Three-Dimensional Structures of Carbohydrates and Where to Find Them
Abstract
1. Introduction
- Primary structure (atom connectivity);
- Monosaccharide ring conformation;
- Rotational states of inter-residue and exocyclic linkages and their energies;
- Ring puckering and transitions of glycosidic linkage conformation on a time scale;
- Large-scale spatial arrangement (tertiary structure).
2. Structural Databases
- Database can be freely accessed through web user interface;
- Database must contain experimentally confirmed and/or predicted 3D structures (preprocessed and/or generated on-the-fly from a primary structure input) of glycans, glycoproteins, or protein-carbohydrate complexes;
- Stored 3D structures must be deposited as atomic coordinates in PDB, MOL, or other format, and the structures must contain a saccharide moiety;
- Databases with records linked to other large 3D data collections (e.g., RCSB PDB, PDBe, PDBj, PDBsum, UniProtKB etc.) are included in Table 1 (as long as database entries contain carbohydrate moiety, e.g., as a part of a lectin or an antibody);
- Databases with derived carbohydrate 3D structural data (conformational maps, conformer energy minima, etc.) are included in Table 1 even if they provide no atomic coordinates (e.g., GlycoMapsDB and GFDB).
3. Carbohydrate 3D Structure Modeling
- Molecular mechanics (MM) and molecular dynamics (MD) calculations [117];
Molecular Mechanics and Dynamics
4. Model Building and Analysis Tools
5. Experimental Data Validation
6. Protein Data Bank and Its Validation
7. 3D Structure Input and Visualization
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AA | All-atom |
| CAZy | Carbohydrate-Active Enzyme |
| CD | Cluster of Differentiation |
| CFG | Consortium for Functional Glycomics |
| CG | Coarse-grained |
| CHI | Carbohydrate Intrinsic |
| CRD | Carbohydrate Recognition Site |
| Cryo-EM | Electron cryo-microscopy |
| CSD | Cambridge Structural Database |
| DFT | Density Functional Theory |
| FUC | α-L-fucopyranose |
| GAG | Glycosaminoglycan |
| GAMD | Gaussian Accelerated MD |
| GBP | Glycan-Binding Protein |
| GM9 | Glc1Man9GlcNAc2 |
| HPLC | High Performance Liquid Chromatography |
| HREX | Hamiltonian Replica-Exchange MD |
| INIOM | Our own N-layered integrated molecular orbital and molecular mechanics |
| LNB | Lacto-N-biose I |
| MD | Molecular Dynamics |
| MM | Molecular Mechanics |
| MS | Mass-spectrometry |
| msesMD | Multidimensional swarm-enhanced sampling MD |
| NAG | 2-acetamido-2-deoxy-β-D-glucopyranose |
| NMR | Nuclear Magnetic Resonance |
| NOE | Nuclear Overhauser Effect |
| PDB | Protein Data Bank |
| PDBe | Protein Data Bank Europe |
| PDBj | Protein Data Bank Japan |
| PDBsum | Database of Structural Summaries of PDB Entries |
| QM | Quantum Mechanics |
| RCSB PDB | Research Collaboratory for Structural Bioinformatics Protein Data Bank |
| REMD | Replica-exchange MD |
| SNFG | Symbol Nomenclature for Glycans |
| UniProtKB | UniProt Knowledgebase |
| wwPDB | Worldwide Protein Data Bank |
References
- Hricovini, M. Structural Aspects of Carbohydrates and the Relation with their Biological Properties. Curr. Med. Chem. 2004, 11, 2565–2583. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Buddhadeb, M.; Dimitrios, M. Applications of Molecular Dynamics Simulations in Immunology: A Useful Computational Method in Aiding Vaccine Design. Curr. Proteom. 2006, 3, 259–270. [Google Scholar]
- Kuttel, M.M.; Ravenscroft, N. The Role of Molecular Modeling in Predicting Carbohydrate Antigen Conformation and Understanding Vaccine Immunogenicity. In Carbohydrate-Based Vaccines: From Concept to Clinic; American Chemical Society: Washington, DC, USA, 2018; Volume 1290, pp. 139–173. [Google Scholar]
- Mishra, S.K.; Calabró, G.; Loeffler, H.H.; Michel, J.; Koča, J. Evaluation of Selected Classical Force Fields for Alchemical Binding Free Energy Calculations of Protein-Carbohydrate Complexes. J. Chem. Theory Comput. 2015, 11, 3333–3345. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.J.; Tessier, M.B. Computational glycoscience: Characterizing the spatial and temporal properties of glycans and glycan–protein complexes. Curr. Opin. Struct. Biol. 2010, 20, 575–583. [Google Scholar] [CrossRef]
- Hadden, J.A.; Tessier, M.B.; Fadda, E.; Woods, R.J. Calculating Binding Free Energies for Protein–Carbohydrate Complexes. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 431–465. [Google Scholar]
- Woods, R.J. Predicting the Structures of Glycans, Glycoproteins, and Their Complexes. Chem. Rev. 2018, 118, 8005–8024. [Google Scholar] [CrossRef]
- Frank, M. Computational Docking as a Tool for the Rational Design of Carbohydrate-Based Drugs. In Carbohydrates as Drugs; Seeberger, P.H., Rademacher, C., Eds.; Springer: Cham, Switzerland, 2014; pp. 53–72. [Google Scholar]
- Purcell, S.C.; Godula, K. Synthetic glycoscapes: Addressing the structural and functional complexity of the glycocalyx. Interface Focus 2019, 9, 20180080. [Google Scholar] [CrossRef]
- Nagae, M.; Yamaguchi, Y. Function and 3D Structure of the N-Glycans on Glycoproteins. Int. J. Mol. Sci. 2012, 13, 8398–8429. [Google Scholar] [CrossRef]
- Aoki-Kinoshita, K.F.; Lisacek, F.; Mazumder, R.; York, W.S.; Packer, N.H. The GlySpace Alliance: Toward a collaborative global glycoinformatics community. Glycobiology 2020, 30, 70–71. [Google Scholar] [CrossRef]
- Yamada, I.; Shiota, M.; Shinmachi, D.; Ono, T.; Tsuchiya, S.; Hosoda, M.; Fujita, A.; Aoki, N.P.; Watanabe, Y.; Fujita, N.; et al. The GlyCosmos Portal: A unified and comprehensive web resource for the glycosciences. Nat. Methods 2020, 17, 649–650. [Google Scholar] [CrossRef]
- Mariethoz, J.; Alocci, D.; Gastaldello, A.; Horlacher, O.; Gasteiger, E.; Rojas-Macias, M.; Karlsson, N.G.; Packer, N.H.; Lisacek, F. Glycomics@ExPASy: Bridging the Gap. Mol. Cell. Proteom. 2018, 17, 2164. [Google Scholar] [CrossRef] [PubMed]
- Kahsay, R.; Vora, J.; Navelkar, R.; Mousavi, R.; Fochtman, B.; Holmes, X.; Pattabiraman, N.; Ranzinger, R.; Mahadik, R.; Williamson, T.; et al. GlyGen data model and processing workflow. Bioinformatics 2020, 36, 3941–3943. [Google Scholar] [CrossRef]
- Maeda, M.; Fujita, N.; Suzuki, Y.; Sawaki, H.; Shikanai, T.; Narimatsu, H. JCGGDB: Japan Consortium for Glycobiology and Glycotechnology Database. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 161–179. [Google Scholar]
- Tiemeyer, M.; Aoki, K.; Paulson, J.; Cummings, R.D.; York, W.S.; Karlsson, N.G.; Lisacek, F.; Packer, N.H.; Campbell, M.P.; Aoki, N.P.; et al. GlyTouCan: An accessible glycan structure repository. Glycobiology 2017, 27, 915–919. [Google Scholar] [CrossRef]
- York, W.S.; Agravat, S.; Aoki-Kinoshita, K.F.; McBride, R.; Campbell, M.P.; Costello, C.E.; Dell, A.; Feizi, T.; Haslam, S.M.; Karlsson, N.; et al. MIRAGE: The minimum information required for a glycomics experiment. Glycobiology 2014, 24, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Comelli, E.M.; Head, S.R.; Gilmartin, T.; Whisenant, T.; Haslam, S.M.; North, S.J.; Wong, N.-K.; Kudo, T.; Narimatsu, H.; Esko, J.D.; et al. A focused microarray approach to functional glycomics: Transcriptional regulation of the glycome. Glycobiology 2006, 16, 117–131. [Google Scholar] [CrossRef]
- Aoki-Kinoshita, K.F. RINGS: A Web Resource of Tools for Analyzing Glycomics Data. In A Practical Guide to Using Glycomics Databases; Aoki-Kinoshita, K.F., Ed.; Springer: Tokyo, Japan, 2017; pp. 299–334. [Google Scholar]
- Gourdine, J.-P.F.; Brush, M.H.; Vasilevsky, N.A.; Shefchek, K.; Köhler, S.; Matentzoglu, N.; Munoz-Torres, M.C.; McMurry, J.A.; Zhang, X.A.; Robinson, P.N.; et al. Representing glycophenotypes: Semantic unification of glycobiology resources for disease discovery. Database 2019, 2019. [Google Scholar] [CrossRef]
- Alocci, D.; Ghraichy, M.; Barletta, E.; Gastaldello, A.; Mariethoz, J.; Lisacek, F. Understanding the glycome: An interactive view of glycosylation from glycocompositions to glycoepitopes. Glycobiology 2018, 28, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.B.; Aoki-Kinoshita, K.F.; Naidoo, K.J. The Glycome Analytics Platform: An integrative framework for glycobioinformatics. Bioinformatics 2016, 32, 3005–3011. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Yamamoto, K. Glycoenzymes in Glycan Analysis and Synthesis. In Glycoscience: Biology and Medicine; Taniguchi, N., Endo, T., Hart, G.W., Seeberger, P.H., Wong, C.-H., Eds.; Springer: Tokyo, Japan, 2015; pp. 379–389. [Google Scholar]
- Montgomery, A.P.; Xiao, K.; Wang, X.; Skropeta, D.; Yu, H. Chapter Two—Computational Glycobiology: Mechanistic Studies of Carbohydrate-Active Enzymes and Implication for Inhibitor Design. In Advances in Protein Chemistry and Structural Biology; Karabencheva-Christova, T., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 109, pp. 25–76. [Google Scholar]
- Copoiu, L.; Malhotra, S. The current structural glycome landscape and emerging technologies. Curr. Opin. Struct. Biol. 2020, 62, 132–139. [Google Scholar] [CrossRef]
- Toukach, P.V.; Egorova, K.S. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016, 44, D1229–D1236. [Google Scholar] [CrossRef]
- Egorova, K.S.; Toukach, P.V. Glycoinformatics: Bridging isolated islands in the sea of data. Angew. Chem. Int. Ed. 2018, 57, 14986–14990. [Google Scholar] [CrossRef] [PubMed]
- Lisacek, F.; Mariethoz, J.; Alocci, D.; Rudd, P.M.; Abrahams, J.L.; Campbell, M.P.; Packer, N.H.; Ståhle, J.; Widmalm, G.; Mullen, E.; et al. Databases and Associated Tools for Glycomics and Glycoproteomics. In High-Throughput Glycomics and Glycoproteomics: Methods and Protocols; Lauc, G., Wuhrer, M., Eds.; Humana Press: New York, NY, USA, 2017; pp. 235–264. [Google Scholar]
- Abrahams, J.L.; Taherzadeh, G.; Jarvas, G.; Guttman, A.; Zhou, Y.; Campbell, M.P. Recent advances in glycoinformatic platforms for glycomics and glycoproteomics. Curr. Opin. Struct. Biol. 2020, 62, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.; Hong, X.; Zhang, Y.; Zou, X. Databases and Bioinformatic Tools for Glycobiology and Glycoproteomics. Int. J. Mol. Sci. 2020, 21, 6727. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.P.; Peterson, R.; Mariethoz, J.; Gasteiger, E.; Akune, Y.; Aoki-Kinoshita, K.F.; Lisacek, F.; Packer, N.H. UniCarbKB: Building a knowledge platform for glycoproteomics. Nucleic Acids Res. 2014, 42, D215–D221. [Google Scholar] [CrossRef]
- Doubet, S.; Bock, K.; Smith, D.; Darvill, A.; Albersheim, P. The Complex Carbohydrate Structure Database. Trends Biochem. Sci. 1989, 14, 475–477. [Google Scholar] [CrossRef]
- Doubet, S.; Albersheim, P. CarbBank. Glycobiology 1992, 2, 505–507. [Google Scholar] [CrossRef]
- von der Lieth, C.W.; Freire, A.A.; Blank, D.; Campbell, M.P.; Ceroni, A.; Damerell, D.R.; Dell, A.; Dwek, R.A.; Ernst, B.; Fogh, R.; et al. EUROCarbDB: An open-access platform for glycoinformatics. Glycobiology 2011, 21, 493–502. [Google Scholar] [CrossRef]
- Ranzinger, R.; Herget, S.; Wetter, T.; von der Lieth, C.W. GlycomeDB—Integration of open-access carbohydrate structure databases. BMC Bioinf. 2008, 9, 384. [Google Scholar] [CrossRef]
- Ranzinger, R.; Frank, M.; von der Lieth, C.W.; Herget, S. Glycome-DB.org: A portal for querying across the digital world of carbohydrate sequences. Glycobiology 2009, 19, 1563–1567. [Google Scholar] [CrossRef]
- Nakahara, T.; Hashimoto, R.; Nakagawa, H.; Monde, K.; Miura, N.; Nishimura, S. Glycoconjugate Data Bank: Structures—An annotated glycan structure database and N-glycan primary structure verification service. Nucleic Acids Res. 2008, 36, D368–D371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cooper, C.A.; Harrison, M.J.; Wilkins, M.R.; Packer, N.H. GlycoSuiteDB: A new curated relational database of glycoprotein glycan structures and their biological sources. Nucleic Acids Res. 2001, 29, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Joshi, H.J.; Harrison, M.J.; Wilkins, M.R.; Packer, N.H. GlycoSuiteDB: A curated relational database of glycoprotein glycan structures and their biological sources. 2003 update. Nucleic Acids Res. 2003, 31, 511–513. [Google Scholar] [CrossRef]
- Toukach, P.V. Bacterial carbohydrate structure database 3: Principles and realization. J. Chem. Inf. Model. 2011, 51, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Toukach, P.V. CSDB_GT: A new curated database on glycosyltransferases. Glycobiology 2017, 27, 285–290. [Google Scholar] [CrossRef]
- Toukach, P.V.; Knirel, Y.A. New database of bacterial carbohydrate structures. Glycoconj. J. 2005, 22, 216–217. [Google Scholar]
- Lütteke, T.; Bohne-Lang, A.; Loss, A.; Goetz, T.; Frank, M.; Von Der Lieth, C.W. GLYCOSCIENCES.de: An Internet portal to support glycomics and glycobiology research. Glycobiology 2006, 16, 71R–81R. [Google Scholar] [CrossRef]
- Lütteke, T. Glycan data retrieval and analysis using GLYCOSCIENCES. de applications. In A Practical Guide to Using Glycomics Databases, 1st ed.; Aoki-Kinoshita, K.F., Ed.; Springer: Tokyo, Japan, 2017; pp. 335–350. [Google Scholar]
- Bohm, M.; Bohne-Lang, A.; Frank, M.; Loss, A.; Rojas-Macias, M.A.; Lutteke, T. Glycosciences.DB: An annotated data collection linking glycomics and proteomics data (2018 update). Nucleic Acids Res. 2019, 47, D1195–D1201. [Google Scholar] [CrossRef]
