Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives
Abstract
:1. Introduction
2. Epidemiology of Stroke
3. Pathophysiology of Stroke
4. Risk Factors for Stroke
4.1. Non-Modifiable Risk Factors
4.2. Modifiable Risk Factors
5. Animal Models of Stroke
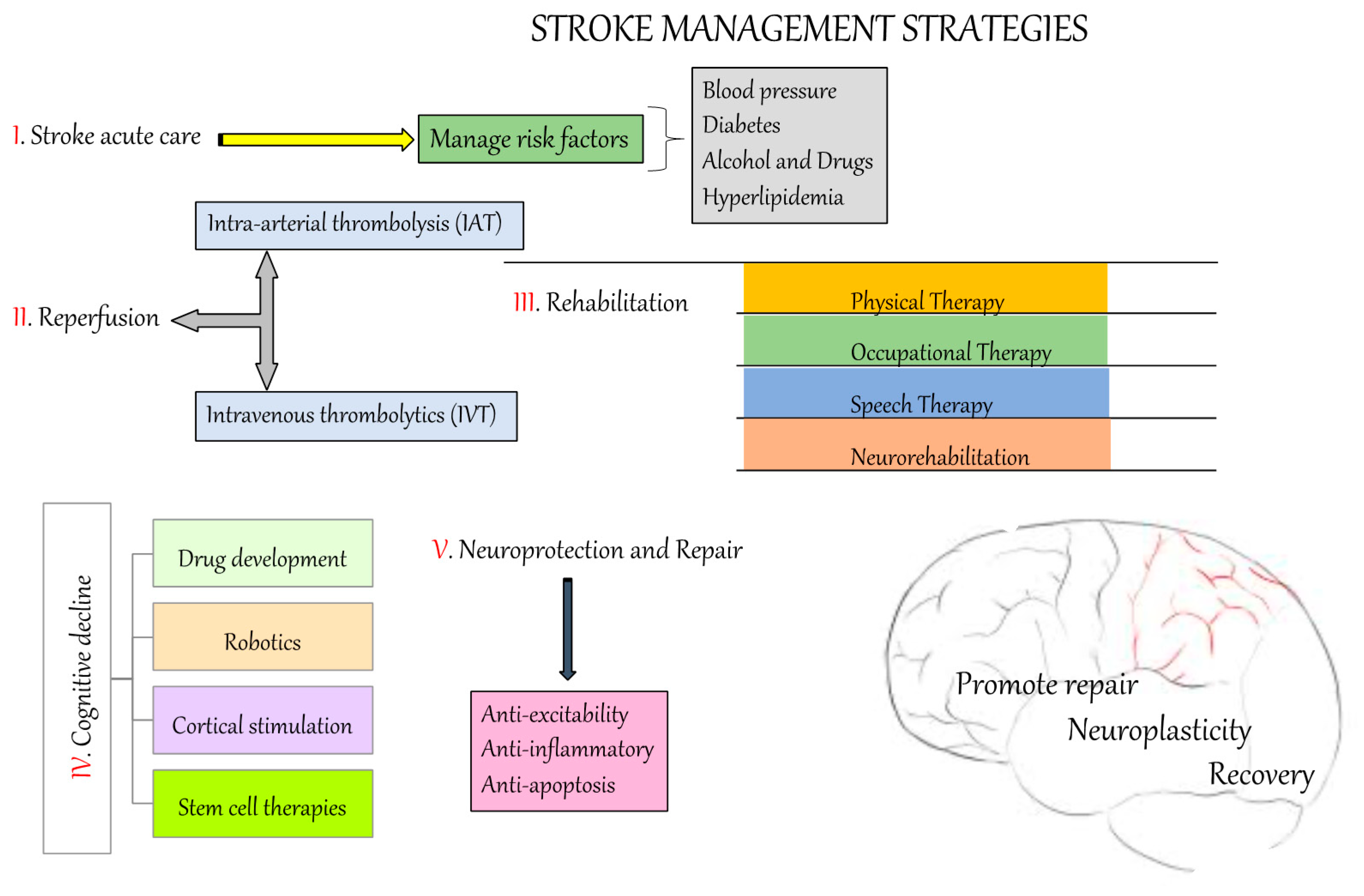
6. Prevention and Treatment Strategies for Stroke
6.1. Reperfusion
6.2. Others
7. Trends in Stroke Research
8. Translational Challenges for the Current Stroke Therapeutic Strategies
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shakir, R. The struggle for stroke reclassification. Nat. Rev. Neurol. 2018, 14, 447–448. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, G.S. Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar]
- Kelly-Hayes, M. Influence of age and health behaviors on stroke risk: Lessons from longitudinal studies. J. Am. Geriatr. Soc. 2010, 58, S325–S328. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Appelros, P.; Stegmayr, B.; Terént, A. Sex differences in stroke epidemiology: A systematic review. Stroke 2009, 40, 1082–1090. [Google Scholar] [CrossRef]
- Reeves, M.J.; Bushnell, C.D.; Howard, G.; Gargano, J.W.; Duncan, P.W.; Lynch, G.; Khatiwoda, A.; Lisabeth, L. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008, 7, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Stuart-Shor, E.M.; Wellenius, G.A.; DelloIacono, D.M.; Mittleman, M.A. Gender differences in presenting and prodromal stroke symptoms. Stroke 2009, 40, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Girijala, R.L.; Sohrabji, F.; Bush, R.L. Sex differences in stroke: Review of current knowledge and evidence. Vasc. Med. 2017, 22, 135–145. [Google Scholar] [CrossRef]
- Chen, J.C. Geographic determinants of stroke mortality: Role of ambient air pollution. Stroke 2010, 41, 839–841. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.L.; Guo, Z.N.; Wu, Y.H.; Liu, H.Y.; Luo, Y.; Sun, M.S.; Xing, Y.Q.; Yang, Y. Prevalence of stroke and associated risk factors: A population based cross sectional study from northeast China. BMJ Open 2017, 7, e015758. [Google Scholar] [CrossRef] [PubMed]
- Kiefe, C.I.; Williams, O.D.; Bild, D.E.; Lewis, C.E.; Hilner, J.E.; Oberman, A. Regional disparities in the incidence of elevated blood pressure among young adults: The CARDIA study. Circulation 1997, 96, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, and 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Nihon Rinsho 2000, 58, 267–275. [Google Scholar]
- Addo, J.; Ayerbe, L.; Mohan, K.M.; Crichton, S.; Sheldenkar, A.; Chen, R.; Wolfe, C.D.; McKevitt, C. Socioeconomic status and stroke: An updated review. Stroke 2012, 43, 1186–1191. [Google Scholar] [CrossRef]
- Sandel, M.E.; Wang, H.; Terdiman, J.; Hoffman, J.M.; Ciol, M.A.; Sidney, S.; Quesenberry, C.; Lu, Q.; Chan, L. Disparities in stroke rehabilitation: Results of a study in an integrated health system in northern California. PM R 2009, 1, 29–40. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wu, D.; Nguyen-Huynh, M.N.; Zhou, Y.; Wang, C.X.; Zhao, X.Q.; Liao, X.L.; Liu, L.P.; Wang, Y.J.; The Prevention of Recurrences of Stroke Study in China (PRESS‐China) Investigators. Antithrombotic management of ischaemic stroke and transient ischaemic attack in China: A consecutive cross-sectional survey. Clin. Exp. Pharmacol. Physiol. 2010, 37, 775–781. [Google Scholar]
- Arrich, J.; Müllner, M.; Lalouschek, W.; Greisenegger, S.; Crevenna, R.; Herkner, H. Influence of socioeconomic status and gender on stroke treatment and diagnostics. Stroke 2008, 39, 2066–2072. [Google Scholar] [CrossRef]
- Kerr, G.D.; Higgins, P.; Walters, M.; Ghosh, S.K.; Wright, F.; Langhorne, P.; Stott, D.J. Socioeconomic status and transient ischaemic attack/stroke: A prospective observational study. Cerebrovasc. Dis. 2011, 31, 130–137. [Google Scholar] [CrossRef]
- Musuka, T.D.; Wilton, S.B.; Traboulsi, M.; Hill, M.D. Diagnosis and management of acute ischemic stroke: Speed is critical. CMAJ 2015, 187, 887–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, B.R.; Reutens, D.C.; Sobey, C.G. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelderblom, M.; Leypoldt, F.; Steinbach, K.; Behrens, D.; Choe, C.U.; Siler, D.A.; Arumugam, T.V.; Orthey, E.; Gerloff, C.; Tolosa, E.; et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009, 40, 1849–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.W.; Shin, B.S.; Ma, H.; Van Hoecke, M.; Brennan, A.M.; Yenari, M.A.; Swanson, R.A. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann. Neurol. 2008, 64, 654–663. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Ali, Z.; Suri, M.F.; Shuaib, A.; Baker, G.; Todd, K.; Guterman, L.R.; Hopkins, L.N. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: An in vivo microdialysis study. Crit. Care Med. 2003, 31, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fields, J.; Zhao, C.; Langer, J.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic. Biol. Med. 2007, 43, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, M.L.; Woo, D.; Haverbusch, M.; Sekar, P.; Khoury, J.; Sauerbeck, L.; Moomaw, C.J.; Schneider, A.; Kissela, B.; Kleindorfer, D.; et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke 2005, 36, 934–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testai, F.D.; Aiyagari, V. Acute hemorrhagic stroke pathophysiology and medical interventions: Blood pressure control, management of anticoagulant-associated brain hemorrhage and general management principles. Neurol. Clin. 2008, 26, 963–985. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Executive summary: Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, 188–197. [Google Scholar]
