropic hydrogel actuator enabling earthworm-like directed peristaltic crawling. Angew. Chem. Int. Ed. 2018, 57, 15772–15776. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Liu, M.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. Thermoresponsive actuation enabled by permittivity switching in an electrostatically anisotropic hydrogel. Nat. Mater. 2015, 14, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Kurcok, P. α-Cyclodextrin-based polypseudorotaxane hydrogels. Materials 2020, 13, 133. [Google Scholar] [CrossRef]
- Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Photoswitchable supramolecular hydrogels formed by cyclodextrins and azobenzene polymers. Angew. Chem. Int. Ed. 2010, 49, 7461–7464. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Harada, A. Redox-responsive macroscopic gel assembly based on discrete dual interactions. Angew. Chem. Int. Ed. 2014, 53, 3617–3621. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Gu, H.; Zhu, C.; Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 2012, 41, 3297–3317. [Google Scholar] [CrossRef]
- Dong, J.; Shokes, J.E.; Scott, R.A.; Lynn, D.G. Modulating amyloid self-assembly and fibril morphology with Zn (II). J. Am. Chem. Soc. 2006, 128, 3540–3542. [Google Scholar] [CrossRef]
- Pires, M.M.; Przybyla, D.E.; Rubert Pérez, C.M.; Chmielewski, J. Metal-mediated tandem coassembly of collagen peptides into banded microstructures. J. Am. Chem. Soc. 2011, 133, 14469–14471. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Verma, S. Metalated peptide fibers derived from a natural metal-binding peptide motif. Tetrahedron Lett. 2007, 48, 2189–2192. [Google Scholar] [CrossRef]
- Hsu, W.; Chen, Y.; Horng, J. Promoting self-assembly of collagen-related peptides into various higher-order structures by metal–histidine coordination. Langmuir 2012, 28, 3194–3199. [Google Scholar] [CrossRef]
- Morgan, D.M.; Dong, J.; Jacob, J.; Lu, K.; Apkarian, R.P.; Thiyagarajan, P.; Lynn, D.G. Metal switch for amyloid formation: Insight into the structure of the nucleus. J. Am. Chem. Soc. 2002, 124, 12644–12645. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Xiao, C.; Dong, M.; Liu, Z.; Sheng, Z.; Castellino, F.J.; Prorok, M. Non-strict strand orientation of the Ca2+-induced dimerization of a conantokin peptide variant with sequence-shifted γ-carboxyglutamate residues. Peptides 2009, 30, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, G.R.; McRorie, D.K.; Lear, J.D.; Sharp, K.A.; DeGrado, W.F.; Pecoraro, V.L. The role of protonation and metal chelation preferences in defining the properties of mercury-binding coiled coils. J. Mol. Biol. 1998, 280, 897–912. [Google Scholar] [CrossRef]
- Kohn, W.D.; Kay, C.M.; Sykes, B.D.; Hodges, R.S. Metal ion induced folding of a de novo designed coiled-coil peptide. J. Am. Chem. Soc. 1998, 120, 1124–1132. [Google Scholar] [CrossRef]
- Albrecht, M.; Stortz, P.; Engeser, M.; Schalley, A. Solid-phase synthesis of a double 4-pyridinyl terminated Leu-Ala-Leu tripeptide and macrocyclization by palladium(II) coordination. Synlett 2004, 15, 2821–2823. [Google Scholar] [CrossRef]
- Ikemi, M.; Kikuchi, T.; Matsumura, S.; Shiba, K.; Fujita, M. Peptide-coated, self-assembled M12 L24 coordination spheres and their immobilization onto an inorganic surface. Chem. Sci. 2010, 1, 68–71. [Google Scholar] [CrossRef]
- LeBruin, L.T.; Banerjee, S.; Rourke, B.D.O.; Case, M.A. Metal ion-assembled micro-collagen heterotrimers. Biopolymers 2011, 95, 792–800. [Google Scholar] [CrossRef]
- Scheib, K.A.; Tavenor, N.A.; Horne, W.S.; Lawless, M.J.; Saxena, S. Understanding and controlling the metal-directed assembly of terpyridine-functionalized coiled-coil peptides. Chem. Commun. 2019, 55, 7752–7755. [Google Scholar] [CrossRef]
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K. Synthetic approaches to construct viral capsid-like spherical nanomaterials. Chem. Commun. 2018, 54, 8944–8959. [Google Scholar] [CrossRef] [PubMed]
- Gačanin, J.; Hedrich, J.; Sieste, S.; Glaßer, G.; Lieberwirth, I.; Schilling, C.; Fischer, S.; Barth, H.; Knöll, B.; Synatschke, C.V.; et al. Autonomous ultrafast self-healing hydrogels by pH-responsive functional nanofiber gelators as cell matrices. Adv. Mater. 2019, 31, 1805044. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Kim, Y.S.; Xie, V.Y.; Smith, B.T.; Watson, E.; Lam, J.; Pearce, H.A.; Engel, P.S.; Mikos, A.G. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5, eaaw7396. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, C.J.; Liyanage, W.; Federation, A.J.; Nilsson, B.L. Tuning β-sheet peptide self-assembly and hydrogelation behavior by modification of sequence hydrophobicity and aromaticity. Biomacromolecules 2011, 12, 2735–2745. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, N.; Muraoka, T. Current Progress in Cross-Linked Peptide Self-Assemblies. Int. J. Mol. Sci. 2020, 21, 7577. https://doi.org/10.3390/ijms21207577
Uchida N, Muraoka T. Current Progress in Cross-Linked Peptide Self-Assemblies. International Journal of Molecular Sciences. 2020; 21(20):7577. https://doi.org/10.3390/ijms21207577
Chicago/Turabian StyleUchida, Noriyuki, and Takahiro Muraoka. 2020. "Current Progress in Cross-Linked Peptide Self-Assemblies" International Journal of Molecular Sciences 21, no. 20: 7577. https://doi.org/10.3390/ijms21207577
APA StyleUchida, N., & Muraoka, T. (2020). Current Progress in Cross-Linked Peptide Self-Assemblies. International Journal of Molecular Sciences, 21(20), 7577. https://doi.org/10.3390/ijms21207577