- Pérez, S.; Sarkar, A.; Rivet, A.; Breton, C.; Imberty, A. Glyco3D: A Portal for Structural Glycosciences. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 241–258. [Google Scholar]
- Pérez, S.; Sarkar, A.; Rivet, A.; Drouillard, S.; Breton, C.; Imberty, A. Glyco3D: A Suite of Interlinked Databases of 3D Structures of Complex Carbohydrates, Lectins, Antibodies, and Glycosyltransferases. In A Practical Guide to Using Glycomics Databases; Aoki-Kinoshita, K.F., Ed.; Springer: Tokyo, Japan, 2017; pp. 133–161. [Google Scholar]
- Sarkar, A.; Pérez, S. PolySac3DB: An annotated data base of 3 dimensional structures of polysaccharides. BMC Bioinf. 2012, 13, 302. [Google Scholar] [CrossRef]
- Kunduru, B.R.; Nair, S.A.; Rathinavelan, T. EK3D: An E. coli K antigen 3-dimensional structure database. Nucleic Acids Res. 2016, 44, D675–D681. [Google Scholar] [CrossRef]
- Veluraja, K.; Selvin, J.F.A.; Venkateshwari, S.; Priyadarzini, T.R.K. 3DSDSCAR—A three dimensional structural database for sialic acid-containing carbohydrates through molecular dynamics simulation. Carbohydr. Res. 2010, 345, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Veluraja, K.; Fermin Angelo Selvin, J.; Jasmine, A.; Hema Thanka Christlet, T. Three Dimensional Structures of Carbohydrates and Glycoinformatics: An Overview. In Current Trends in Bioinformatics: An Insight; Wadhwa, G., Shanmughavel, P., Singh, A.K., Bellare, J.R., Eds.; Springer: Singapore, 2018; pp. 55–87. [Google Scholar]
- Chautard, E.; Fatoux-Ardore, M.; Ballut, L.; Thierry-Mieg, N.; Ricard-Blum, S. MatrixDB, the extracellular matrix interaction database. Nucleic Acids Res. 2011, 39, D235–D240. [Google Scholar] [CrossRef] [PubMed]
- Launay, G.; Salza, R.; Multedo, D.; Thierry-Mieg, N.; Ricard-Blum, S. MatrixDB, the extracellular matrix interaction database: Updated content, a new navigator and expanded functionalities. Nucleic Acids Res. 2015, 43, D321–D327. [Google Scholar] [CrossRef]
- Clerc, O.; Deniaud, M.; Vallet, S.D.; Naba, A.; Rivet, A.; Perez, S.; Thierry-Mieg, N.; Ricard-Blum, S. MatrixDB: Integration of new data with a focus on glycosaminoglycan interactions. Nucleic Acids Res. 2019, 47, D376–D381. [Google Scholar] [CrossRef]
- Birch, J.; Van Calsteren, M.R.; Perez, S.; Svensson, B. The exopolysaccharide properties and structures database: EPS-DB. Application to bacterial exopolysaccharides. Carbohydr. Polym. 2019, 205, 565–570. [Google Scholar] [CrossRef]
- Cao, Y.; Park, S.-J.; Mehta, A.Y.; Cummings, R.D.; Im, W. GlyMDB: Glycan Microarray Database and analysis toolset. Bioinformatics 2020, 36, 2438–2442. [Google Scholar] [CrossRef]
- Raman, R.; Venkataraman, M.; Ramakrishnan, S.; Lang, W.; Raguram, S.; Sasisekharan, R. Advancing glycomics: Implementation strategies at the consortium for functional glycomics. Glycobiology 2006, 16, 82R–90R. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, M.; Sasisekharan, R.; Raman, R. Glycan Array Data Management at Consortium for Functional Glycomics. In Glycoinformatics, 1st ed.; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 181–190. [Google Scholar]
- Yamada, I.; Angata, K.; Watanabe, Y.; Ono, T. Databases for glycoconjugates (GlyCosmos Glycoproteins and Glycolipids, GlycoProtDB, GlycoNAVI:TCarp, GlycoPOST). Glycoforum 2020, 23, A2. [Google Scholar]
- Yamada, I.; Aoki-Kinoshita, K.F. Integration of Glycoscience Data in GlyCosmos Using Semantic Web Technologies. In Program and Abstracts for 2017 Annual Meeting of the Society for Glycobiology, Portland, OR, USA, 5–8 November 2017; Oxford University Press Inc.: Cary, NC, USA, 2017; p. 61. [Google Scholar]
- Shiota, M.; Tsuchiya, S.; Ono, T.; Kuoka, T.; Miura, N.; Hiraki, A.; Yamada, I.; Shinmachi, D.; Aoki, N.P.; Kim, J.-D. The GlyCosmos Web Portal: Glycan structures, glycogenes, glycoproteins, pathways, diseases and more! In Program and Abstracts for 2018 Annual Meeting of the Society for Glycobiology, New Orleans, LA, USA, 5–8 November 2018; Oxford University Press Inc.: Cary, NC, USA, 2018; pp. 1070–1071. [Google Scholar]
- Mariethoz, J.; Khatib, K.; Alocci, D.; Campbell, M.P.; Karlsson, N.G.; Packer, N.H.; Mullen, E.H.; Lisacek, F. SugarBindDB, a resource of glycan-mediated host–pathogen interactions. Nucleic Acids Res. 2016, 44, D1243–D1250. [Google Scholar] [CrossRef]
- Alocci, D.; Mariethoz, J.; Gastaldello, A.; Gasteiger, E.; Karlsson, N.G.; Kolarich, D.; Packer, N.H.; Lisacek, F. GlyConnect: Glycoproteomics Goes Visual, Interactive, and Analytical. J. Proteome Res. 2019, 18, 664–677. [Google Scholar] [CrossRef]
- Choudhary, P.; Nagar, R.; Singh, V.; Bhat, A.H.; Sharma, Y.; Rao, A. ProGlycProt V2.0, a repository of experimentally validated glycoproteins and protein glycosyltransferases of prokaryotes. Glycobiology 2019, 29, 461–468. [Google Scholar] [CrossRef]
- Bhat, A.H.; Mondal, H.; Chauhan, J.S.; Raghava, G.P.S.; Methi, A.; Rao, A. ProGlycProt: A repository of experimentally characterized prokaryotic glycoproteins. Nucleic Acids Res. 2012, 40, D388–D393. [Google Scholar] [CrossRef] [PubMed]
- Copoiu, L.; Torres, P.H.M.; Ascher, D.B.; Blundell, T.L.; Malhotra, S. ProCarbDB: A database of carbohydrate-binding proteins. Nucleic Acids Res. 2020, 48, D368–D375. [Google Scholar] [CrossRef] [PubMed]
- Siva Shanmugam, N.R.; Jino Blessy, J.; Veluraja, K.; Michael Gromiha, M. ProCaff: Protein–carbohydrate complex binding affinity database. Bioinformatics 2020, 36, 3615–3617. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Park, S.-J.; Im, W. A Systematic Analysis of Protein-Carbohydrate Interactions in the PDB. Glycobiology 2020. [Google Scholar] [CrossRef]
- Malik, A.; Firoz, A.; Jha, V.; Ahmad, S. PROCARB: A Database of Known and Modelled Carbohydrate-Binding Protein Structures with Sequence-Based Prediction Tools. Adv. Bioinf. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Bonnardel, F.; Mariethoz, J.; Salentin, S.; Robin, X.; Schroeder, M.; Perez, S.; Lisacek, F.; Imberty, A. UniLectin3D, a database of carbohydrate binding proteins with curated information on 3D structures and interacting ligands. Nucleic Acids Res. 2019, 47, D1236–D1244. [Google Scholar] [CrossRef] [PubMed]
- Bonnardel, F.; Perez, S.; Lisacek, F.; Imberty, A. Structural Database for Lectins and the UniLectin Web Platform. In Lectin Purification and Analysis: Methods and Protocols; Hirabayashi, J., Ed.; Humana: New York, NY, USA, 2020; pp. 1–14. [Google Scholar]
- Hirabayashi, J.; Tateno, H.; Shikanai, T.; Aoki-Kinoshita, K.F.; Narimatsu, H. The Lectin Frontier Database (LfDB), and data generation based on frontal affinity chromatography. Molecules 2015, 20, 951–973. [Google Scholar] [CrossRef]
- Chandra, N.R.; Kumar, N.; Jeyakani, J.; Singh, D.D.; Gowda, S.B.; Prathima, M.N. Lectindb: A plant lectin database. Glycobiology 2006, 16, 938–946. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nakao, H.; Takahashi, E.; Tominaga, T. GlycoEpitope: The integrated database of carbohydrate antigens and antibodies. Trends Glycosci. Glycotechnol. 2006, 18, 267–272. [Google Scholar] [CrossRef][Green Version]
- Kawasaki, T.; Nakao, H.; Tominaga, T. GlycoEpitope: A database of carbohydrate epitopes and antibodies. In Experimental Glycoscience; Taniguchi, N., Suzuki, A., Ito, Y., Narimatsu, H., Kawasaki, T., Hase, S., Eds.; Springer: Tokyo, Japan, 2008; pp. 429–431. [Google Scholar]
- Aoki-Kinoshita, K.F. Using Databases and Web Resources for Glycomics Research. Mol. Cell. Proteom. 2013, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lütteke, T.; Schwartz-Albiez, R. GlycoCD: A repository for carbohydrate-related CD antigens. Bioinformatics 2012, 28, 2553–2555. [Google Scholar] [CrossRef]
- Allcorn, L.C.; Martin, A.C.R. SACS—Self-maintaining database of antibody crystal structure information. Bioinformatics 2002, 18, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, J.; Krawczyk, K.; Leem, J.; Baker, T.; Fuchs, A.; Georges, G.; Shi, J.; Deane, C.M. SAbDab: The structural antibody database. Nucleic Acids Res. 2014, 42, D1140–D1146. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Terrapon, N.; Lombard, V.; Drula, E.; Coutinho, P.M.; Henrissat, B. The CAZy Database/the Carbohydrate-Active Enzyme (CAZy) Database: Principles and Usage Guidelines. In A Practical Guide to Using Glycomics Databases; Aoki-Kinoshita, K.F., Ed.; Springer: Tokyo, Japan, 2017; pp. 117–131. [Google Scholar]
- Lee, T.-Y.; Huang, H.-D.; Hung, J.-H.; Huang, H.-Y.; Yang, Y.-S.; Wang, T.-H. dbPTM: An information repository of protein post-translational modification. Nucleic Acids Res. 2006, 34, D622–D627. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Su, M.-G.; Kao, H.-J.; Hsieh, Y.-C.; Jhong, J.-H.; Cheng, K.-H.; Huang, H.-D.; Lee, T.-Y. dbPTM 2016: 10-year anniversary of a resource for post-translational modification of proteins. Nucleic Acids Res. 2016, 44, D435–D446. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-Y.; Lee, T.-Y.; Kao, H.-J.; Ma, C.-T.; Lee, C.-C.; Lin, T.-H.; Chang, W.-C.; Huang, H.-D. dbPTM in 2019: Exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res. 2019, 47, D298–D308. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.; Schwede, T. The SWISS-MODEL Repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004, 32, D230–D234. [Google Scholar] [CrossRef]
- Kiefer, F.; Arnold, K.; Künzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009, 37, D387–D392. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Frank, M.; Lutteke, T.; Von Der Lieth, C.W. GlycoMapsDB: A database of the accessible conformational space of glycosidic linkages. Nucleic Acids Res. 2007, 35, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Im, W. Glycan fragment database: A database of PDB-based glycan 3D structures. Nucleic Acids Res. 2013, 41, D470–D474. [Google Scholar] [CrossRef]
- Perez, S. X-Ray Diffraction and Crystallography of Oligosaccharides and Polysaccharides. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2767–2777. [Google Scholar]
- Gimeno, A.; Valverde, P.; Ardá, A.; Jiménez-Barbero, J. Glycan structures and their interactions with proteins. A NMR view. Curr. Opin. Struct. Biol. 2020, 62, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Blaum, B.S.; Neu, U.; Peters, T.; Stehle, T. Spin ballet for sweet encounters: Saturation-transfer difference NMR and X-ray crystallography complement each other in the elucidation of protein-glycan interactions. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Battistel, M.D.; Azurmendi, H.F.; Yu, B.; Freedberg, D.I. NMR of glycans: Shedding new light on old problems. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 79, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M. Insights into Carbohydrate Recognition by 3D Structure Determination of Protein–Carbohydrate Complexes Using NMR. In NMR in Glycoscience and Glycotechnology; Kato, K., Peters, T., Eds.; The Royal Society of Chemistry: London, UK, 2017; pp. 101–122. [Google Scholar]
- Cléry, A.; Schubert, M.; Allain, F.H.T. NMR Spectroscopy: An Excellent Tool to Understand RNA and Carbohydrate Recognition by Proteins. Chimia 2012, 66, 741–746. [Google Scholar] [CrossRef]
- Valverde, P.; Delgado, S.; Martínez, J.D.; Vendeville, J.-B.; Malassis, J.; Linclau, B.; Reichardt, N.-C.; Cañada, F.J.; Jiménez-Barbero, J.; Ardá, A. Molecular Insights into DC-SIGN Binding to Self-Antigens: The Interaction with the Blood Group A/B Antigens. ACS Chem. Biol. 2019, 14, 1660–1671. [Google Scholar] [CrossRef]
- Aeschbacher, T.; Zierke, M.; Smieško, M.; Collot, M.; Mallet, J.-M.; Ernst, B.; Allain, F.H.-T.; Schubert, M. A Secondary Structural Element in a Wide Range of Fucosylated Glycoepitopes. Chem. Eur. J. 2017, 23, 11598–11610. [Google Scholar] [CrossRef]
- Zierke, M.; Smieško, M.; Rabbani, S.; Aeschbacher, T.; Cutting, B.; Allain, F.H.T.; Schubert, M.; Ernst, B. Stabilization of Branched Oligosaccharides: Lewisx Benefits from a Nonconventional C–H···O Hydrogen Bond. J. Am. Chem. Soc. 2013, 135, 13464–13472. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, B.M.; Almond, A. Is N-acetyl-d-glucosamine a rigid 4C1 chair? Glycobiology 2011, 21, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- França, B.A.; da Silva, C.O. Specific rotation of monosaccharides: A global property bringing local information. Phys. Chem. Chem. Phys. 2014, 16, 13096–13102. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Edwards, J.V.; Nam, S.; Xu, F.; French, A.D. Conformational analysis of xylobiose by DFT quantum mechanics. Cellulose 2020, 27, 1207–1224. [Google Scholar] [CrossRef]
- McMahon, C.M.; Isabella, C.R.; Windsor, I.W.; Kosma, P.; Raines, R.T.; Kiessling, L.L. Stereoelectronic Effects Impact Glycan Recognition. J. Am. Chem. Soc. 2020, 142, 2386–2395. [Google Scholar] [CrossRef]
- Zhang, W.; Meredith, R.; Pan, Q.; Wang, X.; Woods, R.J.; Carmichael, I.; Serianni, A.S. Use of Circular Statistics To Model αMan-(1→2)-αMan and αMan-(1→3)-α/βMan O-Glycosidic Linkage Conformation in 13C-Labeled Disaccharides and High-Mannose Oligosaccharides. Biochemistry 2019, 58, 546–560. [Google Scholar] [CrossRef]
- Saraboji, K.; Håkansson, M.; Genheden, S.; Diehl, C.; Qvist, J.; Weininger, U.; Nilsson, U.J.; Leffler, H.; Ryde, U.; Akke, M.; et al. The Carbohydrate-Binding Site in Galectin-3 Is Preorganized To Recognize a Sugarlike Framework of Oxygens: Ultra-High-Resolution Structures and Water Dynamics. Biochemistry 2012, 51, 296–306. [Google Scholar] [CrossRef]