- George, M.G.; Tong, X.; Kuklina, E.V.; Labarthe, D.R. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann. Neurol. 2011, 70, 713–721. [Google Scholar] [CrossRef]
- Kapral, M.K.; Fang, J.; Hill, M.D.; Silver, F.; Richards, J.; Jaigobin, C.; Cheung, A.M.; Investigators of the Registry of the Canadian Stroke Network. Sex differences in stroke care and outcomes: Results from the Registry of the Canadian Stroke Network. Stroke 2005, 36, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Flores, S.; Rabinstein, A.; Biller, J.; Elkind, M.S.; Griffith, P.; Gorelick, P.B.; Howard, G.; Leira, E.C.; Morgenstern, L.B.; Ovbiagele, B.; et al. Racial-ethnic disparities in stroke care: The American experience: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2091–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleindorfer, D.; Broderick, J.; Khoury, J.; Flaherty, M.; Woo, D.; Alwell, K.; Moomaw, C.J.; Schneider, A.; Miller, R.; Shukla, R.; et al. The unchanging incidence and case-fatality of stroke in the 1990s: A population-based study. Stroke 2006, 37, 2473–2478. [Google Scholar] [CrossRef]
- Zahuranec, D.B.; Brown, D.L.; Lisabeth, L.D.; Gonzales, N.R.; Longwell, P.J.; Eden, S.V.; Smith, M.A.; Garcia, N.M.; Morgenstern, L.B. Differences in intracerebral hemorrhage between Mexican Americans and non-Hispanic whites. Neurology 2006, 66, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Falcão, I.; Rodrigues, G.; Canhão, P.; Melo, T.P.; Oliveira, V.; Pinto, A.N.; Crespo, M.; Salgado, A.V. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996, 27, 2225–2229. [Google Scholar] [CrossRef]
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009, 40, 2276–2293. [Google Scholar]
- Seshadri, S.; Beiser, A.; Pikula, A.; Himali, J.J.; Kelly-Hayes, M.; Debette, S.; DeStefano, A.L.; Romero, J.R.; Kase, C.S.; Wolf, P.A. Parental occurrence of stroke and risk of stroke in their children: The Framingham study. Circulation 2010, 121, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Touzé, E.; Rothwell, P.M. Sex differences in heritability of ischemic stroke: A systematic review and meta-analysis. Stroke 2008, 39, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Matarin, M.; Brown, W.M.; Singleton, A.; Hardy, J.A.; Meschia, J.F.; For the ISGS Investigators. Whole genome analyses suggest ischemic stroke and heart disease share an association with polymorphisms on chromosome 9p21. Stroke 2008, 39, 1586–1589. [Google Scholar] [CrossRef]
- Bevan, S.; Traylor, M.; Adib-Samii, P.; Malik, R.; Paul, N.L.; Jackson, C.; Farrall, M.; Rothwell, P.M.; Sudlow, C.; Dichgans, M.; et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke 2012, 43, 3161–3167. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Collaboration, P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Collins, R.; Peto, R.; MacMahon, S.; Hebert, P.; Fiebach, N.H.; Eberlein, K.A.; Godwin, J.; Qizilbash, N.; Taylor, J.O.; Hennekens, C.H. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet 1990, 335, 827–838. [Google Scholar] [CrossRef]
- Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons with Isolated Systolic Hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991, 265, 3255–3264. [Google Scholar] [CrossRef]
- Staessen, J.A.; Fagard, R.; Thijs, L.; Celis, H.; Arabidze, G.G.; Birkenhäger, W.H.; Bulpitt, C.J.; de Leeuw, P.W.; Dollery, C.T.; Fletcher, A.E.; et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997, 350, 757–764. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Sandee, W.; Algra, A.; Koudstaal, P.J.; Kappelle, L.J.; Dippel, D.W.; Dutch TIA Trial Study Group. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke 2006, 37, 1413–1417. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, C.; Moon, Y.P.; Paik, M.C.; Rundek, T.; Mora-McLaughlin, C.; Vieira, J.R.; Sacco, R.L.; Elkind, M.S. Duration of diabetes and risk of ischemic stroke: The Northern Manhattan Study. Stroke 2012, 43, 1212–1217. [Google Scholar] [CrossRef] [Green Version]
