Effect of Silymarin Supplementation on Physical Performance, Muscle and Myocardium Histological Changes, Bodyweight, and Food Consumption in Rats Subjected to Regular Exercise Training
Abstract
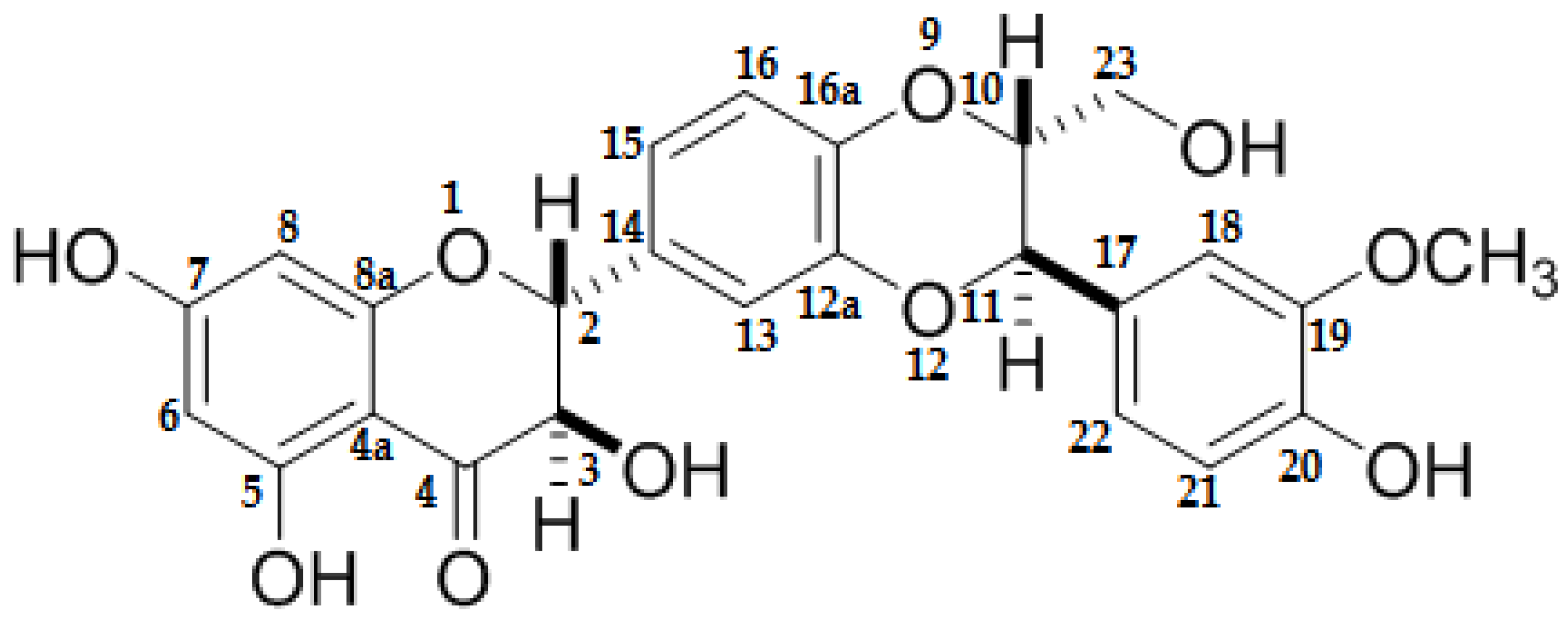
:1. Introduction
2. Results
2.1. Bodyweight
2.2. Food Consumption, Energy, and Nutrient Intake
2.3. Exercise-Endurance Capacity Test
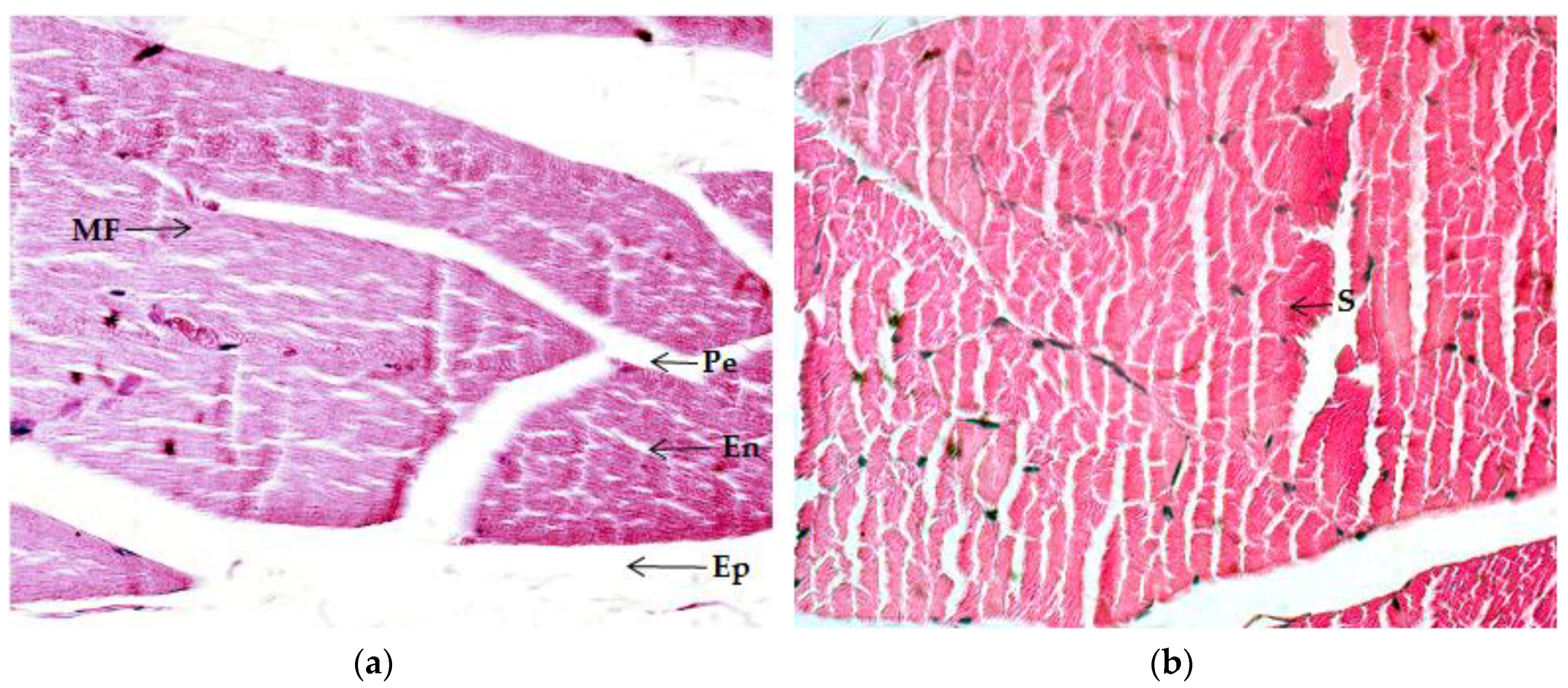
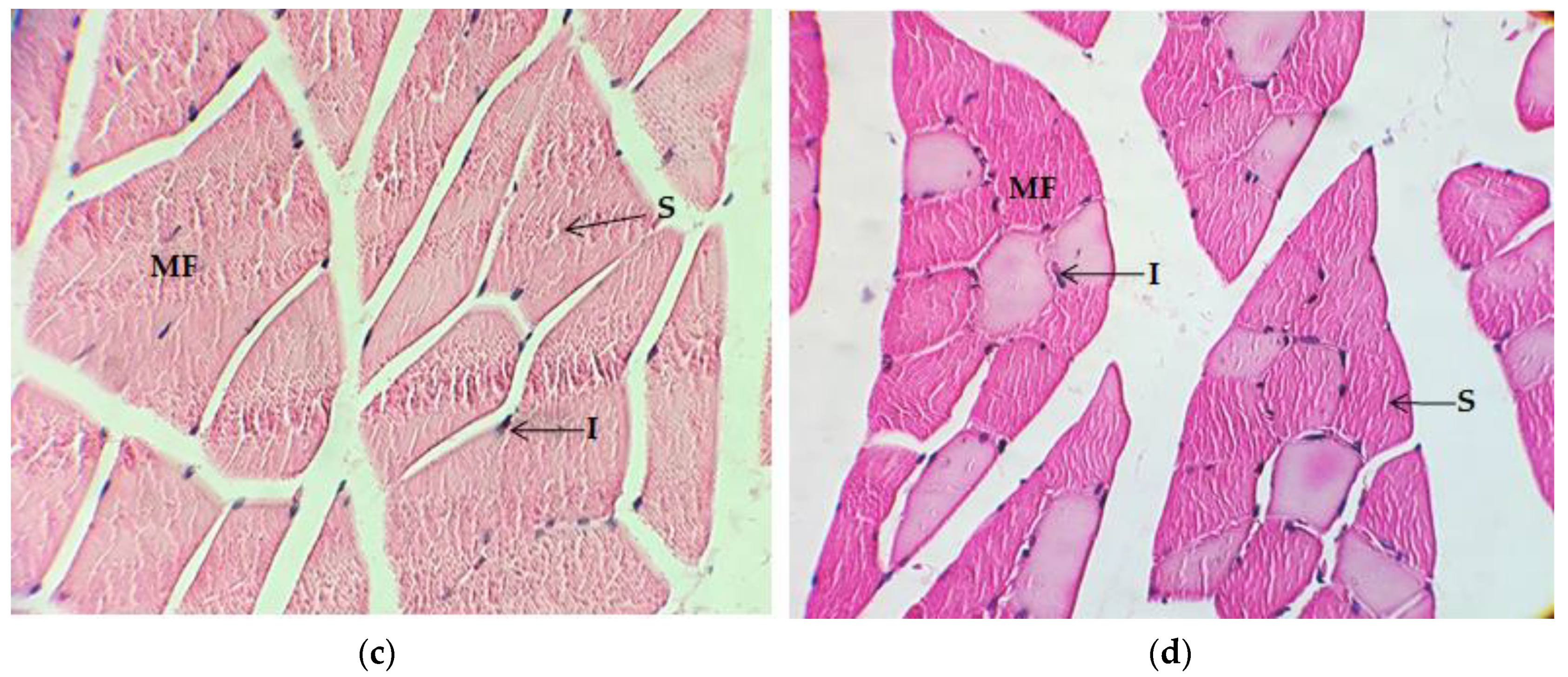
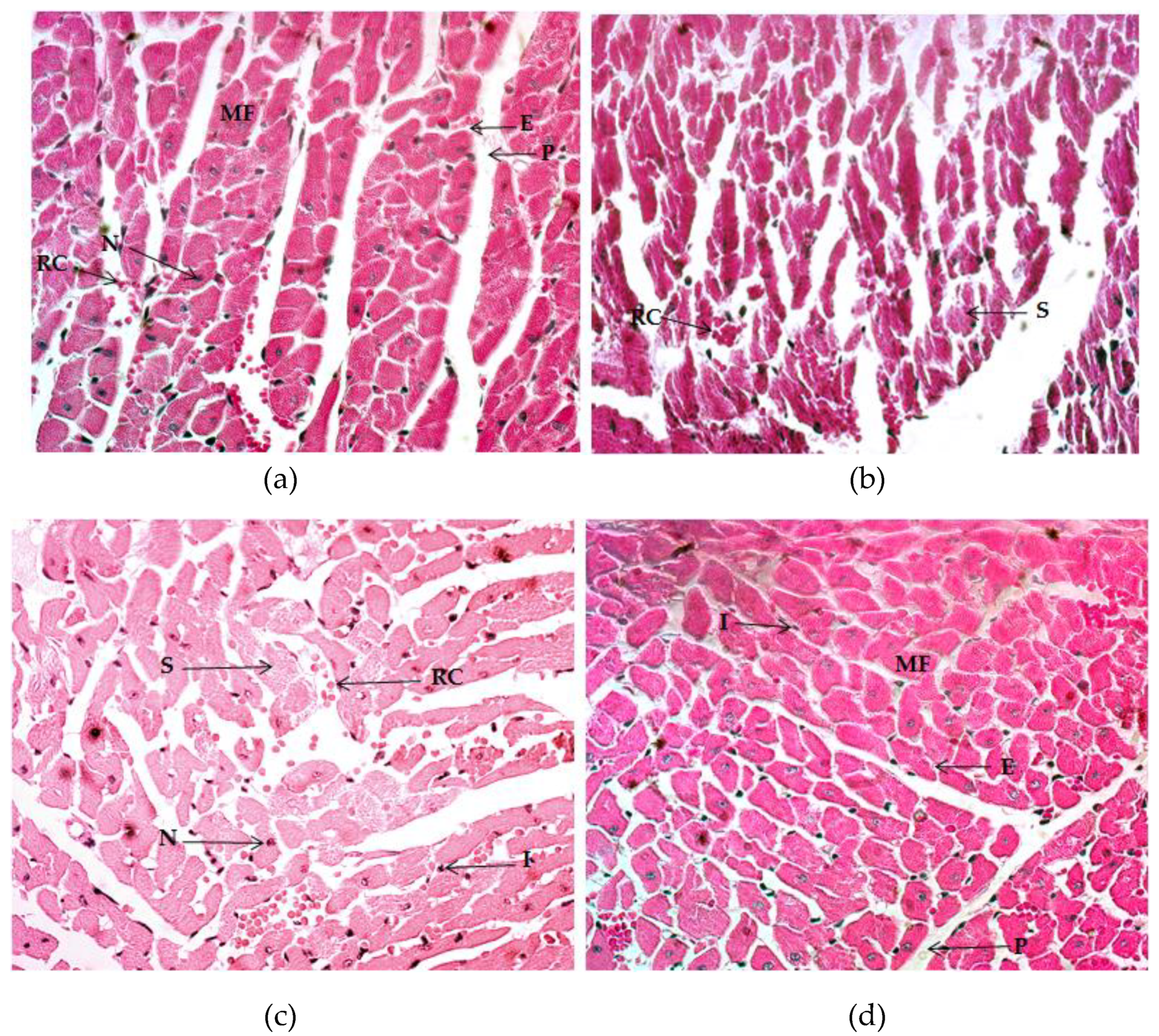
2.4. Histological Analysis
2.4.1. Quadriceps Muscle
2.4.2. Gastrocnemius Muscle
2.4.3. Myocardium
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Exercise-Training Model
4.3. Food Intake and Bodyweight Control
4.4. Exercise Endurance-Capacity Test
4.5. Sample Collection
4.6. Histological and Microscopy Analyses
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J.; Wackerhage, H.; Spangenburg, E.E.; Booth, F.W. Control of the size of the human muscle mass. Annu. Rev. Physiol. 2004, 66, 799–828. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, G.R.; West, D.W.; Baar, K. The molecular basis for load-induced skeletal muscle hypertrophy. Calcif. Tissue Int. 2015, 96, 196–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barany, M. ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 1967, 50, 197–218. [Google Scholar] [CrossRef] [Green Version]