- Turupcu, A.; Bowen, A.M.; Di Paolo, A.; Matagne, A.; Oostenbrink, C.; Redfield, C.; Smith, L.J. An NMR and MD study of complexes of bacteriophage lambda lysozyme with tetra- and hexa-N-acetylchitohexaose. Proteins Struct. Funct. Bioinf. 2020, 88, 82–93. [Google Scholar] [CrossRef]
- Turupcu, A.; Oostenbrink, C. Modeling of Oligosaccharides within Glycoproteins from Free-Energy Landscapes. J. Chem. Inf. Model. 2017, 57, 2222–2236. [Google Scholar] [CrossRef]
- Zhang, W.; Turney, T.; Meredith, R.; Pan, Q.; Sernau, L.; Wang, X.; Hu, X.; Woods, R.J.; Carmichael, I.; Serianni, A.S. Conformational Populations of β-(1→4) O-Glycosidic Linkages Using Redundant NMR J-Couplings and Circular Statistics. J. Phys. Chem. B 2017, 121, 3042–3058. [Google Scholar] [CrossRef]
- Sattelle, B.M.; Almond, A. Shaping up for structural glycomics: A predictive protocol for oligosaccharide conformational analysis applied to N-linked glycans. Carbohydr. Res. 2014, 383, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Frank, M. Conformational Analysis of Carbohydrates—A Historical Overview. In Bioinformatics for Glycobiology and Glycomics: An Introduction; Von Der Lieth, C.W., Lütteke, T., Frank, M., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 335–357. [Google Scholar]
- Frank, M. Predicting Carbohydrate 3D Structures Using Theoretical Methods. In Bioinformatics for Glycobiology and Glycomics: An Introduction; Von Der Lieth, C.W., Lütteke, T., Frank, M., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 359–388. [Google Scholar]
- Toukach, F.V.; Ananikov, V.P. Recent advances in computational predictions of NMR parameters for the structure elucidation of carbohydrates: Methods and limitations. Chem. Soc. Rev. 2013, 42, 8376–8415. [Google Scholar] [CrossRef]
- French, A.D. Computerized Models of Carbohydrates. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1397–1440. [Google Scholar]
- French, A.D.; Johnson, G.P. Computerized Molecular Modeling of Carbohydrates. In The Plant Cell Wall: Methods and Protocols; Popper, Z.A., Ed.; Humana: New York, NY, USA, 2020; pp. 513–539. [Google Scholar]
- Feng, T.; Li, M.; Zhou, J.; Zhuang, H.; Chen, F.; Ye, R.; Campanella, O.; Fang, Z. Application of molecular dynamics simulation in food carbohydrate research—A review. Innov. Food Sci. Emerg. Technol. 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Dowd, M.K.; Kiely, D.E.; Zhang, J. Monte Carlo-based searching as a tool to study carbohydrate structure. Carbohydr. Res. 2011, 346, 1140–1148. [Google Scholar] [CrossRef]
- Zhang, W.; Howell, S.C.; Wright, D.W.; Heindel, A.; Qiu, X.; Chen, J.; Curtis, J.E. Combined Monte Carlo/torsion-angle molecular dynamics for ensemble modeling of proteins, nucleic acids and carbohydrates. J. Mol. Graph. Modell. 2017, 73, 179–190. [Google Scholar] [CrossRef]
- Rahal-Sekkal, M.; Sekkal, N.; Kleb, D.C.; Bleckmann, P. Structures and energies of D-galactose and galabiose conformers as calculated by ab initio and semiempirical methods. J. Comput. Chem. 2003, 24, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.B.; Naidoo, K.J. Ring Puckering: A Metric for Evaluating the Accuracy of AM1, PM3, PM3CARB-1, and SCC-DFTB Carbohydrate QM/MM Simulations. J. Phys. Chem. B 2010, 114, 17142–17154. [Google Scholar] [CrossRef]
- Govender, K.; Gao, J.; Naidoo, K.J. AM1/d-CB1: A Semiempirical Model for QM/MM Simulations of Chemical Glycobiology Systems. J. Chem. Theory Comput. 2014, 10, 4694–4707. [Google Scholar] [CrossRef]
- Govender, K.K.; Naidoo, K.J. Evaluating AM1/d-CB1 for Chemical Glycobiology QM/MM Simulations. J. Chem. Theory Comput. 2014, 10, 4708–4717. [Google Scholar] [CrossRef]
- Gould, I.R.; Bettley, H.A.-A.; Bryce, R.A. Correlated ab initio quantum chemical calculations of di- and trisaccharide conformations. J. Comput. Chem. 2007, 28, 1965–1973. [Google Scholar] [CrossRef]
- French, A.D.; Johnson, G.P.; Cramer, C.J.; Csonka, G.I. Conformational analysis of cellobiose by electronic structure theories. Carbohydr. Res. 2012, 350, 68–76. [Google Scholar] [CrossRef]
- Schnupf, U.; Momany, F.A. DFT Energy Optimization of a Large Carbohydrate: Cyclomaltohexaicosaose (CA-26). J. Phys. Chem. B 2012, 116, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, A.; Markutsya, S.; Lamm, M.H.; Cheng, X.; Smith, J.C.; Baluyut, J.Y.; Kholod, Y.; Gordon, M.S.; Windus, T.L. Ab Initio Study of Molecular Interactions in Cellulose Iα. J. Phys. Chem. B 2013, 117, 10430–10443. [Google Scholar] [CrossRef]
- Chan, B. Aqueous-Phase Conformations of Lactose, Maltose, and Sucrose and the Assessment of Low-Cost DFT Methods with the DSCONF Set of Conformers for the Three Disaccharides. J. Phys. Chem. A 2020, 124, 582–590. [Google Scholar] [CrossRef]
- Ishida, T. Computational analysis of carbohydrate recognition based on hybrid QM/MM modeling: A case study of norovirus capsid protein in complex with Lewis antigen. Phys. Chem. Chem. Phys. 2018, 20, 4652–4665. [Google Scholar] [CrossRef]
- Tafazzoli, M.; Ghiasi, M. Structure and conformation of α-, β- and γ-cyclodextrin in solution: Theoretical approaches and experimental validation. Carbohydr. Polym. 2009, 78, 10–15. [Google Scholar] [CrossRef]
- Ardèvol, A.; Rovira, C. Reaction Mechanisms in Carbohydrate-Active Enzymes: Glycoside Hydrolases and Glycosyltransferases. Insights from ab Initio Quantum Mechanics/Molecular Mechanics Dynamic Simulations. J. Am. Chem. Soc. 2015, 137, 7528–7547. [Google Scholar] [CrossRef]
- Tvaroška, I. Atomistic insight into the catalytic mechanism of glycosyltransferases by combined quantum mechanics/molecular mechanics (QM/MM) methods. Carbohydr. Res. 2015, 403, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.P.; Petersen, L.; French, A.D.; Reilly, P.J. Twisting of glycosidic bonds by hydrolases. Carbohydr. Res. 2009, 344, 2157–2166. [Google Scholar] [CrossRef]
- Chung, L.W.; Sameera, W.M.C.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Liu, F.; et al. The ONIOM Method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef]
- Burnham, J.F. Scopus database: A review. Biomed. Digit. Libr. 2006, 3, 1. [Google Scholar] [CrossRef]
- Frank, M.; Schloissnig, S. Bioinformatics and molecular modeling in glycobiology. Cell. Mol. Life Sci. 2010, 67, 2749–2772. [Google Scholar] [CrossRef]
- Sapay, N.; Nurisso, A.; Imberty, A. Simulation of Carbohydrates, from Molecular Docking to Dynamics in Water. In Biomolecular Simulations: Methods and Protocols; Monticelli, L., Salonen, E., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 469–483. [Google Scholar]
- Pérez, S.; Tvaroška, I. Chapter 1—Carbohydrate–Protein Interactions: Molecular Modeling Insights. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 9–136. [Google Scholar]
- Fadda, E.; Woods, R.J. Molecular simulations of carbohydrates and protein–carbohydrate interactions: Motivation, issues and prospects. Drug Discov. Today 2010, 15, 596–609. [Google Scholar] [CrossRef]
- Yongye, A.B.; Gonzalez-Outeiriño, J.; Glushka, J.; Schultheis, V.; Woods, R.J. The Conformational Properties of Methyl α-(2,8)-Di/Trisialosides and Their N-Acyl Analogues: Implications for Anti-Neisseria meningitidis B Vaccine Design. Biochemistry 2008, 47, 12493–12514. [Google Scholar] [CrossRef]
- Re, S.; Nishima, W.; Miyashita, N.; Sugita, Y. Conformational flexibility of N-glycans in solution studied by REMD simulations. Biophys. Rev. 2012, 4, 179–187. [Google Scholar] [CrossRef][Green Version]
- Patel, D.S.; Pendrill, R.; Mallajosyula, S.S.; Widmalm, G.; MacKerell, A.D. Conformational Properties of α- or β-(1→6)-Linked Oligosaccharides: Hamiltonian Replica Exchange MD Simulations and NMR Experiments. J. Phys. Chem. B 2014, 118, 2851–2871. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kara, M.; Zacharias, M.; Koča, J. Enhanced conformational sampling of carbohydrates by Hamiltonian replica-exchange simulation. Glycobiology 2014, 24, 70–84. [Google Scholar] [CrossRef]
- Mallajosyula, S.S.; MacKerell, A.D. Influence of Solvent and Intramolecular Hydrogen Bonding on the Conformational Properties of O-Linked Glycopeptides. J. Phys. Chem. B 2011, 115, 11215–11229. [Google Scholar] [CrossRef] [PubMed]
- Alibay, I.; Burusco, K.K.; Bruce, N.J.; Bryce, R.A. Identification of Rare Lewis Oligosaccharide Conformers in Aqueous Solution Using Enhanced Sampling Molecular Dynamics. J. Phys. Chem. B 2018, 122, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Alibay, I.; Bryce, R.A. Ring Puckering Landscapes of Glycosaminoglycan-Related Monosaccharides from Molecular Dynamics Simulations. J. Chem. Inf. Model. 2019, 59, 4729–4741. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.; Komáromi, I.; Bereczky, Z. The mechanism of high affinity pentasaccharide binding to antithrombin, insights from Gaussian accelerated molecular dynamics simulations. J. Biomol. Struct. Dyn. 2019, 38, 4718–4732. [Google Scholar] [CrossRef]
- Balogh, G.; Gyöngyösi, T.; Timári, I.; Herczeg, M.; Borbás, A.; Fehér, K.; Kövér, K.E. Comparison of Carbohydrate Force Fields Using Gaussian Accelerated Molecular Dynamics Simulations and Development of Force Field Parameters for Heparin-Analogue Pentasaccharides. J. Chem. Inf. Model. 2019, 59, 4855–4867. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kajino, M.; Yanaka, S.; Zhu, T.; Yagi, H.; Satoh, T.; Yamaguchi, T.; Kato, K. Conformational Analysis of a High-Mannose-Type Oligosaccharide Displaying Glucosyl Determinant Recognised by Molecular Chaperones Using NMR-Validated Molecular Dynamics Simulation. ChemBioChem 2017, 18, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sakae, Y.; Zhang, Y.; Yamamoto, S.; Okamoto, Y.; Kato, K. Exploration of Conformational Spaces of High-Mannose-Type Oligosaccharides by an NMR-Validated Simulation. Angew. Chem. Int. Ed. 2014, 53, 10941–10944. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.L.; Tessier, M.B.; Woods, R.J. Carbohydrate force fields. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 652–697. [Google Scholar] [CrossRef]
- Kozmon, S.; Matuška, R.; Spiwok, V.; Koča, J. Dispersion interactions of carbohydrates with condensate aromatic moieties: Theoretical study on the CH–π interaction additive properties. Phys. Chem. Chem. Phys. 2011, 13, 14215–14222. [Google Scholar] [CrossRef]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Park, S.; Mortenson, D.E.; Foley, B.L.; Wang, X.; Woods, R.J.; Case, D.A.; Powers, E.T.; Wong, C.-H.; Dyson, H.J.; et al. The Dependence of Carbohydrate–Aromatic Interaction Strengths on the Structure of the Carbohydrate. J. Am. Chem. Soc. 2016, 138, 7636–7648. [Google Scholar] [CrossRef]
- Stanković, I.M.; Blagojević Filipović, J.P.; Zarić, S.D. Carbohydrate—Protein aromatic ring interactions beyond CH/π interactions: A Protein Data Bank survey and quantum chemical calculations. Int. J. Biol. Macromol. 2020, 157, 1–9. [Google Scholar] [CrossRef]
- Nivedha, A.K.; Makeneni, S.; Foley, B.L.; Tessier, M.B.; Woods, R.J. Importance of ligand conformational energies in carbohydrate docking: Sorting the wheat from the chaff. J. Comput. Chem. 2014, 35, 526–539. [Google Scholar] [CrossRef]
- Nivedha, A.K.; Thieker, D.F.; Hu, H.; Woods, R.J. Vina-Carb: Improving Glycosidic Angles during Carbohydrate Docking. J. Chem. Theory Comput. 2016, 12, 892–901. [Google Scholar] [CrossRef]
- Pérez, S. 2.11—Molecular Modeling in Glycoscience. In Comprehensive Glycoscience; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; Volume 2, pp. 347–388. [Google Scholar]
- Stortz, C.A.; French, A.D. Disaccharide conformational maps: Adiabaticity in analogues with variable ring shapes. Mol. Simul. 2008, 34, 373–389. [Google Scholar] [CrossRef]
- Allinger, N.L.; Yuh, Y.H.; Lii, J.H. Molecular mechanics. The MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 1989, 111, 8551–8566. [Google Scholar] [CrossRef]
- Allinger, N.L.; Rahman, M.; Lii, J.H. A molecular mechanics force field (MM3) for alcohols and ethers. J. Am. Chem. Soc. 1990, 112, 8293–8307. [Google Scholar] [CrossRef]
- Stortz, C.A. Comparative performance of MM3(92) and two TINKER™ MM3 versions for the modeling of carbohydrates. J. Comput. Chem. 2005, 26, 471–483. [Google Scholar] [CrossRef]
- Stortz, C.A.; Johnson, G.P.; French, A.D.; Csonka, G.I. Comparison of different force fields for the study of disaccharides. Carbohydr. Res. 2009, 344, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.A.; Richards, M.R.; Lowary, T.L. Conformational analysis of furanoside-containing mono- and oligosaccharides. Chem. Rev. 2013, 113, 1851–1876. [Google Scholar] [CrossRef]
- Stortz, C.A. Additive effects in the modeling of oligosaccharides with mm3 at high dielectric constants: An approach to the ‘multiple minimum problem’. Carbohydr. Res. 2006, 341, 663–671. [Google Scholar] [CrossRef]
- Stortz, C.A. mm3 Potential energy surfaces of trisaccharide models of λ-, μ-, and ν-carrageenans. Carbohydr. Res. 2006, 341, 2531–2542. [Google Scholar] [CrossRef]
- Xiong, X.M.; Chen, Z.Q.; Cossins, B.P.; Xu, Z.J.; Shao, Q.; Ding, K.; Zhu, W.L.; Shi, J.Y. Force fields and scoring functions for carbohydrate simulation. Carbohydr. Res. 2015, 401, 73–81. [Google Scholar] [CrossRef]
- CHARMM Force Field Files. Available online: https://www.charmm.org/charmm/resources/charmm-force-fields/#charmm (accessed on 31 July 2020).