- Lukovits, T.G.; Mazzone, T.M.; Gorelick, T.M. Diabetes mellitus and cerebrovascular disease. Neuroepidemiology 1999, 18, 1–14. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Romero, J.R.; Morris, J.; Pikula, A. Stroke prevention: Modifying risk factors. Ther. Adv. Cardiovasc. Dis. 2008, 2, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Disertori, M.; Quintarelli, S.; Grasso, M.; Pilotto, A.; Narula, N.; Favalli, V.; Canclini, C.; Diegoli, M.; Mazzola, S.; Marini, M.; et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of Natriuretic Peptide Precursor A. Circ. Cardiovasc. Genet. 2013, 6, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iribarren, C.; Jacobs, D.R.; Sadler, M.; Claxton, A.J.; Sidney, S. Low total serum cholesterol and intracerebral hemorrhagic stroke: Is the association confined to elderly men? The Kaiser Permanente Medical Care Program. Stroke 1996, 27, 1993–1998. [Google Scholar] [CrossRef]
- Denti, L.; Cecchetti, A.; Annoni, V.; Merli, M.F.; Ablondi, F.; Valenti, G. The role of lipid profile in determining the risk of ischemic stroke in the elderly: A case-control study. Arch. Gerontol. Geriatr. 2003, 37, 51–62. [Google Scholar] [CrossRef]
- Iso, H.; Jacobs, D.R.; Wentworth, D.; Neaton, J.D.; Cohen, J.D. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N. Engl. J. Med. 1989, 320, 904–910. [Google Scholar] [CrossRef]
- Gill, J.S.; Zezulka, A.V.; Shipley, M.J.; Gill, S.K.; Beevers, D.G. Stroke and alcohol consumption. N. Engl. J. Med. 1986, 315, 1041–1046. [Google Scholar] [CrossRef]
- Hillbom, M.; Numminen, H.; Juvela, S. Recent heavy drinking of alcohol and embolic stroke. Stroke 1999, 30, 2307–2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatsky, A.L.; Armstrong, M.A.; Friedman, G.D.; Sidney, S. Alcohol drinking and risk of hospitalization for ischemic stroke. Am. J. Cardiol. 2001, 88, 703–706. [Google Scholar] [CrossRef]
- Esse, K.; Fossati-Bellani, M.; Traylor, A.; Martin-Schild, S. Epidemic of illicit drug use, mechanisms of action/addiction and stroke as a health hazard. Brain Behav. 2011, 1, 44–54. [Google Scholar] [CrossRef]
- Kaku, D.A.; Lowenstein, D.H. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann. Intern. Med. 1990, 113, 821–827. [Google Scholar] [CrossRef]
- Brust, J.C. Neurologic complications of substance abuse. J. Acquir. Immune Defic. Syndr. 2002, 31, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.M.; Cole, J.W.; Sorkin, J.D.; Wozniak, M.A.; Malarcher, A.M.; Giles, W.H.; Stern, B.J.; Kittner, S.J. Dose-response relationship between cigarette smoking and risk of ischemic stroke in young women. Stroke 2008, 39, 2439–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.M.; Cho, H.J. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: A cohort study in korean men. Stroke 2008, 39, 2432–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinton, R.; Beevers, G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989, 298, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.L.; Zhu, L.; Wang, J.; Hang, C.H.; Shi, J.X. The inflammation in the gut after experimental subarachnoid hemorrhage. J. Surg. Res. 2007, 137, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Krolewski, A.S.; Rosner, B.; Arky, R.A.; Speizer, F.E.; Hennekens, C.H. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 1991, 151, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary potassium intake and risk of stroke: A dose-response meta-analysis of prospective studies. Stroke 2011, 42, 2746–2750. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Martínez-González, M.A. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 676–677. [Google Scholar] [CrossRef] [Green Version]
- Appel, L.J.; Brands, M.W.; Daniels, S.R.; Karanja, N.; Elmer, P.J.; Sacks, F.M.; Association, A.H. Dietary approaches to prevent and treat hypertension: A scientific statement from the American Heart Association. Hypertension 2006, 47, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Cai, X.L.; Bian, P.D.; Hu, L.R. High salt intake and stroke: Meta-analysis of the epidemiologic evidence. CNS Neurosci. Ther. 2012, 18, 691–701. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ogden, L.G.; Vupputuri, S.; Bazzano, L.A.; Loria, C.; Whelton, P.K. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA 1999, 282, 2027–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagundes, D.J.; Omar, T.M. Animal disease model: Choice’s criteria and current animals specimens. Acta Cir. Bras. 2004, 19, 59–65. [Google Scholar] [CrossRef]
- Rollin, B.E. The Experimental Animal in Biomedical Research: Care, Husbandry and Well-Being: An Overview by Species; Kesel, M.L., Ed.; CRC Press: Boston, MA, USA, 1995. [Google Scholar]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Devel. Ther. 2015, 9, 3445–3454. [Google Scholar] [PubMed] [Green Version]
- Bogousslavsky, J.; Van Melle, G.; Regli, F. The Lausanne Stroke Registry: Analysis of 1,000 consecutive patients with first stroke. Stroke 1988, 19, 1083–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howells, D.W.; Porritt, M.J.; Rewell, S.S.; O’Collins, V.; Sena, E.S.; van der Worp, H.B.; Traystman, R.J.; Macleod, M.R. Different strokes for different folks: The rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1412–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhen, G.; Meloni, B.P.; Campbell, K.; Winn, H.R. Rodent stroke model guidelines for preclinical stroke trials (1st edition). J. Exp. Stroke Transl. Med. 2009, 2, 2–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VE, O.C.; GA, D.; MR, M.; DW, H. Animal models of stroke versus clinical stroke: Comparison of infarct size, cause, location, study design, and efficacy of experimental therapies. In Animal Models for the Study of Human Disease; Michael Conn, P., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 531–568. [Google Scholar]