- Schiaffino, S. Fibre types in skeletal muscle: A personal account. Acta Physiol. 2010, 199, 451–463. [Google Scholar] [CrossRef]
- D’Andrea, A.; Formisano, T.; Riegler, L.; Scarafile, R.; America, R.; Martone, F.; di Maio, M.; Russo, M.G.; Bossone, E.; Galderisi, M.; et al. Acute and Chronic Response to Exercise in Athletes: The “Supernormal Heart”. Adv. Exp. Med. Biol. 2017, 999, 21–41. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [Green Version]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Mendoza, N.; Madrigal-Santillan, E.; Morales-Gonzalez, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; Garcia-Luna, Y.G.-R.M.; Gayosso-de-Lucio, J.A.; Morales-Gonzalez, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-Gonzalez, A.; Morales-Martinez, M.; Soriano-Ursua, M.A.; Delgado-Olivares, L.; Sandoval-Gallegos, E.M.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Madrigal-Santillan, E.; Morales-Gonzalez, J.A. Flavolignans from Silymarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines 2020, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of Exercise in the Older Population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Chanoit, G.P.; Lefebvre, H.P.; Orcel, K.; Laroute, V.; Toutain, P.L.; Braun, J.P. Use of plasma creatine kinase pharmacokinetics to estimate the amount of excercise-induced muscle damage in Beagles. Am. J. Vet. Res. 2001, 62, 1375–1380. [Google Scholar] [CrossRef]