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Mackerell, A.D., Jr.; Feig, M.; Brooks, C.L., III. Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 2004, 25, 1400–1415. [Google Scholar] [CrossRef]
- Guvench, O.; Hatcher, E.; Venable, R.M.; Pastor, R.W.; MacKerell, A.D. CHARMM Additive All-Atom Force Field for Glycosidic Linkages between Hexopyranoses. J. Chem. Theory Comput. 2009, 5, 2353–2370. [Google Scholar] [CrossRef]
- Guvench, O.; Greene, S.N.; Kamath, G.; Brady, J.W.; Venable, R.M.; Pastor, R.W.; Mackerell, A.D., Jr. Additive empirical force field for hexopyranose monosaccharides. J. Comput. Chem. 2008, 29, 2543–2564. [Google Scholar] [CrossRef] [PubMed]
- Raman, E.P.; Guvench, O.; MacKerell, A.D. CHARMM Additive All-Atom Force Field for Glycosidic Linkages in Carbohydrates Involving Furanoses. J. Phys. Chem. B 2010, 114, 12981–12994. [Google Scholar] [CrossRef] [PubMed]
- Guvench, O.; Mallajosyula, S.S.; Raman, E.P.; Hatcher, E.; Vanommeslaeghe, K.; Foster, T.J.; Jamison, F.W.; MacKerell, A.D. CHARMM Additive All-Atom Force Field for Carbohydrate Derivatives and Its Utility in Polysaccharide and Carbohydrate—Protein Modeling. J. Chem. Theory Comput. 2011, 7, 3162–3180. [Google Scholar] [CrossRef]
- Mallajosyula, S.S.; Guvench, O.; Hatcher, E.; MacKerell, A.D. CHARMM Additive All-Atom Force Field for Phosphate and Sulfate Linked to Carbohydrates. J. Chem. Theory Comput. 2012, 8, 759–776. [Google Scholar] [CrossRef]
- Cloutier, T.; Sudrik, C.; Sathish, H.A.; Trout, B.L. Kirkwood–Buff-Derived Alcohol Parameters for Aqueous Carbohydrates and Their Application to Preferential Interaction Coefficient Calculations of Proteins. J. Phys. Chem. B 2018, 122, 9350–9360. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]
- Tessier, M.B.; DeMarco, M.L.; Yongye, A.B.; Woods, R.J. Extension of the GLYCAM06 biomolecular force field to lipids, lipid bilayers and glycolipids. Mol. Simul. 2008, 34, 349–364. [Google Scholar] [CrossRef]
- DeMarco, M.L.; Woods, R.J. Atomic-resolution conformational analysis of the GM3 ganglioside in a lipid bilayer and its implications for ganglioside-protein recognition at membrane surfaces. Glycobiology 2009, 19, 344–355. [Google Scholar] [CrossRef][Green Version]
- DeMarco, M.L. Molecular Dynamics Simulations of Membrane- and Protein-Bound Glycolipids Using GLYCAM. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 379–390. [Google Scholar]
- Kirschner, K.N.; Lins, R.D.; Maass, A.; Soares, T.A. A Glycam-Based Force Field for Simulations of Lipopolysaccharide Membranes: Parametrization and Validation. J. Chem. Theory Comput. 2012, 8, 4719–4731. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tessier, M.B.; Pederson, K.; Wang, X.; Venot, A.P.; Boons, G.-J.; Prestegard, J.H.; Woods, R.J. Extension and validation of the GLYCAM force field parameters for modeling glycosaminoglycans. Can. J. Chem. 2016, 94, 927–935. [Google Scholar] [CrossRef]
- Lins, R.D.; Hünenberger, P.H. A new GROMOS force field for hexopyranose-based carbohydrates. J. Comput. Chem. 2005, 26, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S.; Hünenberger, P.H. A reoptimized GROMOS force field for hexopyranose-based carbohydrates accounting for the relative free energies of ring conformers, anomers, epimers, hydroxymethyl rotamers, and glycosidic linkage conformers. J. Comput. Chem. 2011, 32, 998–1032. [Google Scholar] [CrossRef]
- Pontes, F.J.S.; Rusu, V.H.; Soares, T.A.; Lins, R.D. The Effect of Temperature, Cations, and Number of Acyl Chains on the Lamellar to Non-Lamellar Transition in Lipid-A Membranes: A Microscopic View. J. Chem. Theory Comput. 2012, 8, 3830–3838. [Google Scholar] [CrossRef]
- Pol-Fachin, L.; Rusu, V.H.; Verli, H.; Lins, R.D. GROMOS 53A6GLYC, an Improved GROMOS Force Field for Hexopyranose-Based Carbohydrates. J. Chem. Theory Comput. 2012, 8, 4681–4690. [Google Scholar] [CrossRef] [PubMed]
- Pol-Fachin, L.; Verli, H.; Lins, R.D. Extension and validation of the GROMOS 53A6glyc parameter set for glycoproteins. J. Comput. Chem. 2014, 35, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Plazinski, W.; Lonardi, A.; Hünenberger, P.H. Revision of the GROMOS 56A6CARBO force field: Improving the description of ring-conformational equilibria in hexopyranose-based carbohydrates chains. J. Comput. Chem. 2016, 37, 354–365. [Google Scholar] [CrossRef]
- Naumov, V.S.; Ignatov, S.K. Modification of 56ACARBO force field for molecular dynamic calculations of chitosan and its derivatives. J. Mol. Model. 2017, 23, 244. [Google Scholar] [CrossRef]
- Panczyk, K.; Gaweda, K.; Drach, M.; Plazinski, W. Extension of the GROMOS 56a6CARBO/CARBO_R Force Field for Charged, Protonated, and Esterified Uronates. J. Phys. Chem. B 2018, 122, 3696–3710. [Google Scholar] [CrossRef] [PubMed]
- Nester, K.; Gaweda, K.; Plazinski, W. A GROMOS Force Field for Furanose-Based Carbohydrates. J. Chem. Theory Comput. 2019, 15, 1168–1186. [Google Scholar] [CrossRef]
- Pol-Fachin, L.; Fernandes, C.L.; Verli, H. GROMOS96 43a1 performance on the characterization of glycoprotein conformational ensembles through molecular dynamics simulations. Carbohydr. Res. 2009, 344, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.L.; Sachett, L.G.; Pol-Fachin, L.; Verli, H. GROMOS96 43a1 performance in predicting oligosaccharide conformational ensembles within glycoproteins. Carbohydr. Res. 2010, 345, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kony, D.; Damm, W.; Stoll, S.; Van Gunsteren, W.F. An improved OPLS–AA force field for carbohydrates. J. Comput. Chem. 2002, 23, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Damm, W.; Frontera, A.; Tirado–Rives, J.; Jorgensen, W.L. OPLS all-atom force field for carbohydrates. J. Comput. Chem. 1997, 18, 1955–1970. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jamali, S.H.; Westen, T.V.; Moultos, O.A.; Vlugt, T.J.H. Optimizing Nonbonded Interactions of the OPLS Force Field for Aqueous Solutions of Carbohydrates: How to Capture Both Thermodynamics and Dynamics. J. Chem. Theory Comput. 2018, 14, 6690–6700. [Google Scholar] [CrossRef]
- Patel, D.S.; He, X.; MacKerell, A.D. Polarizable Empirical Force Field for Hexopyranose Monosaccharides Based on the Classical Drude Oscillator. J. Phys. Chem. B 2015, 119, 637–652. [Google Scholar] [CrossRef]
- Yang, M.; Aytenfisu, A.H.; MacKerell, A.D. Proper balance of solvent-solute and solute-solute interactions in the treatment of the diffusion of glucose using the Drude polarizable force field. Carbohydr. Res. 2018, 457, 41–50. [Google Scholar] [CrossRef]
- Jana, M.; MacKerell, A.D. CHARMM Drude Polarizable Force Field for Aldopentofuranoses and Methyl-aldopentofuranosides. J. Phys. Chem. B 2015, 119, 7846–7859. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Aytenfisu, A.H.; MacKerell, A.D.; Mallajosyula, S.S. Drude Polarizable Force Field Parametrization of Carboxylate and N-Acetyl Amine Carbohydrate Derivatives. J. Chem. Theory Comput. 2019, 15, 4982–5000. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lopes, P.E.M.; MacKerell, A.D., Jr. Polarizable Empirical Force Field for Acyclic Polyalcohols Based on the Classical Drude Oscillator. Biopolymers 2013, 99, 724–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aytenfisu, A.H.; Yang, M.; MacKerell, A.D. CHARMM Drude Polarizable Force Field for Glycosidic Linkages Involving Pyranoses and Furanoses. J. Chem. Theory Comput. 2018, 14, 3132–3143. [Google Scholar] [CrossRef]
- López, C.A.; Rzepiela, A.J.; De Vries, A.H.; Dijkhuizen, L.; Hünenberger, P.H.; Marrink, S.J. Martini Coarse-Grained Force Field: Extension to Carbohydrates. J. Chem. Theory Comput. 2009, 5, 3195–3210. [Google Scholar] [CrossRef]
- Schmalhorst, P.S.; Deluweit, F.; Scherrers, R.; Heisenberg, C.-P.; Sikora, M. Overcoming the Limitations of the MARTINI Force Field in Simulations of Polysaccharides. J. Chem. Theory Comput. 2017, 13, 5039–5053. [Google Scholar] [CrossRef]
- Shivgan, A.; Marzinek, J.K.; Huber, R.G.; Krah, A.; Henchman, R.H.; Matsudaira, P.T.; Verma, C.S.; Bond, P.J. Extending the Martini Coarse-Grained Forcefield to N-Glycans. J. Chem. Inf. Model. 2020, 60, 3864–3883. [Google Scholar] [CrossRef]
- López, C.A.; Sovova, Z.; van Eerden, F.J.; De Vries, A.H.; Marrink, S.J. Martini Force Field Parameters for Glycolipids. J. Chem. Theory Comput. 2013, 9, 1694–1708. [Google Scholar] [CrossRef]
- Rusu, V.H.; Baron, R.; Lins, R.D. PITOMBA: Parameter Interface for Oligosaccharide Molecules Based on Atoms. J. Chem. Theory Comput. 2014, 10, 5068–5080. [Google Scholar] [CrossRef]
- Spiwok, V.; Lipovová, P.; Skálová, T.; Vondráčková, E.; Dohnálek, J.; Hašek, J.; Králová, B. Modelling of carbohydrate–aromatic interactions: Ab initio energeticsand force field performance. J. Comput. Aided Mol. Des. 2005, 19, 887–901. [Google Scholar] [CrossRef]
- Wimmerová, M.; Kozmon, S.; Nečasová, I.; Mishra, S.K.; Komárek, J.; Koča, J. Stacking interactions between carbohydrate and protein quantified by combination of theoretical and experimental methods. PLoS ONE 2012, 7, e46032. [Google Scholar] [CrossRef]
- Makeneni, S.; Thieker, D.F.; Woods, R.J. Applying Pose Clustering and MD Simulations To Eliminate False Positives in Molecular Docking. J. Chem. Inf. Model. 2018, 58, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Grinter, S.Z.; Zou, X. Scoring functions and their evaluation methods for protein–ligand docking: Recent advances and future directions. Phys. Chem. Chem. Phys. 2010, 12, 12899–12908. [Google Scholar] [CrossRef]
- Vandenbussche, S.; Díaz, D.; Fernández-Alonso, M.C.; Pan, W.; Vincent, S.P.; Cuevas, G.; Cañada, F.J.; Jiménez-Barbero, J.; Bartik, K. Aromatic–Carbohydrate Interactions: An NMR and Computational Study of Model Systems. Chem. Eur. J. 2008, 14, 7570–7578. [Google Scholar] [CrossRef]
- Hill, A.D.; Reilly, P.J. A Gibbs free energy correlation for automated docking of carbohydrates. J. Comput. Chem. 2008, 29, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Baig, M.H.; Manavalan, B. Protein-Carbohydrate Interactions. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Elsevier: Oxford, UK, 2019; Volume 3, pp. 666–677. [Google Scholar]
- Samsonov, S.A.; Teyra, J.; Pisabarro, M.T. Docking glycosaminoglycans to proteins: Analysis of solvent inclusion. J. Comput. Aided Mol. Des. 2011, 25, 477–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samsonov, S.A.; Gehrcke, J.-P.; Pisabarro, M.T. Flexibility and Explicit Solvent in Molecular-Dynamics-Based Docking of Protein–Glycosaminoglycan Systems. J. Chem. Inf. Model. 2014, 54, 582–592. [Google Scholar] [CrossRef]
- Gerlits, O.O.; Coates, L.; Woods, R.J.; Kovalevsky, A. Mannobiose Binding Induces Changes in Hydrogen Bonding and Protonation States of Acidic Residues in Concanavalin a As Revealed by Neutron Crystallography. Biochemistry 2017, 56, 4747–4750. [Google Scholar] [CrossRef] [PubMed]
- Yuriev, E.; Holien, J.; Ramsland, P.A. Improvements, trends, and new ideas in molecular docking: 2012–2013 in review. J. Mol. Recognit. 2015, 28, 581–604. [Google Scholar] [CrossRef]
- Mishra, N.K.; Kříž, Z.; Wimmerová, M.; Koča, J. Recognition of selected monosaccharides by Pseudomonas aeruginosa Lectin II analyzed by molecular dynamics and free energy calculations. Carbohydr. Res. 2010, 345, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Adam, J.; Wimmerová, M.; Koča, J. In Silico Mutagenesis and Docking Study of Ralstonia solanacearum RSL Lectin: Performance of Docking Software to Predict Saccharide Binding. J. Chem. Inf. Model. 2012, 52, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, A.; Mosier, P.D.; Desai, U.R. Finding a Needle in a Haystack: Development of a Combinatorial Virtual Screening Approach for Identifying High Specificity Heparin/Heparan Sulfate Sequence(s). J. Med. Chem. 2006, 49, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, S.A.; Pisabarro, M.T. Computational analysis of interactions in structurally available protein–glycosaminoglycan complexes. Glycobiology 2016, 26, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Gehrcke, J.-P.; Pisabarro, M.T. Identification and characterization of a glycosaminoglycan binding site on interleukin-10 via molecular simulation methods. J. Mol. Graph. Modell. 2015, 62, 97–104. [Google Scholar] [CrossRef]
- Agostino, M.; Sandrin, M.S.; Thompson, P.E.; Yuriev, E.; Ramsland, P.A. In silico analysis of antibody–carbohydrate interactions and its application to xenoreactive antibodies. Mol. Immunol. 2009, 47, 233–246. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Lee, J.; Hitzenberger, M.; Rieger, M.; Kern, N.R.; Zacharias, M.; Im, W. CHARMM-GUI supports the Amber force fields. J. Chem. Phys. 2020, 153, 035103. [Google Scholar] [CrossRef]