- Connolly, E.S.; Winfree, C.J.; Stern, D.M.; Solomon, R.A.; Pinsky, D.J. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery 1996, 38, 523–531, discussion 532. [Google Scholar] [PubMed]
- Popa-Wagner, A.; Schröder, E.; Schmoll, H.; Walker, L.C.; Kessler, C. Upregulation of MAP1B and MAP2 in the rat brain after middle cerebral artery occlusion: Effect of age. J. Cereb. Blood Flow Metab. 1999, 19, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimori, H.; Yao, H.; Ooboshi, H.; Ibayashi, S.; Iida, M. Krypton laser-induced photothrombotic distal middle cerebral artery occlusion without craniectomy in mice. Brain Res. Brain Res. Protoc. 2004, 13, 189–196. [Google Scholar] [CrossRef] [PubMed]
- McAuley, M.A. Rodent models of focal ischemia. Cerebrovasc. Brain Metab. Rev. 1995, 7, 153–180. [Google Scholar]
- Derugin, N.; Ferriero, D.M.; Vexler, Z.S. Neonatal reversible focal cerebral ischemia: A new model. Neurosci. Res. 1998, 32, 349–353. [Google Scholar] [CrossRef]
- Tsuji, M.; Ohshima, M.; Taguchi, A.; Kasahara, Y.; Ikeda, T.; Matsuyama, T. A novel reproducible model of neonatal stroke in mice: Comparison with a hypoxia-ischemia model. Exp. Neurol. 2013, 247, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Ago, T.; Wakisaka, Y.; Kuroda, J.; Shijo, M.; Yoshikawa, Y.; Komori, M.; Nishimura, A.; Makihara, N.; Nakamura, K.; et al. Early Reperfusion After Brain Ischemia Has Beneficial Effects Beyond Rescuing Neurons. Stroke 2017, 48, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud, A.; Guiraut, C.; Chevin, M.; Chabrier, S.; Sébire, G. Role of Perinatal Inflammation in Neonatal Arterial Ischemic Stroke. Front. Neurol. 2017, 8, 612. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Koo, Y.S.; Shin, M.J.; Kim, S.Y.; Shin, Y.B.; Choi, B.T.; Yun, Y.J.; Lee, S.Y.; Shin, H.K. Combination of Constraint-Induced Movement Therapy with Electroacupuncture Improves Functional Recovery following Neonatal Hypoxic-Ischemic Brain Injury in Rats. Biomed. Res. Int. 2018, 2018, 8638294. [Google Scholar] [CrossRef] [Green Version]
- Gennaro, M.; Mattiello, A.; Pizzorusso, T. Rodent Models of Developmental Ischemic Stroke for Translational Research: Strengths and Weaknesses. Neural Plast. 2019, 2019, 5089321. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef]
- Hu, X.; Wester, P.; Brännström, T.; Watson, B.D.; Gu, W. Progressive and reproducible focal cortical ischemia with or without late spontaneous reperfusion generated by a ring-shaped, laser-driven photothrombotic lesion in rats. Brain Res. Brain Res. Protoc. 2001, 7, 76–85. [Google Scholar] [CrossRef]
- Yu, C.L.; Zhou, H.; Chai, A.P.; Yang, Y.X.; Mao, R.R.; Xu, L. Whole-scale neurobehavioral assessments of photothrombotic ischemia in freely moving mice. J. Neurosci. Methods 2015, 239, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Macrae, I.M.; Todd, M.; Reid, J.L.; McCulloch, J. Reduction of local cerebral blood flow to pathological levels by endothelin-1 applied to the middle cerebral artery in the rat. Neurosci. Lett. 1990, 118, 269–272. [Google Scholar] [CrossRef]
- Biernaskie, J.; Corbett, D.; Peeling, J.; Wells, J.; Lei, H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn. Reson. Med. 2001, 46, 827–830. [Google Scholar] [CrossRef] [PubMed]
- del Zoppo, G.J.; Schmid-Schönbein, G.W.; Mori, E.; Copeland, B.R.; Chang, C.M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991, 22, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Saggu, R. Characterisation of endothelin-1-induced intrastriatal lesions within the juvenile and adult rat brain using MRI and 31P MRS. Transl. Stroke Res. 2013, 4, 351–367. [Google Scholar] [CrossRef]
- Tsenov, G.; Mátéffyová, A.; Mares, P.; Otáhal, J.; Kubová, H. Intrahippocampal injection of endothelin-1: A new model of ischemia-induced seizures in immature rats. Epilepsia 2007, 48, 7–13. [Google Scholar] [CrossRef]
- Hossmann, K.A. Cerebral ischemia: Models, methods and outcomes. Neuropharmacology 2008, 55, 257–270. [Google Scholar] [CrossRef]
- Gerriets, T.; Li, F.; Silva, M.D.; Meng, X.; Brevard, M.; Sotak, C.H.; Fisher, M. The macrosphere model: Evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. J. Neurosci. Methods 2003, 122, 201–211. [Google Scholar] [CrossRef]
- Overgaard, K.; Sereghy, T.; Boysen, G.; Pedersen, H.; Høyer, S.; Diemer, N.H. A rat model of reproducible cerebral infarction using thrombotic blood clot emboli. J. Cereb. Blood Flow Metab. 1992, 12, 484–490. [Google Scholar] [CrossRef]