- Labonte, J.W.; Adolf-Bryfogle, J.; Schief, W.R.; Gray, J.J. Residue-centric modeling and design of saccharide and glycoconjugate structures. J. Comput. Chem. 2017, 38, 276–287. [Google Scholar] [CrossRef]
- Whitmore, E.K.; Vesenka, G.; Sihler, H.; Guvench, O. Efficient Construction of Atomic-Resolution Models of Non-Sulfated Chondroitin Glycosaminoglycan Using Molecular Dynamics Data. Biomolecules 2020, 10, 537. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.; Qi, Y.F.; Kern, N.R.; Lee, H.S.; Jo, S.; Joung, I.; Joo, K.; Lee, J.; Im, W. CHARMM-GUI Glycan Modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology 2019, 29, 320–331. [Google Scholar] [CrossRef]
- Lemmin, T.; Soto, C. Glycosylator: A Python framework for the rapid modeling of glycans. BMC Bioinf. 2019, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Alford, R.F.; Leaver-Fay, A.; Jeliazkov, J.R.; O’Meara, M.J.; DiMaio, F.P.; Park, H.; Shapovalov, M.V.; Renfrew, P.D.; Mulligan, V.K.; Kappel, K.; et al. The Rosetta All-Atom Energy Function for Macromolecular Modeling and Design. J. Chem. Theory Comput. 2017, 13, 3031–3048. [Google Scholar] [CrossRef] [PubMed]
- Roy Burman, S.S.; Nance, M.L.; Jeliazkov, J.R.; Labonte, J.W.; Lubin, J.H.; Biswas, N.; Gray, J.J. Novel sampling strategies and a coarse-grained score function for docking homomers, flexible heteromers, and oligosaccharides using Rosetta in CAPRI rounds 37–45. Proteins Struct. Funct. Bioinf. 2020, 88, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Leman, J.K.; Weitzner, B.D.; Lewis, S.M.; Adolf-Bryfogle, J.; Alam, N.; Alford, R.F.; Aprahamian, M.; Baker, D.; Barlow, K.A.; Barth, P.; et al. Macromolecular modeling and design in Rosetta: Recent methods and frameworks. Nat. Methods 2020, 17, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Arroyuelo, A.; Vila, J.A.; Martin, O.A. Azahar: A PyMOL plugin for construction, visualization and analysis of glycan molecules. J. Comput. Aided Mol. Des. 2016, 30, 619–624. [Google Scholar] [CrossRef]
- Rosen, J.; Miguet, L.; Pérez, S. Shape: Automatic conformation prediction of carbohydrates using a genetic algorithm. J. Cheminf. 2009, 1, 16. [Google Scholar] [CrossRef]
- Frank, M.; Bohne-Lang, A.; Wetter, T.; Von Der Lieth, C.-W. Rapid Generation of a Representative Ensemble of N-Glycan Conformations. In Silico Biol. 2002, 2, 427–439. [Google Scholar]
- Nahmany, A.; Strino, F.; Rosen, J.; Kemp, G.J.L.; Nyholm, P.-G. The use of a genetic algorithm search for molecular mechanics (MM3)-based conformational analysis of oligosaccharides. Carbohydr. Res. 2005, 340, 1059–1064. [Google Scholar] [CrossRef]
- Xia, J.; Daly, R.P.; Chuang, F.-C.; Parker, L.; Jensen, J.H.; Margulis, C.J. Sugar Folding: A Novel Structural Prediction Tool for Oligosaccharides and Polysaccharides 1. J. Chem. Theory Comput. 2007, 3, 1620–1628. [Google Scholar] [CrossRef]
- Xia, J.; Daly, R.P.; Chuang, F.-C.; Parker, L.; Jensen, J.H.; Margulis, C.J. Sugar Folding: A Novel Structural Prediction Tool for Oligosaccharides and Polysaccharides 2. J. Chem. Theory Comput. 2007, 3, 1629–1643. [Google Scholar] [CrossRef]
- Xia, J.; Margulis, C. A tool for the prediction of structures of complex sugars. J. Biomol. NMR 2008, 42, 241–256. [Google Scholar] [CrossRef]
- Xia, J.; Margulis, C.J. Computational Study of the Conformational Structures of Saccharides in Solution Based on J Couplings and the “Fast Sugar Structure Prediction Software”. Biomacromolecules 2009, 10, 3081–3088. [Google Scholar] [CrossRef] [PubMed]
- Bohne-Lang, A.; Von Der Lieth, C.-W. GlyProt: In silico glycosylation of proteins. Nucleic Acids Res. 2005, 33, W214–W219. [Google Scholar] [CrossRef]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkoczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.-W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D: Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Tessier, M.B.; Grant, O.C.; Heimburg-Molinaro, J.; Smith, D.; Jadey, S.; Gulick, A.M.; Glushka, J.; Deutscher, S.L.; Rittenhouse-Olson, K.; Woods, R.J. Computational screening of the human TF-glycome provides a structural definition for the specificity of anti-tumor antibody JAA-F11. PLoS ONE 2013, 8, e54874. [Google Scholar] [CrossRef]
- Grant, O.C.; Smith, H.M.; Firsova, D.; Fadda, E.; Woods, R.J. Presentation, presentation, presentation! Molecular-level insight into linker effects on glycan array screening data. Glycobiology 2014, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.C.; Woods, R.J. Recent advances in employing molecular modelling to determine the specificity of glycan-binding proteins. Curr. Opin. Struct. Biol. 2014, 28, 47–55. [Google Scholar] [CrossRef]
- Grant, O.C.; Xue, X.; Ra, D.; Khatamian, A.; Foley, B.L.; Woods, R.J. Gly-Spec: A webtool for predicting glycan specificity by integrating glycan array screening data and 3D structure. Glycobiology 2016, 26, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.C.; Tessier, M.B.; Meche, L.; Mahal, L.K.; Foley, B.L.; Woods, R.J. Combining 3D structure with glycan array data provides insight into the origin of glycan specificity. Glycobiology 2016, 26, 772–783. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Gao, Y.; Lee, J.; Widmalm, G.; Im, W. Modeling and Simulation of Bacterial Outer Membranes with Lipopolysaccharides and Enterobacterial Common Antigen. J. Phys. Chem. B 2020, 124, 5948–5956. [Google Scholar] [CrossRef]
- Baltoumas, F.A.; Hamodrakas, S.J.; Iconomidou, V.A. The gram-negative outer membrane modeler: Automated building of lipopolysaccharide-rich bacterial outer membranes in four force fields. J. Comput. Chem. 2019, 40, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Krüger, D.M.; Kamerlin, S.C.L. Micelle Maker: An Online Tool for Generating Equilibrated Micelles as Direct Input for Molecular Dynamics Simulations. ACS Omega 2017, 2, 4524–4530. [Google Scholar] [CrossRef]
- Dashti, H.; Westler, W.M.; Wedell, J.R.; Demler, O.V.; Eghbalnia, H.R.; Markley, J.L.; Mora, S. Probabilistic identification of saccharide moieties in biomolecules and their protein complexes. Sci. Data 2020, 7, 210. [Google Scholar] [CrossRef]
- Woods, R. GlyFinder and GlyProbity: New Online Tools for Locating and Curating Carbohydrate Structures in wwPDB. In Time-Proof Perspectives on Glycoscience—Beilstein Glyco-Bioinformatics Symposium, Limburg, Germany, 25–27 June 2019; Beilstein-Institut: Frankfurt, Germany, 2019; pp. 82–83. [Google Scholar]
- Woods, R.J.; Montgomery, D.W.; Young, J.; Grant, O.C.; Wentworth, D.; Foley, B.L. Tools to Find Glycoproteins in the Protein Data Bank and Generate Realistic 3D Structures for Them. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Lütteke, T.; Frank, M.; von der Lieth, C.-W. Data mining the protein data bank: Automatic detection and assignment of carbohydrate structures. Carbohydr. Res. 2004, 339, 1015–1020. [Google Scholar] [CrossRef]
- Jo, S.; Song, K.C.; Desaire, H.; MacKerell, A.D., Jr.; Im, W. Glycan reader: Automated sugar identification and simulation preparation for carbohydrates and glycoproteins. J. Comput. Chem. 2011, 32, 3135–3141. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, J.; Patel, D.S.; Ma, H.; Lee, H.S.; Jo, S.; Im, W. Glycan Reader is improved to recognize most sugar types and chemical modifications in the Protein Data Bank. Bioinformatics 2017, 33, 3051–3057. [Google Scholar] [CrossRef]
- Danne, R.; Poojari, C.; Martinez-Seara, H.; Rissanen, S.; Lolicato, F.; Róg, T.; Vattulainen, I. doGlycans–Tools for Preparing Carbohydrate Structures for Atomistic Simulations of Glycoproteins, Glycolipids, and Carbohydrate Polymers for GROMACS. J. Chem. Inf. Model. 2017, 57, 2401–2406. [Google Scholar] [CrossRef]
- Bohne, A.; Lang, E.; von der Lieth, C.-W. W3-SWEET: Carbohydrate Modeling by Internet. J. Mol. Model. 1998, 4, 33–43. [Google Scholar] [CrossRef]
- Bohne, A.; Lang, E.; von der Lieth, C.W. SWEET—WWW-based rapid 3D construction of oligo- and polysaccharides. Bioinformatics 1999, 15, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, I.Y.; Toukach, P.V. REStLESS: Automated translation of glycan sequences from residue-based notation to SMILES and atomic coordinates. Bioinformatics 2018, 34, 2679–2681. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, S.B.; Cros, S.; Mackie, W.; Pérez, S. A molecular builder for carbohydrates: Application to polysaccharides and complex carbohydrates. Biopolymers 1996, 39, 417–433. [Google Scholar] [CrossRef]
- Engelsen, S.B.; Hansen, P.I.; Pérez, S. POLYS 2.0: An open source software package for building three-dimensional structures of polysaccharides. Biopolymers 2014, 101, 733–743. [Google Scholar] [CrossRef]
- Kuttel, M.; Mao, Y.; Widmalm, G.; Lundborg, M. CarbBuilder: An Adjustable Tool for Building 3D Molecular Structures of Carbohydrates for Molecular Simulation. In Proceedings of the 2011 IEEE Seventh International Conference on eScience, Stockholm, Sweden, 5–8 December 2011; pp. 395–402. [Google Scholar]
- Kuttel, M.M.; Stahle, J.; Widmalm, G. CarbBuilder: Software for building molecular models of complex oligo- and polysaccharide structures. J. Comput. Chem. 2016, 37, 2098–2105. [Google Scholar] [CrossRef]
- Clerc, O.; Mariethoz, J.; Rivet, A.; Lisacek, F.; Pérez, S.; Ricard-Blum, S. A pipeline to translate glycosaminoglycan sequences into 3D models. Application to the exploration of glycosaminoglycan conformational space. Glycobiology 2019, 29, 36–44. [Google Scholar] [CrossRef]
- Singh, A.; Montgomery, D.; Xue, X.; Foley, B.L.; Woods, R.J. GAG Builder: A web-tool for modeling 3D structures of glycosaminoglycans. Glycobiology 2019, 29, 515–518. [Google Scholar] [CrossRef]
- Kerzmann, A.; Neumann, D.; Kohlbacher, O. SLICK—Scoring and Energy Functions for Protein−Carbohydrate Interactions. J. Chem. Inf. Model. 2006, 46, 1635–1642. [Google Scholar] [CrossRef]
- Kerzmann, A.; Fuhrmann, J.; Kohlbacher, O.; Neumann, D. BALLDock/SLICK: A New Method for Protein-Carbohydrate Docking. J. Chem. Inf. Model. 2008, 48, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Mottarella, S.E.; Beglov, D.; Beglova, N.; Nugent, M.A.; Kozakov, D.; Vajda, S. Docking Server for the Identification of Heparin Binding Sites on Proteins. J. Chem. Inf. Model. 2014, 54, 2068–2078. [Google Scholar] [CrossRef]
- Sankaranarayanan, N.V.; Nagarajan, B.; Desai, U.R. So you think computational approaches to understanding glycosaminoglycan–protein interactions are too dry and too rigid? Think again! Curr. Opin. Struct. Biol. 2018, 50, 91–100. [Google Scholar] [CrossRef]
- Griffith, A.R.; Rogers, C.J.; Miller, G.M.; Abrol, R.; Hsieh-Wilson, L.C.; Goddard, W.A. Predicting glycosaminoglycan surface protein interactions and implications for studying axonal growth. Proc. Natl. Acad. Sci. USA 2017, 114, 13697–13702. [Google Scholar] [CrossRef] [PubMed]
- Eric, B.; Jed, B.; Neha, G.; Vito, F. GlycoTorch Vina: Improved Docking of Sulfated Sugars Using QM-derived Scoring Functions. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Frank, M. Conformational Analysis of Oligosaccharides and Polysaccharides Using Molecular Dynamics Simulations. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 359–377. [Google Scholar]
- Makeneni, S.; Foley, B.L.; Woods, R.J. BFMP: A Method for Discretizing and Visualizing Pyranose Conformations. J. Chem. Inf. Model. 2014, 54, 2744–2750. [Google Scholar] [CrossRef]
- Chalmers, G.; Glushka, J.N.; Foley, B.L.; Woods, R.J.; Prestegard, J.H. Direct NOE simulation from long MD trajectories. J. Magn. Reson. 2016, 265, 1–9. [Google Scholar] [CrossRef]
- Lee, H.S.; Jo, S.; Mukherjee, S.; Park, S.-J.; Skolnick, J.; Lee, J.; Im, W. GS-align for glycan structure alignment and similarity measurement. Bioinformatics 2015, 31, 2653–2659. [Google Scholar] [CrossRef][Green Version]
- Lütteke, T.; Frank, M.; von der Lieth, C.-W. Carbohydrate Structure Suite (CSS): Analysis of carbohydrate 3D structures derived from the PDB. Nucleic Acids Res. 2005, 33, D242–D246. [Google Scholar] [CrossRef]
- Rojas-Macias, M.A.; Lütteke, T. Statistical Analysis of Amino Acids in the Vicinity of Carbohydrate Residues Performed by GlyVicinity. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 215–226. [Google Scholar]
- Marchetti, R.; Perez, S.; Arda, A.; Imberty, A.; Jimenez-Barbero, J.; Silipo, A.; Molinaro, A. Rules of Engagement of Protein-Glycoconjugate Interactions: A Molecular View Achievable by using NMR Spectroscopy and Molecular Modeling. ChemistryOpen 2016, 5, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Delbianco, M. Conformational Studies of Oligosaccharides. Chem. Eur. J. 2020, 26, 9814–9825. [Google Scholar] [CrossRef]
- Imberty, A.; Pérez, S. Structure, Conformation, and Dynamics of Bioactive Oligosaccharides: Theoretical Approaches and Experimental Validations. Chem. Rev. 2000, 100, 4567–4588. [Google Scholar] [CrossRef]
- Wormald, M.R.; Petrescu, A.J.; Pao, Y.-L.; Glithero, A.; Elliott, T.; Dwek, R.A. Conformational Studies of Oligosaccharides and Glycopeptides: Complementarity of NMR, X-ray Crystallography, and Molecular Modelling. Chem. Rev. 2002, 102, 371–386. [Google Scholar] [CrossRef]
- Lutteke, T. Analysis and validation of carbohydrate three-dimensional structures. Acta Crystallogr. Sect. D Struct. Biol. 2009, 65, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Blanco Capurro, J.I.; Di Paola, M.; Gamarra, M.D.; Martí, M.A.; Modenutti, C.P. An efficient use of X-ray information, homology modeling, molecular dynamics and knowledge-based docking techniques to predict protein–monosaccharide complexes. Glycobiology 2019, 29, 124–136. [Google Scholar] [CrossRef]
- Coxon, B. Chapter 3 Developments in the Karplus Equation as they Relate to the NMR Coupling Constants of Carbohydrates. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 2009; Volume 62, pp. 17–82. [Google Scholar]
- Widmalm, G. A perspective on the primary and three-dimensional structures of carbohydrates. Carbohydr. Res. 2013, 378, 123–132. [Google Scholar] [CrossRef]
- Slynko, V.; Schubert, M.; Numao, S.; Kowarik, M.; Aebi, M.; Allain, F.H.T. NMR Structure Determination of a Segmentally Labeled Glycoprotein Using In vitro Glycosylation. J. Am. Chem. Soc. 2009, 131, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.A.G.; Queiroz, I.N.L.; Pomin, V.H. NMR structural biology of sulfated glycans. J. Biomol. Struct. Dyn. 2017, 35, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- van Beusekom, B.; Lutteke, T.; Joosten, R.P. Making glycoproteins a little bit sweeter with PDB-REDO. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 463–472. [Google Scholar] [CrossRef]
- Frenz, B.; Ramisch, S.; Borst, A.J.; Walls, A.C.; Adolf-Bryfogle, J.; Schief, W.R.; Veesler, D.; DiMaio, F. Automatically Fixing Errors in Glycoprotein Structures with Rosetta. Structure 2019, 27, 134–139. [Google Scholar] [CrossRef]
- Bagdonas, H.; Ungar, D.; Agirre, J. Leveraging glycomics data in glycoprotein 3D structure validation with Privateer. Beilstein J. Org. Chem. 2020, 16, 2523–2533. [Google Scholar] [CrossRef]
- Casañal, A.; Lohkamp, B.; Emsley, P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci. 2020, 29, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Agirre, J.; Davies, G.; Wilson, K.; Cowtan, K. Carbohydrate anomalies in the PDB. Nat. Chem. Biol. 2015, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, J.; Tran, V.; Sanejouand, Y.-H. Numerous severely twisted N-acetylglucosamine conformations found in the protein databank. Proteins Struct. Funct. Bioinf. 2020, 88, 1376–1383. [Google Scholar] [CrossRef]
- Atanasova, M.; Bagdonas, H.; Agirre, J. Structural glycobiology in the age of electron cryo-microscopy. Curr. Opin. Struct. Biol. 2020, 62, 70–78. [Google Scholar] [CrossRef]
- Agirre, J. Strategies for carbohydrate model building, refinement and validation. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 171–186. [Google Scholar] [CrossRef]
- Pallesen, J.; Murin, C.D.; De Val, N.; Cottrell, C.A.; Hastie, K.M.; Turner, H.L.; Fusco, M.L.; Flyak, A.I.; Zeitlin, L.; Crowe, J.E.; et al. Structures of Ebola virus GP and sGP in complex with therapeutic antibodies. Nat. Microbiol. 2016, 1, 16128. [Google Scholar] [CrossRef]
- Lee, J.H.; Ozorowski, G.; Ward, A.B. Cryo-EM structure of a native, fully glycosylated, cleaved HIV-1 envelope trimer. Science 2016, 351, 1043–1048. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concepts Magn. Reson. Part A 2003, 19A, 1–19. [Google Scholar] [CrossRef]
- Ardá, A.; Coelho, H.; de Toro, B.F.; Galante, S.; Gimeno, A.; Poveda, A.; Sastre, J.; Unione, L.; Valverde, P.; Cañada, F.J.; et al. Recent advances in the application of NMR methods to uncover the conformation and recognition features of glycans. In Carbohydrate Chemistry; The Royal Society of Chemistry: London, UK, 2017; Volume 42, pp. 47–82. [Google Scholar]
- Ardá, A.; Jiménez-Barbero, J. The recognition of glycans by protein receptors. Insights from NMR spectroscopy. Chem. Commun. 2018, 54, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Valverde, P.; Quintana, J.I.; Santos, J.I.; Ardá, A.; Jiménez-Barbero, J. Novel NMR Avenues to Explore the Conformation and Interactions of Glycans. ACS Omega 2019, 4, 13618–13630. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Angles d’Ortoli, T.; Säwén, E.; Jana, M.; Widmalm, G.; MacKerell, A.D. Delineating the conformational flexibility of trisaccharides from NMR spectroscopy experiments and computer simulations. Phys. Chem. Chem. Phys. 2016, 18, 18776–18794. [Google Scholar] [CrossRef]
- Säwén, E.; Hinterholzinger, F.; Landersjö, C.; Widmalm, G. Conformational flexibility of the pentasaccharide LNF-2 deduced from NMR spectroscopy and molecular dynamics simulations. Org. Biomol. Chem. 2012, 10, 4577–4585. [Google Scholar] [CrossRef]
- Turupcu, A.; Blaukopf, M.; Kosma, P.; Oostenbrink, C. Molecular Conformations of Di-, Tri-, and Tetra-α-(2→8)-Linked Sialic Acid from NMR Spectroscopy and MD Simulations. Int. J. Mol. Sci. 2020, 21, 30. [Google Scholar] [CrossRef]
- Frank, M.; Collins, P.M.; Peak, I.R.; Grice, I.D.; Wilson, J.C. An unusual carbohydrate conformation is evident in Moraxella catarrhalis oligosaccharides. Molecules 2015, 20, 14234–14253. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The single global macromolecular structure archive. In Protein Crystallography; Wlodawer, A., Dauter, Z., Jaskolski, M., Eds.; Humana Press: New York, NY, USA, 2017; pp. 627–641. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- De Meirelles, J.L.; Nepomuceno, F.C.; Peña-García, J.; Schmidt, R.R.; Pérez-Sánchez, H.; Verli, H. Current Status of Carbohydrates Information in the Protein Data Bank. J. Chem. Inf. Model. 2020, 60, 684–699. [Google Scholar] [CrossRef]
- Lütteke, T.; von der Lieth, C.W. Data Mining the PDB for Glyco-Related Data. In Glycomics: Methods and Protocols; Packer, N.H., Karlsson, N.G., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 293–310. [Google Scholar]
- Agirre, J.; Davies, G.J.; Wilson, K.S.; Cowtan, K.D. Carbohydrate structure: The rocky road to automation. Curr. Opin. Struct. Biol. 2017, 44, 39–47. [Google Scholar] [CrossRef]
- Crispin, M.; Stuart, D.I.; Jones, E.Y. Building meaningful models of glycoproteins. Nat. Struct. Mol. Biol. 2007, 14, 354. [Google Scholar] [CrossRef]
- Joosten, R.P.; Lütteke, T. Carbohydrate 3D structure validation. Curr. Opin. Struct. Biol. 2017, 44, 9–17. [Google Scholar] [CrossRef]
- Speciale, G.; Thompson, A.J.; Davies, G.J.; Williams, S.J. Dissecting conformational contributions to glycosidase catalysis and inhibition. Curr. Opin. Struct. Biol. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Fushinobu, S. Conformations of the type-1 lacto-N-biose I unit in protein complex structures. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Zardecki, C.; Shao, C.; Feng, Z.; Westbrook, J.D.; Persikova, I.; Burley, S.K.; Young, J.Y. Collaborating with Glycoscience Community To Improve Data Representation of Carbohydrates in the Protein Data Bank. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Van Beusekom, B.; Wezel, N.; Hekkelman, M.L.; Perrakis, A.; Emsley, P.; Joosten, R.P. Building and rebuilding N-glycans in protein structure models. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 416–425. [Google Scholar] [CrossRef]
- Lütteke, T.; von der Lieth, C.W. pdb-care (PDB carbohydrate residue check): A program to support annotation of complex carbohydrate structures in PDB files. BMC Bioinf. 2004, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Brunger, A.T.; Adams, P.D.; Clore, G.M.; DeLano, W.L.; Gros, P.; Grosse-Kunstleve, R.W.; Jiang, J.-S.; Kuszewski, J.; Nilges, M.; Pannu, N.S.; et al. Crystallography & NMR System: A New Software Suite for Macromolecular Structure Determination. Acta Crystallogr. Sect. D Struct. Biol. 1998, 54, 905–921. [Google Scholar]
- Brunger, A.T. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2007, 2, 2728–2733. [Google Scholar] [CrossRef]
- Emsley, P.; Brunger, A.T.; Lütteke, T. Tools to Assist Determination and Validation of Carbohydrate 3D Structure Data. In Glycoinformatics; Lütteke, T., Frank, M., Eds.; Humana Press: New York, NY, USA, 2015; pp. 229–240. [Google Scholar]
- Feng, Y. Compatible topologies and parameters for NMR structure determination of carbohydrates by simulated annealing. PLoS ONE 2017, 12, e0189700. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Crispin, M. Structural analysis of glycoproteins: Building N-linked glycans with Coot. Acta Crystallogr. Sect. D Struct. Biol. 2018, 74, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Agirre, J.; Iglesias-Fernandez, J.; Rovira, C.; Davies, G.J.; Wilson, K.S.; Cowtan, K.D. Privateer: Software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 2015, 22, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Vařeková, R.S.; Jaiswal, D.; Sehnal, D.; Ionescu, C.-M.; Geidl, S.; Pravda, L.; Horský, V.; Wimmerová, M.; Koča, J. MotiveValidator: Interactive web-based validation of ligand and residue structure in biomolecular complexes. Nucleic Acids Res. 2014, 42, W227–W233. [Google Scholar] [CrossRef]
- Sehnal, D.; Svobodová Vařeková, R.; Pravda, L.; Ionescu, C.-M.; Geidl, S.; Horský, V.; Jaiswal, D.; Wimmerová, M.; Koča, J. ValidatorDB: Database of up-to-date validation results for ligands and non-standard residues from the Protein Data Bank. Nucleic Acids Res. 2015, 43, D369–D375. [Google Scholar] [CrossRef]
- Aoki-Kinoshita, K.F. A Practical Guide to Using Glycomics Databases, 1st ed.; Springer: Tokyo, Japan, 2017; 370p. [Google Scholar]
- Tsuchiya, S.; Aoki, N.P.; Shinmachi, D.; Matsubara, M.; Yamada, I.; Aoki-Kinoshita, K.F.; Narimatsu, H. Implementation of GlycanBuilder to draw a wide variety of ambiguous glycans. Carbohydr. Res. 2017, 445, 104–116. [Google Scholar] [CrossRef]
- Mehta, A.Y.; Cummings, R.D. GlycoGlyph: A glycan visualizing, drawing and naming application. Bioinformatics 2020, 36, 3613–3614. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O’Boyle, N.; Packer, N.; Stanley, P.; Toukach, P.; et al. Updates to the Symbol Nomenclature For Glycans (SNFG) Guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef]
- Lal, K.; Bermeo, R.; Perez, S. Computational tools for drawing, building and displaying carbohydrates: A visual guide. Beilstein J. Org. Chem. 2020, 16, 2448–2468. [Google Scholar] [CrossRef]
- Damerell, D.; Ceroni, A.; Maass, K.; Ranzinger, R.; Dell, A.; Haslam, S.M. The GlycanBuilder and GlycoWorkbench glycoinformatics tools: Updates and new developments. Biol. Chem. 2012, 393, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Akune, Y.; Hosoda, M.; Kaiya, S.; Shinmachi, D.; Aoki-Kinoshita, K.F. The RINGS Resource for Glycome Informatics Analysis and Data Mining on the Web. OMICS 2010, 14, 475–486. [Google Scholar] [CrossRef]
- Alocci, D.; Suchánková, P.; Costa, R.; Hory, N.; Mariethoz, J.; Svobodová Vařeková, R.; Toukach, P.; Lisacek, F. SugarSketcher: Quick and intuitive online glycan drawing. Molecules 2018, 23, 3206. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, Y.; Neelamegham, S. DrawGlycan-SNFG: A robust tool to render glycans and glycopeptides with fragmentation information. Glycobiology 2017, 27, 200–205. [Google Scholar] [CrossRef]
- Cheng, K.; Pawlowski, G.; Yu, X.; Zhou, Y.; Neelamegham, S. DrawGlycan-SNFG and gpAnnotate: Rendering glycans and annotating glycopeptide mass spectra. Bioinformatics 2020, 36, 1942–1943. [Google Scholar] [CrossRef] [PubMed]
- Hypercube, Inc. HyperChem. Available online: http://www.hyper.com/?tabid=360 (accessed on 31 July 2020).
- Schrödinger, Inc. The PyMOL Molecular Graphics System. Available online: https://pymol.org/2/ (accessed on 31 July 2020).
- Dalby, A.; Nourse, J.G.; Hounshell, W.D.; Gushurst, A.K.I.; Grier, D.L.; Leland, B.A.; Laufer, J. Description of several chemical structure file formats used by computer programs developed at Molecular Design Limited. J. Chem. Inf. Comput. Sci. 1992, 32, 244–255. [Google Scholar] [CrossRef]
- Callaway, J.; Cummings, M.; Deroski, B.; Esposito, P.; Forman, A.; Langdon, P.; Libeson, M.; McCarthy, J.; Sikora, J.; Xue, D.; et al. Protein Data Bank Contents Guide: Atomic Coordinate Entry Format Description. Brookhaven Natl. Lab. 1996. Available online: https://cdn.rcsb.org/wwpdb/docs/documentation/file-format/PDB_format_Dec_1996.pdf (accessed on 20 December 1996).