- Smith, W.S.; Sung, G.; Starkman, S.; Saver, J.L.; Kidwell, C.S.; Gobin, Y.P.; Lutsep, H.L.; Nesbit, G.M.; Grobelny, T.; Rymer, M.M.; et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke 2005, 36, 1432–1438. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, M.; Xiao, T.; Jolkkonen, J.; Zhao, C. Constraint-induced movement therapy overcomes the intrinsic axonal growth-inhibitory signals in stroke rats. Stroke 2013, 44, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.U.; Lappalainen, R.S.; Narkilahti, S.; Suuronen, R.; Corbett, D.; Sivenius, J.; Hovatta, O.; Jolkkonen, J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: Cell survival and functional recovery. Eur. J. Neurosci. 2009, 29, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Vigaru, B.; Lambercy, O.; Graber, L.; Fluit, R.; Wespe, P.; Schubring-Giese, M.; Luft, A.; Gassert, R. A small-scale robotic manipulandum for motor training in stroke rats. IEEE Int. Conf. Rehabil. Robot. 2011, 2011, 5975349. [Google Scholar] [PubMed]
- Modo, M.; Crum, W.R.; Gerwig, M.; Vernon, A.C.; Patel, P.; Jackson, M.J.; Rose, S.; Jenner, P.; Iravani, M.M. Magnetic resonance imaging and tensor-based morphometry in the MPTP non-human primate model of Parkinson’s disease. PLoS ONE 2017, 12, e0180733. [Google Scholar] [CrossRef] [Green Version]
- Nitzsche, B.; Frey, S.; Collins, L.D.; Seeger, J.; Lobsien, D.; Dreyer, A.; Kirsten, H.; Stoffel, M.H.; Fonov, V.S.; Boltze, J. A stereotaxic, population-averaged T1w ovine brain atlas including cerebral morphology and tissue volumes. Front. Neuroanat. 2015, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Modo, M.M.; Jolkkonen, J.; Zille, M.; Boltze, J. Future of Animal Modeling for Poststroke Tissue Repair. Stroke 2018, 49, 1099–1106. [Google Scholar] [CrossRef]
- Sutherland, B.A.; Minnerup, J.; Balami, J.S.; Arba, F.; Buchan, A.M.; Kleinschnitz, C. Neuroprotection for ischaemic stroke: Translation from the bench to the bedside. Int. J. Stroke 2012, 7, 407–418. [Google Scholar] [CrossRef]
- Hoyte, L.; Barber, P.A.; Buchan, A.M.; Hill, M.D. The rise and fall of NMDA antagonists for ischemic stroke. Curr. Mol. Med. 2004, 4, 131–136. [Google Scholar] [CrossRef]
- Wahlgren, N.G.; Bornhov, S.; Sharma, A.; Cederin, B.; Rosolacci, T.; Ashwood, T.; Claesson, L.; CLASS Study Group. The clomethiazole acute stroke study (CLASS): Efficacy results in 545 patients classified as total anterior circulation syndrome (TACS). J. Stroke Cerebrovasc. Dis. 1999, 8, 231–239. [Google Scholar] [CrossRef]
- Carter, A.J. The importance of voltage-dependent sodium channels in cerebral ischaemia. Amino Acids 1998, 14, 159–169. [Google Scholar] [CrossRef]
- Hewitt, K.E.; Stys, P.K.; Lesiuk, H.J. The use-dependent sodium channel blocker mexiletine is neuroprotective against global ischemic injury. Brain Res. 2001, 898, 281–287. [Google Scholar] [CrossRef]
- Kotoda, M.; Hishiyama, S.; Ishiyama, T.; Mitsui, K.; Matsukawa, T. Amiodarone exacerbates brain injuries after hypoxic-ischemic insult in mice. BMC Neurosci. 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Segura, T.; Calleja, S.; Jordan, J. Recommendations and treatment strategies for the management of acute ischemic stroke. Expert Opin. Pharmacother. 2008, 9, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Verdecchia, P.; Reboldi, G.P.; Gattobigio, R.; Bentivoglio, M.; Staessen, J.A.; Porcellati, C. Calcium channel blockade to prevent stroke in hypertension: A meta-analysis of 13 studies with 103,793 subjects. Am. J. Hypertens. 2004, 17, 817–822. [Google Scholar] [CrossRef]
- Bowler, R.P.; Sheng, H.; Enghild, J.J.; Pearlstein, R.D.; Warner, D.S.; Crapo, J.D. A catalytic antioxidant (AEOL 10150) attenuates expression of inflammatory genes in stroke. Free Radic. Biol. Med. 2002, 33, 1141–1152. [Google Scholar] [CrossRef]
- Ono, S.; Hishikawa, T.; Ogawa, T.; Nishiguchi, M.; Onoda, K.; Tokunaga, K.; Sugiu, K.; Date, I. Effect of deferoxamine-activated hypoxia inducible factor-1 on the brainstem following subarachnoid haemorrhage. In Cerebral Vasospasm; Acta Neurochirurgica Supplement, Volume 104; Springer: Vienna, Austria, 2008; pp. 69–73. [Google Scholar]
- Mu, D.; Chang, Y.S.; Vexler, Z.S.; Ferriero, D.M. Hypoxia-inducible factor 1alpha and erythropoietin upregulation with deferoxamine salvage after neonatal stroke. Exp. Neurol. 2005, 195, 407–415. [Google Scholar] [CrossRef]
- Shuaib, A.; Lees, K.R.; Lyden, P.; Grotta, J.; Davalos, A.; Davis, S.M.; Diener, H.C.; Ashwood, T.; Wasiewski, W.W.; Emeribe, U.; et al. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007, 357, 562–571. [Google Scholar] [CrossRef]
- Lees, K.R.; Zivin, J.A.; Ashwood, T.; Davalos, A.; Davis, S.M.; Diener, H.C.; Grotta, J.; Lyden, P.; Shuaib, A.; Hårdemark, H.G.; et al. NXY-059 for acute ischemic stroke. N. Engl. J. Med. 2006, 354, 588–600. [Google Scholar] [CrossRef]
- Shirley, R.; Ord, E.N.; Work, L.M. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef] [Green Version]