- Bohne, A. PDB2MultiGIF: A Web Tool to Create Animated Images of Molecules. J. Mol. Model. 1998, 4, 344–346. [Google Scholar] [CrossRef]
- Sayle, R.A.; Milner-White, E.J. RASMOL: Biomolecular graphics for all. Trends Biochem. Sci. 1995, 20, 374–376. [Google Scholar] [CrossRef]
- Willighagen, E.; Howard, M. Fast and Scriptable Molecular Graphics in Web Browsers without Java3D. Nat. Prec. 2007. [Google Scholar] [CrossRef]
- Rose, A.S.; Hildebrand, P.W. NGL Viewer: A web application for molecular visualization. Nucleic Acids Res. 2015, 43, W576–W579. [Google Scholar] [CrossRef]
- Rose, A.S.; Bradley, A.R.; Valasatava, Y.; Duarte, J.M.; Prlić, A.; Rose, P.W. NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics 2018, 34, 3755–3758. [Google Scholar] [CrossRef]
- Sehnal, D.; Deshpande, M.; Vařeková, R.S.; Mir, S.; Berka, K.; Midlik, A.; Pravda, L.; Velankar, S.; Koča, J. LiteMol suite: Interactive web-based visualization of large-scale macromolecular structure data. Nat. Methods 2017, 14, 1121–1122. [Google Scholar] [CrossRef]
- Sehnal, D.; Rose, A.; Koca, J.; Burley, S.; Velankar, S. Mol: Towards a Common Library and Tools for Web Molecular Graphics. In Workshop on Molecular Graphics and Visual Analysis of Molecular Data, Brno, Czech Republic, 4 June 2018; Byska, J., Krone, M., Sommer, B., Eds.; Eurographics Association: Geneva, Switzerland, 2018; pp. 29–33. [Google Scholar]
- Consortium, T.U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Kuttel, M.; Gain, J.; Burger, A.; Eborn, I. Techniques for visualization of carbohydrate molecules. J. Mol. Graph. Modell. 2006, 25, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.; Kuttel, M.M.; Stone, J.E.; Gain, J.E. Visualisation of cyclic and multi-branched molecules with VMD. J. Mol. Graph. Modell. 2009, 28, 131–139. [Google Scholar] [CrossRef]
- Eborn, I.; Burger, A.; Kuttel, M.; Gain, J. Carbohydra: Rendering Carbohydrate Cartoons; University of Cape Town: Cape Town, South Africa, 2004. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Perez, S.; Tubiana, T.; Imberty, A.; Baaden, M. Three-dimensional representations of complex carbohydrates and polysaccharides—SweetUnityMol: A video game-based computer graphic software. Glycobiology 2015, 25, 483–491. [Google Scholar] [CrossRef]
- Besançon, C.; Guillot, A.; Blaise, S.; Dauchez, M.; Belloy, N.; Prévoteau-Jonquet, J.; Baud, S. New visualization of dynamical flexibility of N-Glycans: Umbrella Visualization in UnityMol. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; pp. 291–298. [Google Scholar]
- Besançon, C.; Guillot, A.; Blaise, S.; Dauchez, M.; Belloy, N.; Prévoteau-Jonquet, J.; Baud, S. Umbrella Visualization: A method of analysis dedicated to glycan flexibility with UnityMol. Methods 2020, 173, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, D.; Grant, O.C. Rapidly Display Glycan Symbols in 3D Structures: 3D-SNFG in LiteMol. J. Proteome Res. 2019, 18, 770–774. [Google Scholar] [CrossRef]
- Thieker, D.F.; Hadden, J.A.; Schulten, K.; Woods, R.J. 3D implementation of the symbol nomenclature for graphical representation of glycans. Glycobiology 2016, 26, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E.M. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. Sect. D Struct. Biol. 2011, 67, 386–394. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, S.; Agirre, J. Glycoblocks: A schematic three-dimensional representation for glycans and their interactions. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 187–194. [Google Scholar] [CrossRef]
- Pendrill, R.; Jonsson, K.H.M.; Widmalm, G. Glycan synthesis, structure, and dynamics: A selection. Pure Appl. Chem. 2013, 85, 1759. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Marth, J.D.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. Symbol nomenclature for glycan representation. Proteomics 2009, 9, 5398–5399. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Aoki-Kinoshita, K.F. Development of Carbohydrate Nomenclature and Representation. In A Practical Guide to Using Glycomics Databases; Aoki-Kinoshita, K.F., Ed.; Springer: Tokyo, Japan, 2017; pp. 7–25. [Google Scholar]


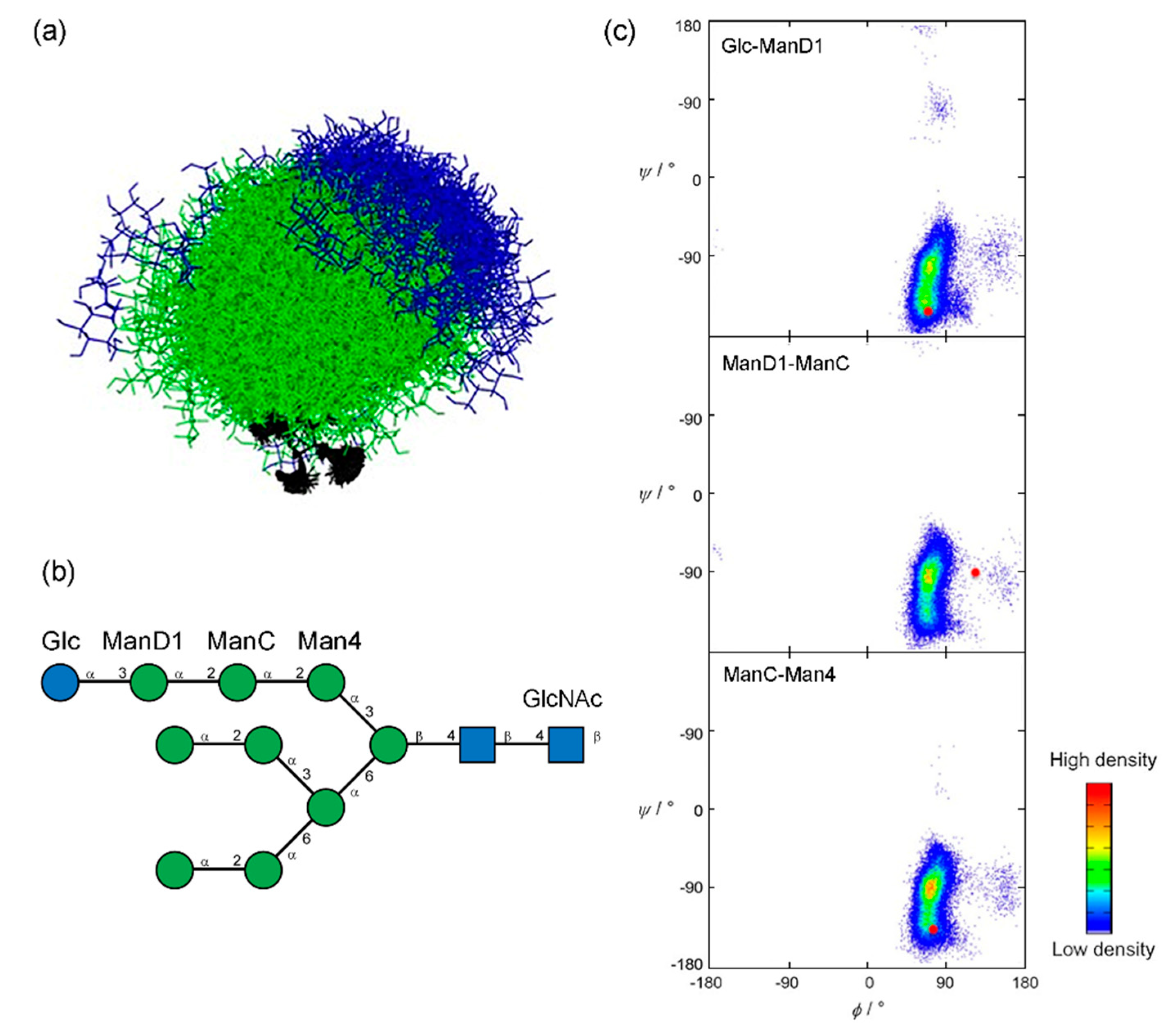
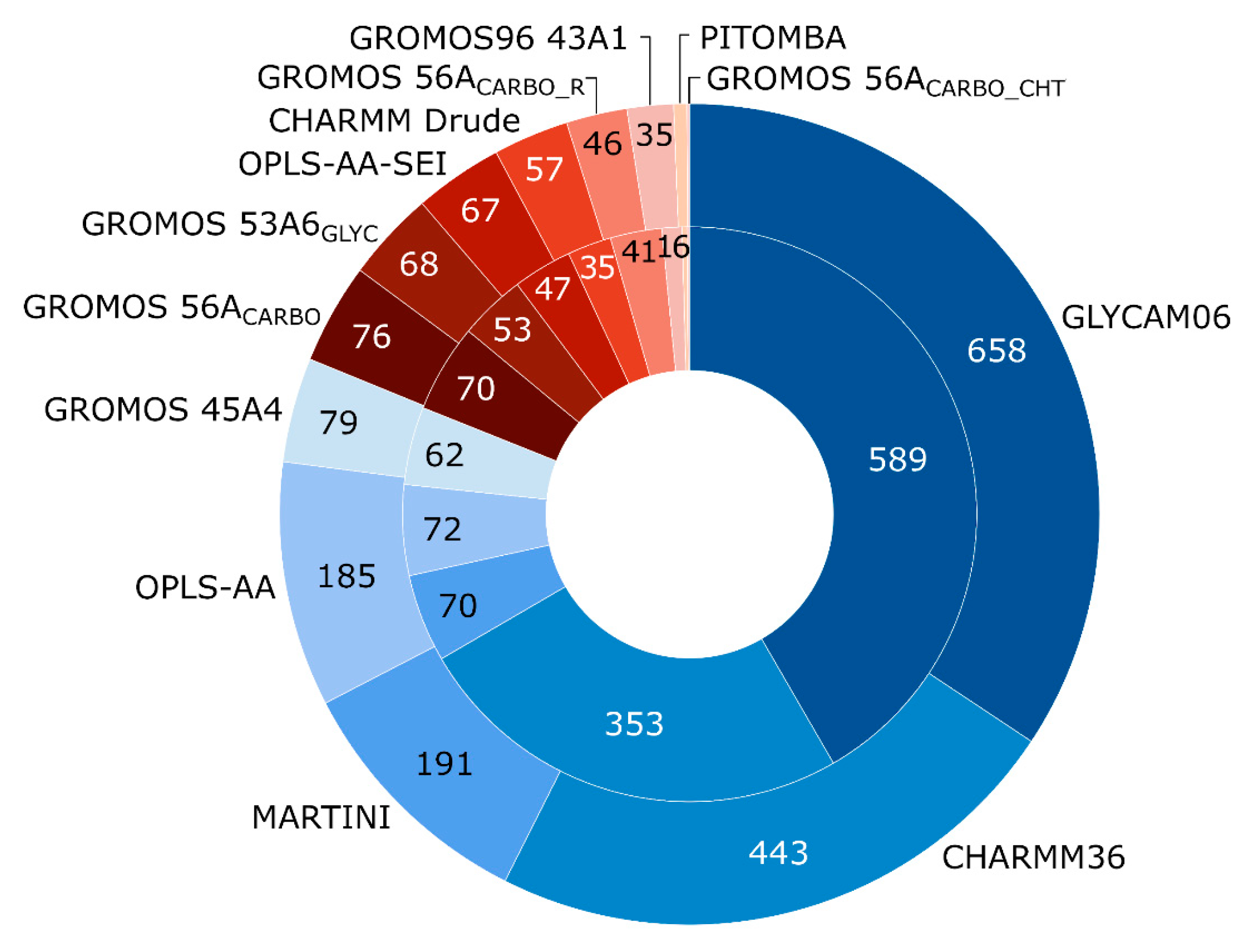



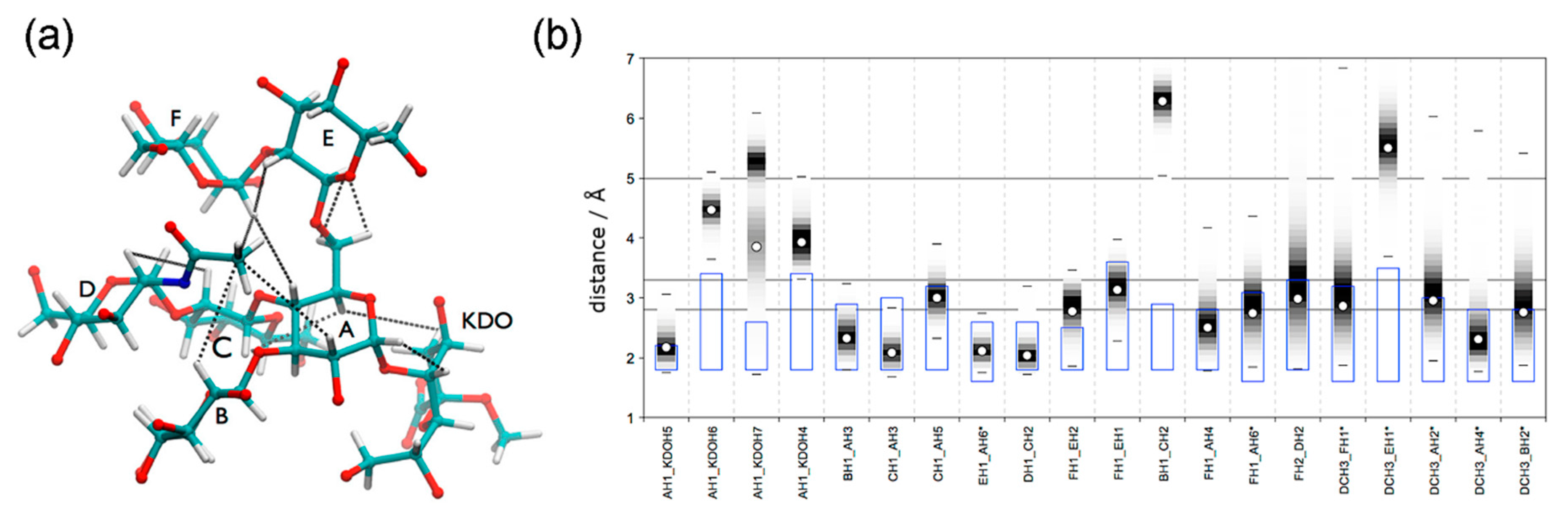




| Database | Years a | Description b | Data Coverage | Carbohydrate 3D Structures | References |
|---|---|---|---|---|---|
| Structure-centric | |||||
Carbohydrate Structure Database (CSDB) | 2005– present |
|
|
| [28,42,43,44] (http://csdb.glycoscience.ru/database) |
Glycosciences.DE | 1997– present |
|
|
| [45,46,47] (http://www.glycosciences.de/) |
Glyco3D | 2015– present |
|
|
| [48,49] (http://glyco3d.cermav.cnrs.fr/home.php) |
PolySac3DB | 2012– present |
|
|
| [50] (http://glyco3d.cermav.cnrs.fr/home.php) |
EK3D | 2016– present |
|
|
| [51] (www.iith.ac.in/EK3D/) |
3DSDSCAR | 2010– present |
|
|
| [52,53] (http://aliffishbay.com/Domains/3dsdscar.org/3dsdscar.html) |
MatrixDB | 2011– present |
|
|
| [54,55,56] (http://matrixdb.univ-lyon1.fr/) |
EPS-DB | 2017– present |
|
|
| [57] (http://www.epsdatabase.com) |
GlyMDB | 2020– present |
|
|
| [58] (http://www.glycanstructure.org/glymdb/) |
CFG Glycan Structures Database | 2006– present |
|
|
| [59,60] (http://www.functionalglycomics.org/glycomics/molecule/jsp/carbohydrate/carbMoleculeHome.jsp) (http://www.functionalglycomics.org/glycomics/publicdata/selectedScreens.jsp) |
| Glycoproteomic | |||||
GlycoNAVI Tcarp | 2020– present |
|
|
| [61] (https://glyconavi.org/TCarp/) |
GlyCosmos | 2017– present |
| 109854 glycans glycolipids * 50113 glycoproteins 1238 lectins 20580 glycogenes |
| [13,62,63] (https://glycosmos.org/) |
SugarBind | 2010– present |
|
|
| [64] (https://sugarbind.expasy.org/) |
GlyConnect | 2019– present |
|
|
| [65] (https://glyconnect.expasy.org/) |
ProGlycProt | 2012– present |
|
|
| [66,67] (http://www.proglycprot.org/) |
ProCarbDB | 2020– present |
|
|
| [68] (http://www.procarbdb.science/procarb/) |
Procaff | 2019– present |
|
|
| [69] (https://web.iitm.ac.in/bioinfo2/procaff/index.html) |
GBSDB | 2020– present |
|
|
| [70] (http://www.glycanstructure.org/gbs-db/pdb/) |
PROCARB | 2010– present |
|
|
| [71] (http://www.procarb.org/procarbdb/) |
| UniLectin3D | 2019– present |
|
|
| [72,73] (https://www.unilectin.eu/unilectin3D/) |
Lectin Frontier | 2015– present |
|
|
| [74] (https://acgg.asia/lfdb2/) |
LectinDB | 2006– present |
|
|
| [75] (http://proline.physics.iisc.ernet.in/lectindb/) |
GlycoEpitope | 2006– present |
|
|
| [76,77,78] (https://www.glycoepitope.jp/epitopes) |
| GlycoCD | 2012– present |
|
|
| [79] (http://www.glycosciences.de/glyco-cd/) |
| SACS | 2002– present |
|
|
| [80] (http://www.bioinf.org.uk/abs/sacs/xslt.cgi?src=antibodies.xml&xsl=summary.xsl) |
SabDab | 2014– present |
|
|
| [81] (http://opig.stats.ox.ac.uk/webapps/newsabdab/sabdab/) |
CAZy | 1998– present |
|
|
| [82,83,84] (http://www.cazy.org/) |
dbPTM | 2006– present |
|
|
| [85,86,87] (http://dbptm.mbc.nctu.edu.tw/) |
SWISS-MODEL Repository | 2004– present |
|
|
| [88,89,90] (https://swissmodel.expasy.org/repository) |
| Specialized | |||||
GlycoMaps DB | 2004– present |
|
|
| [91] (http://www.glycosciences.de/modeling/glycomapsdb/) |
GFDB | 2013– present |
|
|
| [92] (http://www.glycanstructure.org/fragment-db) |
GLYCAM-Web | 2013– present |
|
|
| (http://glycam.org/Pre-builtLibraries.jsp) |
| Tool | Description | Type a | Reference |
|---|---|---|---|
| Structure modeling | |||
| CHARMM-GUI Glycan Modeler | In silico N-/O-glycosylation of proteins;modeling of carbohydrate-only systems | Web-service | [230] (http://www.charmm-gui.org/?doc=input/glycan) |
| CHARMM-GUI Glycolipid/LPS Modeler | Glycolipid and lipoglycan structure modeling | Web-service | [230] (http://charmm-gui.org/?doc=input/glycolipid) (http://charmm-gui.org/?doc=input/lps) |
| Glycosylator | Rapid modeling of glycans and glycoproteins (including glycosylation) based on CHARMM force field | Python framework | [231] (https://github.com/tlemmin/glycosylator) |
| RosettaCarbohydrate | Modeling a wide variety of saccharide and glycoconjugate structures (including loop modeling, glyco-ligand docking and glycosylation) | Python framework | [228,232,233,234] (https://www.rosettacommons.org/docs/latest/application_documentation/carbohydrates/WorkingWithGlycans) |
| Azahar | Monte Carlo conformational search and trajectory analysis of glycans | Python framework; PyMol plugin | [235] (https://github.com/BIOS-IMASL/Azahar) |
| Shape | Carbohydrate-dedicated fully automated MM3-based conformation simulation | Standalone software | [236] (https://sourceforge.net/projects/shapega/) |
| Glydict | MM3-based N-glycan structure prediction based on MD simulations | Web-service | [237] (http://www.glycosciences.de/modeling/glydict/) |
| GLYGAL | MM3-based conformational analysis of oligosaccharides | Standalone software | [238] |
| Fast Sugar Structure Prediction Software (FSPS) | Automatic structure prediction tool for oligo- and polysaccharides in solution | Standalone software | [239,240,241,242] |
| Glycosylation modeling and grafting | |||
| GLYCAM-Web Glycoprotein Builder | Attaching a glycan (user input) to a protein (PDB file) | Web-service | (http://glycam.org/gp) |
| GlyProt | In silico generation of N-glycosylated 3D models of proteins | Web-service | [243] (http://www.glycosciences.de/modeling/glyprot/php/main.php) |
| Phenix CarboLoad | Loading a carbohydrate structure into protein model and PDB file generation | Python framework | [244] (https://www.phenix-online.org/documentation/reference/carbo_load.html) |