- Barreto, A.D. Intravenous thrombolytics for ischemic stroke. Neurotherapeutics 2011, 8, 388–399. [Google Scholar] [CrossRef] [Green Version]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Külkens, S.; Hacke, W. Thrombolysis with alteplase for acute ischemic stroke: Review of SITS-MOST and other Phase IV studies. Expert Rev. Neurother. 2007, 7, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Higashida, R.; Wechsler, L.; Gent, M.; Rowley, H.; Kase, C.; Pessin, M.; Ahuja, A.; Callahan, F.; Clark, W.M.; et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999, 282, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Mori, E.; Minematsu, K.; Taki, W.; Takahashi, A.; Nemoto, S.; Miyamoto, S.; Sasaki, M.; Inoue, T.; The MELT Japan Study Group. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: The middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke 2007, 38, 2633–2639. [Google Scholar] [CrossRef]
- Abou-Chebl, A.; Bajzer, C.T.; Krieger, D.W.; Furlan, A.J.; Yadav, J.S. Multimodal therapy for the treatment of severe ischemic stroke combining GPIIb/IIIa antagonists and angioplasty after failure of thrombolysis. Stroke 2005, 36, 2286–2288. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.I.; Harris-Lane, P.; Kirmani, J.F.; Janjua, N.; Divani, A.A.; Mohammad, Y.M.; Suarez, J.I.; Montgomery, M.O. Intra-arterial reteplase and intravenous abciximab in patients with acute ischemic stroke: An open-label, dose-ranging, phase I study. Neurosurgery 2006, 59, 789–796, discussion 796–787. [Google Scholar] [CrossRef]
- Investigators, I.I.T. The Interventional Management of Stroke (IMS) II Study. Stroke 2007, 38, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Mazighi, M.; Meseguer, E.; Labreuche, J.; Amarenco, P. Bridging therapy in acute ischemic stroke: A systematic review and meta-analysis. Stroke 2012, 43, 1302–1308. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Stapf, C.; Arnold, M. Stroke unit management and revascularisation in acute ischemic stroke. Eur. Neurol. 2015, 73, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sun, D.; Liu, M.; Zhang, S.; Ren, C. Defibrinogen Therapy for Acute Ischemic Stroke: 1332 Consecutive Cases. Sci. Rep. 2018, 8, 9489. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, M.; Counsell, C.; Wardlaw, J.M.; Lin, S.; Zhao, X. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Trammel, J.; Wasiewski, W.W.; For the Ancrod Stroke Program (ASP) Study Team. Ancrod for acute ischemic stroke: A new dosing regimen derived from analysis of prior ancrod stroke studies. J. Stroke Cerebrovasc. Dis. 2009, 18, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, B.; Asplund, K.; Hägg, E. Factors influencing admission blood pressure levels in patients with acute stroke. Stroke 1991, 22, 527–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohwaki, K.; Yano, E.; Nagashima, H.; Hirata, M.; Nakagomi, T.; Tamura, A. Blood pressure management in acute intracerebral hemorrhage: Relationship between elevated blood pressure and hematoma enlargement. Stroke 2004, 35, 1364–1367. [Google Scholar] [CrossRef]
- Owens, W.B. Blood pressure control in acute cerebrovascular disease. J. Clin. Hypertens. 2011, 13, 205–211. [Google Scholar] [CrossRef]
- Schrader, J.; Lüders, S.; Kulschewski, A.; Berger, J.; Zidek, W.; Treib, J.; Einhäupl, K.; Diener, H.C.; Dominiak, P.; On behalf of the ACCESS Study Group. The ACCESS Study: Evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke 2003, 34, 1699–1703. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.G.; Potter, J.F.; Ford, G.A.; Bulpitt, C.J.; Chernova, J.; Jagger, C.; James, M.A.; Knight, J.; Markus, H.S.; Mistri, A.K.; et al. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): A prospective, randomised, open, blinded-endpoint trial. Lancet Neurol. 2010, 9, 767–775. [Google Scholar] [CrossRef]
- Potter, J.; Mistri, A.; Brodie, F.; Chernova, J.; Wilson, E.; Jagger, C.; James, M.; Ford, G.; Robinson, T. Controlling hypertension and hypotension immediately post stroke (CHHIPS)--a randomised controlled trial. Health Technol. Assess. 2009, 13, 1–73. [Google Scholar] [CrossRef] [Green Version]
- Hankey, G.J. Lowering blood pressure in acute stroke: The SCAST trial. Lancet 2011, 377, 696–698. [Google Scholar] [CrossRef]
- Lindsberg, P.J.; Roine, R.O. Hyperglycemia in acute stroke. Stroke 2004, 35, 363–364. [Google Scholar] [CrossRef] [Green Version]
- Wada, S.; Yoshimura, S.; Inoue, M.; Matsuki, T.; Arihiro, S.; Koga, M.; Kitazono, T.; Makino, H.; Hosoda, K.; Ihara, M.; et al. Outcome Prediction in Acute Stroke Patients by Continuous Glucose Monitoring. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackam, D.G.; Spence, J.D. Antiplatelet Therapy in Ischemic Stroke and Transient Ischemic Attack. Stroke 2019, 50, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Stringberg, A.; Camden, R.; Qualls, K.; Naqvi, S.H. Update on Dual Antiplatelet Therapy for Secondary Stroke Prevention. Mo. Med. 2019, 116, 303–307. [Google Scholar]
- Borlongan, C.V.; Koutouzis, T.K.; Jorden, J.R.; Martinez, R.; Rodriguez, A.I.; Poulos, S.G.; Freeman, T.B.; McKeown, P.; Cahill, D.W.; Nishino, H.; et al. Neural transplantation as an experimental treatment modality for cerebral ischemia. Neurosci. Biobehav. Rev. 1997, 21, 79–90. [Google Scholar] [CrossRef]
- Park, Y.J.; Niizuma, K.; Mokin, M.; Dezawa, M.; Borlongan, C.V. Cell-Based Therapy for Stroke: Musing With Muse Cells. Stroke 2020, 51, 2854–2862. [Google Scholar] [CrossRef]
- Aizman, I.; Vinodkumar, D.; McGrogan, M.; Bates, D. Cell Injury-Induced Release of Fibroblast Growth Factor 2: Relevance to Intracerebral Mesenchymal Stromal Cell Transplantations. Stem Cells Dev. 2015, 24, 1623–1634. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.; Tate, C.C.; McGrogan, M.; Case, C.C. Comparing the angiogenic potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells. J. Transl. Med. 2013, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, X.; Wei, J.; Feng, M.; Lu, S.; Li, G.; Dou, W.; Ma, W.; Ma, S.; An, Y.; Qin, C.; et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011, 1367, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Kim, S.S.; Lee, S.Y.; Lee, H.S.; Kim, H.S.; Lee, Y.D.; Suh-Kim, H. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp. Mol. Med. 2008, 40, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Aizman, I.; Tate, C.C.; McGrogan, M.; Case, C.C. Extracellular matrix produced by bone marrow stromal cells and by their derivative, SB623 cells, supports neural cell growth. J. Neurosci. Res. 2009, 87, 3198–3206. [Google Scholar] [CrossRef] [PubMed]
- Chopp, M.; Li, Y.; Zhang, Z.G. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 2009, 40, S143–S145. [Google Scholar] [CrossRef] [Green Version]
- Cramer, S.C.; Abila, B.; Scott, N.E.; Simeoni, M.; Enney, L.A.; Investigators, M.S. Safety, pharmacokinetics, and pharmacodynamics of escalating repeat doses of GSK249320 in patients with stroke. Stroke 2013, 44, 1337–1342. [Google Scholar] [CrossRef] [Green Version]
- Emerick, A.J.; Neafsey, E.J.; Schwab, M.E.; Kartje, G.L. Functional reorganization of the motor cortex in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. J. Neurosci. 2003, 23, 4826–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.T.; Duncan, P.W.; Lai, S.M.; Studenski, S. The relation between impairments and functional outcomes poststroke. Arch. Phys. Med. Rehabil. 2000, 81, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. The Clinical Science of Neurologic Rehabilitation; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Dobkin, B.H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Iacoboni, M.; Woods, R.P.; Brass, M.; Bekkering, H.; Mazziotta, J.C.; Rizzolatti, G. Cortical mechanisms of human imitation. Science 1999, 286, 2526–2528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbik, F.; Hirsch, J.A.; Chandra, R.V.; Frei, D.; Patel, A.B.; Rabinov, J.D.; Rost, N.; Schwamm, L.H.; Leslie-Mazwi, T.M. Telestroke-the promise and the challenge. Part one: Growth and current practice. J. Neurointerv. Surg. 2017, 9, 357–360. [Google Scholar] [CrossRef]
- Bowry, R.; Parker, S.; Rajan, S.S.; Yamal, J.M.; Wu, T.C.; Richardson, L.; Noser, E.; Persse, D.; Jackson, K.; Grotta, J.C. Benefits of Stroke Treatment Using a Mobile Stroke Unit Compared With Standard Management: The BEST-MSU Study Run-In Phase. Stroke 2015, 46, 3370–3374. [Google Scholar] [CrossRef] [Green Version]
- Association, A.H. New Recommendations for Stroke Systems of Care to Improve Patient Outcomes; ScienceDaily: Rockville, MD, USA, 2019. [Google Scholar]
- Arienti, C.; Lazzarini, S.G.; Pollock, A.; Negrini, S. Rehabilitation interventions for improving balance following stroke: An overview of systematic reviews. PLoS ONE 2019, 14, e0219781. [Google Scholar] [CrossRef] [Green Version]
- Bonini-Rocha, A.C.; de Andrade, A.L.S.; Moraes, A.M.; Gomide Matheus, L.B.; Diniz, L.R.; Martins, W.R. Effectiveness of Circuit-Based Exercises on Gait Speed, Balance, and Functional Mobility in People Affected by Stroke: A Meta-Analysis. PM R 2018, 10, 398–409. [Google Scholar] [CrossRef]
- Eng, J.J.; Bird, M.L.; Godecke, E.; Hoffmann, T.C.; Laurin, C.; Olaoye, O.A.; Solomon, J.; Teasell, R.; Watkins, C.L.; Walker, M.F. Moving stroke rehabilitation research evidence into clinical practice: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2019, 14, 766–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Macrae, I.M.; Allan, S.M. Stroke: The past, present and future. Brain Neurosci. Adv. 2018, 2, 2398212818810689. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.W.; Liu, Z.Y.; Hou, K.; Liu, S.Y.; Hu, Y.X.; Zhang, L.; Zhang, F.L.; Lv, K.Y.; Kang, Q.; Hu, W.Y.; et al. Wip1 knockout inhibits neurogenesis by affecting the Wnt/β-catenin signaling pathway in focal cerebral ischemia in mice. Exp. Neurol. 2018, 309, 44–53. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Zhao, T.; Wu, K.W.; Watanabe, K.; Xiao, Z.C.; Zhu, L.L.; Fan, M. Loss of NB-3 aggravates cerebral ischemia by impairing neuron survival and neurite growth. Stroke 2011, 42, 2910–2916. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Liu, X.; Zhu, Y.; Chen, S.; Zhou, D.; Wang, Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci. Lett. 2013, 534, 123–127. [Google Scholar] [CrossRef]