| GLYCAM-Web GlySpec (Grafting) | Prediction of glycan specificity by integrating glycan array screening data and 3D structure | Web-service | [245,246,247,248,249] (http://glycam.org/djdev/grafting/) |
| Biological membranes and micelles | |||
| CHARMM-GUI Membrane Builder | Building complex glycolipid-/LPS-/LOS-containing biological membrane systems | Web-service | [230,250,251,252,253] (http://www.charmm-gui.org/?doc=input/membrane.bilayer) |
| GNOMM (gram-negative outer membrane modeler) | Automated building of lipopolysaccharide-rich bacterial outer membranes (3D model preparation for MD simulations in GROMACS) | Standalone software | [254] (http://thalis.biol.uoa.gr/GNOMM/) |
| Micelle Maker | Micelle building based on broad range of starting lipids and glycolipids (3D model preparation using AMBER software package and GLYCAM library) | Web-service | [255] (http://micelle.icm.uu.se/) |
| Carbohydrate moiety identification | |||
| Cheminformatics Tool for Probabilistic Identification of Carbohydrates (CTPIC) | Identification of small saccharides and their derivatives (input in SDF or MOL format) | Web-service | [256] (http://ctpic.nmrfam.wisc.edu/) (https://github.com/htdashti/ctpic) |
| Sails | Automated identification of linked sugars | Python framework | (https://github.com/glycojones/sails) |
| GlyFinder | Locating relevant carbohydrate-containing structures in Protein Data Bank | Part of web-service pipeline | [257,258] (https://dev.glycam.org/portal/gf_home/) |
| pdb2linucs | Extraction of carbohydrate data from a PDB file | Web-tool | [259] (http://www.glycosciences.de/tools/pdb2linucs/) |
| GLYCAM-Web PDB-preprocessor | Processing of PDB files with (glyco-)proteins for AMBER-style output | Web-service | (http://glycam.org/pdb) |
| Sugar identification program | Identifying the residue names of carbohydrates in a PDB file | Standalone software | (http://glycam.org/docs/othertoolsservice/downloads/downloads-software/) |
| Glycan Reader | Automated sugar identification and simulation preparation for carbohydrates and glycoproteins in PDB files | Web-service | [260,261] (http://glycanstructure.org/glycanreader/) (http://www.charmm-gui.org/?doc=input/glycan) |
| Structure building and model preparation | |||
| doGlycans | Preparing carbohydrate structures (including polysaccharides, glycolipids and glycoproteins) for GROMACS atomistic simulations | Python framework | [262] (https://bitbucket.org/biophys-uh/doglycans/src/master/) |
| GLYCAM-Web Carbohydrate builder | 3D structure prediction of carbohydrates and related macromolecules using GLYCAM06 force field and MD in AMBER (successor of GLYCAM Biomolecule Builder (http://glycam.org/old/biombuilder/biomb_index.jsp)) | Web-service | [177] (http://glycam.org/) |
| SWEET-II | Rapid 3D model construction of oligo- and polysaccharides with MM3 optimization | Web-service | [263,264] (http://www.glycosciences.de/modeling/sweet2/) |
| REStLESS API | 3D structure generation of carbohydrates and derivatives from CSDB Linear notation with MMFF94 optimization (including aglycone moiety) | Web-service | [265] (http://csdb.glycoscience.ru/database/core/translate.html#from) |
| Polysaccharide builders | |||
| POLYS | 3D structure generation of poly- and complex oligosaccharides from MM2-precalculated glycosidic linkage torsions and energy minimization | Web-service | [266,267] (https://bitbucket.org/polys/polys/src/default/) (http://glycan-builder.cermav.cnrs.fr/) |
| CarbBuilder | Building of 3D structures of polysaccharides in CHARMM force field from pre-calculated glycosidic linkage torsions | Standalone software | [268,269] (https://people.cs.uct.ac.za/~mkuttel/Downloads.html) |
| GAG-builder | Translating of GAG sequences into 3D models based on POLYS glycan builder | Web-service | [270] (http://glycan-builder.cermav.cnrs.fr/gag/) (http://matrixdb.univ-lyon1.fr/) |
| GLYCAM-Web GAG Builder | Modeling of GAG 3D structure in GLYCAM06 force field using AMBER MD package | Web-service | [271] (http://glycam.org/gag) |
| Docking | |||
| BALLDock/SLICK | Protein-carbohydrate complex docking software | Standalone software, a module in docking software | [272,273] (https://ball-project.org/download/) |
| HADDOCK | Modeling of biomolecular complexes with support of glycosylated proteins | Web-service | [274] (https://wenmr.science.uu.nl/haddock2.4/library) |
| Vina-Carb | CHI-energy functions implemented in AutoDock Vina software | Standalone software | [156,157] (http://glycam.org/docs/othertoolsservice/download-docs/publication-materials/vina-carb/) |
| GLYCAM-Web Antibody docking | Docking of an antibody (from a PDB file) to a glycan antigen (from a library or user input) | Web- service | (http://glycam.org/ad) |
| Cluspro | Sulfated GAG docking (as one of options) | Web-service | [275,276] (https://cluspro.bu.edu/login.php) |
| GAGDock (DarwinDock) | Modification of DarwinDock method for sulfated glycosaminoglycans | Algorithm | [277] |
| GlycoTorch Vina | Docking of sulfated glycosaminoglycans based on Vina-Carb | Standalone software | [278] (http://ericboittier.pythonanywhere.com/) |
| Structural data analysis | |||
| Conformational Analysis Tool (CAT) | Analysis of carbohydrate molecular trajectory data derived from MD simulations | Standalone software | [279] (http://www.md-simulations.de/CAT/) |
| Best-fit, Four-Membered Plane (BFMP) | Analysis of conformational data from crystal structures and MD simulations of carbohydrates | Standalone software | [280] (http://glycam.org/docs/othertoolsservice/download-docs/publication-materials/bfmp/) |
| Distance Mapping | Estimation of nuclear Overhauser effects in disaccharides | Web-tool | (http://www.glycosciences.de/modeling/distmap/) |
| MD2NOE | Calculation of Nuclear Overhauser effect build-up curves from long MD trajectories | Standalone software | [281] (http://glycam.org/docs/othertoolsservice/download-docs/publication-materials/md2noe/) |
| GS-align | Glycan structure alignment and similarity calculation | Standalone software | [282] (http://www.glycanstructure.org/gsalign) |
| GlyTorsion | Analysis of torsion angles in carbohydrates from Protein Data Bank | Web-tool | [283] (http://www.glycosciences.de/tools/glytorsion/) |
| GlyVicinity | Analysis of amino acids in the vicinity of carbohydrate residues derived from Protein Data Bank | Web-tool | [284] (http://www.glycosciences.de/tools/glyvicinity/) |
| Tool | Description | Type a | Reference |
|---|---|---|---|
| CNS | Macromolecular structure determination and refinement (including carbohydrates and glycoproteins) based on X-ray and NMR data | Standalone software | [327,328,329,330] (http://cns-online.org/v1.3/) |
| pdb-care | Identification and assigning carbohydrate structures using atom types and coordinates from PDB files | Web-tool | [326] (http://www.glycosciences.de/tools/pdb-care/) |
| CARP | Glycoprotein 3D quality evaluation based on the analysis of glycosidic torsion angles from PDB | Web-tool | [283] (http://www.glycosciences.de/tools/carp/) |
| GlyProbity | Accuracy and internal consistency check of carbohydrate 3D structures | Part of web-service pipeline | [257] (https://dev.glycam.org/portal/gf_home/) |
| PDB2Glycan | 3D structure analysis and validation of glycoprotein PDB entries | Part of web-service pipeline | [61] (https://glyconavi.org/TCarp/) (https://gitlab.com/glyconavi/pdb2glycan) |
| PDB-REDO | Glycoprotein structure model improvement and validation | Web-service; standalone software | [295,325] (https://pdb-redo.eu/) |
| Coot | Refinement and validation of glycoprotein 3D structure from cryoEM and X-ray crystallography data | Standalone software | [298,331] (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/) |
| Rosetta Carbohydrate | Refinement of glycoprotein 3D structure from cryoEM and X-ray crystallography data, based on correction of conformational and configurational errors in carbohydrates | Python framework | [296] (https://www.rosettacommons.org/docs/latest/application_documentation/carbohydrates/WorkingWithGlycans) |
| Privateer | Automated validation of carbohydrate conformation data based on 3D structure analysis | Standalone software | [297,332] (https://smb.slac.stanford.edu/facilities/software/ccp4/html/privateer.html) |
| Phenix | Determination, refinement and validation of macromolecular structure (including carbohydrates and glycoproteins) from cryoEM, X-ray diffraction and neutron diffraction crystallography data | Standalone software | [244] (http://phenix-online.org/) |
| Motive Validator | Automatic custom residue validation in biomolecules, including carbohydrates | Web-service | [333] (https://webchem.ncbr.muni.cz/Platform/MotiveValidator) |
| ValidatorDB | Pre-computed validation results of ligands and non-standard residues in PDB (including carbohydrates) | Web-service | [334] (http://webchem.ncbr.muni.cz/Platform/ValidatorDb) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherbinina, S.I.; Toukach, P.V. Three-Dimensional Structures of Carbohydrates and Where to Find Them. Int. J. Mol. Sci. 2020, 21, 7702. https://doi.org/10.3390/ijms21207702
Scherbinina SI, Toukach PV. Three-Dimensional Structures of Carbohydrates and Where to Find Them. International Journal of Molecular Sciences. 2020; 21(20):7702. https://doi.org/10.3390/ijms21207702
Chicago/Turabian StyleScherbinina, Sofya I., and Philip V. Toukach. 2020. "Three-Dimensional Structures of Carbohydrates and Where to Find Them" International Journal of Molecular Sciences 21, no. 20: 7702. https://doi.org/10.3390/ijms21207702
APA StyleScherbinina, S. I., & Toukach, P. V. (2020). Three-Dimensional Structures of Carbohydrates and Where to Find Them. International Journal of Molecular Sciences, 21(20), 7702. https://doi.org/10.3390/ijms21207702