- Qiu, C.W.; Liu, Z.Y.; Zhang, F.L.; Zhang, L.; Li, F.; Liu, S.Y.; He, J.Y.; Xiao, Z.C. Post-stroke gastrodin treatment ameliorates ischemic injury and increases neurogenesis and restores the Wnt/β-Catenin signaling in focal cerebral ischemia in mice. Brain Res. 2019, 1712, 7–15. [Google Scholar] [CrossRef]
- Rudd, A.G.; Bladin, C.; Carli, P.; De Silva, D.A.; Field, T.S.; Jauch, E.C.; Kudenchuk, P.; Kurz, M.W.; Lærdal, T.; Ong, M.; et al. Utstein recommendation for emergency stroke care. Int. J. Stroke 2020, 15, 555–564. [Google Scholar] [CrossRef]
- Chollet, F.; Cramer, S.C.; Stinear, C.; Kappelle, L.J.; Baron, J.C.; Weiller, C.; Azouvi, P.; Hommel, M.; Sabatini, U.; Moulin, T.; et al. Pharmacological therapies in post stroke recovery: Recommendations for future clinical trials. J. Neurol. 2014, 261, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- Khandelwal, P.; Yavagal, D.R.; Sacco, R.L. Acute Ischemic Stroke Intervention. J. Am. Coll. Cardiol. 2016, 67, 2631–2644. [Google Scholar] [CrossRef]
- Boltze, J.; Ayata, C. Challenges and Controversies in Translational Stroke Research—An Introduction. Transl. Stroke Res. 2016, 7, 355–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endres, M.; Engelhardt, B.; Koistinaho, J.; Lindvall, O.; Meairs, S.; Mohr, J.P.; Planas, A.; Rothwell, N.; Schwaninger, M.; Schwab, M.E.; et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovasc. Dis. 2008, 25, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerna, C.; Hill, M.D.; Boltze, J. Towards Improved Translational Stroke Research: Progress and Perspectives of the Recent National Institute of Neurological Disorders and Stroke Consensus Group Meeting. Stroke 2017, 48, 2341–2342. [Google Scholar] [CrossRef] [PubMed]
- Boltze, J.; Nitzsche, F.; Jolkkonen, J.; Weise, G.; Pösel, C.; Nitzsche, B.; Wagner, D.C. Concise Review: Increasing the Validity of Cerebrovascular Disease Models and Experimental Methods for Translational Stem Cell Research. Stem Cells 2017, 35, 1141–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H.; Group, S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef]
- Boltze, J.; Lukomska, B.; Jolkkonen, J.; For the MEMS–IRBI Consortium. Mesenchymal stromal cells in stroke: Improvement of motor recovery or functional compensation? J. Cereb. Blood Flow Metab. 2014, 34, 1420–1421. [Google Scholar] [CrossRef]
- Boltze, J.; Wagner, D.C.; Barthel, H.; Gounis, M.J. Academic-industry Collaborations in Translational Stroke Research. Transl. Stroke Res. 2016, 7, 343–353. [Google Scholar] [CrossRef]
- Wang, L.; Plump, A.; Ringel, M. Racing to define pharmaceutical R&D external innovation models. Drug Discov. Today 2015, 20, 361–370. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |


| Stroke Models | Advantages | Disadvantages |
|---|---|---|
| Intraluminal suture MCAo model | Mimics human ischemic stroke, Exhibits a penumbra, Highly reproducible, No craniectomy | Hyper-/hypothermia, Increased haemorrhage, Not suitable for thrombolysis studies |
| Craniotomy model | High long-term survival rates, Visual confirmation of successful MCAo | Highly invasive and procedural complications, Requires surgical skills |
| Photo-thrombosis model | Enables well-defined localization of an ischemic lesion, Highly reproducible, Less invasive | Causes early vasogenic edema, Not suitable for investigating neuroprotective agents |
| Endothelin-1 model | Less invasive, Induction of ischemic lesion in cortical regions, Low mortality | Duration of ischemia not controllable, Induction of astrocytosis and axonal sprouting |
| Embolic stroke model | Mimics the pathogenesis of human stroke | Low reproducibility of infarcts, Spontaneous recanalization |
| Neurorehabilitation | Rapid establishment of independence in activities of daily living Improves outcomes for cognitive, language, and motor skills | Develop cost-effective rehabilitative services Lack of in-depth studies on efficacy related to neurorehabilitation |
| Biomaterial testing | Reduction in lesion volume Bridge the lesion with neural tissue for neural reorganization Reduce secondary damage Improve neurological behaviour | Long-term experiments with the same biomaterial are challenging because of the degradation of material which might affect the treatment |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. https://doi.org/10.3390/ijms21207609
Kuriakose D, Xiao Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. International Journal of Molecular Sciences. 2020; 21(20):7609. https://doi.org/10.3390/ijms21207609
Chicago/Turabian StyleKuriakose, Diji, and Zhicheng Xiao. 2020. "Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives" International Journal of Molecular Sciences 21, no. 20: 7609. https://doi.org/10.3390/ijms21207609
APA StyleKuriakose, D., & Xiao, Z. (2020). Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. International Journal of Molecular Sciences, 21(20), 7609. https://doi.org/10.3390/ijms21207609