- Mengyan, Z. Protection of Lotus Seedpod Proanthocyanidins on Organs and Tissues under High-intensity Excercise. Open Biomed. Eng. J. 2015, 9, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Kim, E.K.; Jeoung, N.H.; Kim, S.H. Effect of silymarin on gluconeogenesis and lactate production in exercising rats. Food Sci. Biotechnol. 2016, 25, 119–124. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Kwak, H.B.; Seo, K.W.; McGregor, R.A.; Yeo, J.Y.; Ko, T.H.; Bolorerdene, S.; Kim, N.; Ko, K.S.; et al. Voluntary stand-up physical activity enhances endurance exercise capacity in rats. Korean J. Physiol. Pharmacol. 2016, 20, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Leeuwenburgh, C.; Heinecke, J.W. Oxidative stress and antioxidants in exercise. Curr. Med. Chem. 2001, 8, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J.M.; Rodriguez, D.A.; Hord, J.M. Mitochondria in the middle: Exercise preconditioning protection of striated muscle. J. Physiol. 2016, 594, 5161–5183. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jager, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, V.H.; Valente, H.F.; Casal, S.I.; Marques, A.F.; Moreira, P.A. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med. Sci. Sports Exerc. 2009, 41, 1752–1760. [Google Scholar] [CrossRef]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: Reversal by vitamin C supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci. Rep. 2019, 9, 4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, F.F.; Guo, Y.; Li, J.W.; Yuan, K. Antifatigue Effect of Luteolin-6-C-Neohesperidoside on Oxidative Stress Injury Induced by Forced Swimming of Rats through Modulation of Nrf2/ARE Signaling Pathways. Oxidative Med. Cell. Longev. 2017, 2017, 3159358. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Karimian, J.; Feizi, A.; Paknahad, Z.; Sharifirad, G.; Hajishafiei, M. Does quercetin and vitamin C improve exercise performance, muscle damage, and body composition in male athletes? J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 328–331. [Google Scholar]
- Casuso, R.A.; Martínez-Amat, A.; Martínez-Lópe, E.J.; Camiletti-Moirón, D.; Porres, J.M.; Aranda, P. Ergogenic effects of quercetin supplementation in trained rats. J. Int. Soc. Sports Nutr. 2013, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, Z.W.; Ralston, E. Nuclear domains in muscle cells. Cell 1989, 59, 771–772. [Google Scholar] [CrossRef]
- Roy, R.R.; Monke, S.R.; Allen, D.L.; Edgerton, V.R. Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. J. Appl. Physiol. 1999, 87, 634–642. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Mobley, C.B.; Osburn, S.C.; Mumford, P.W.; Roberson, P.A.; Romero, M.A.; Fox, C.D.; Parry, H.A.; Kavazis, A.N.; et al. Pre-training Skeletal Muscle Fiber Size and Predominant Fiber Type Best Predict Hypertrophic Responses to 6 Weeks of Resistance Training in Previously Trained Young Men. Front. Physiol. 2019, 10, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murach, K.A.; Dungan, C.M.; Peterson, C.A.; McCarthy, J.J. Muscle Fiber Splitting Is a Physiological Response to Extreme Loading in Animals. Exerc. Sport Sci. Rev. 2019, 47, 108–115. [Google Scholar] [CrossRef]
- Antonio, J.; Gonyea, W.J. Skeletal muscle fiber hyperplasia. Med. Sci. Sports Exerc. 1993, 25, 1333–1345. [Google Scholar] [CrossRef]
- Antonio, J.; Gonyea, W.J. Muscle fiber splitting in stretch-enlarged avian muscle. Med. Sci. Sports Exerc. 1994, 26, 973–977. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.M.; Schultz, E. Mechanisms of nascent fiber formation during avian skeletal muscle hypertrophy. Dev. Biol. 1992, 150, 319–334. [Google Scholar] [CrossRef]
- Winchester, P.K.; Gonyea, W.J. Regional injury and the terminal differentiation of satellite cells in stretched avian slow tonic muscle. Dev. Biol. 1992, 151, 459–472. [Google Scholar] [CrossRef]
- Mackey, A.L.; Kjaer, M. The breaking and making of healthy adult human skeletal muscle in vivo. Skelet. Muscle 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDougall, J.D.; Sale, D.G.; Elder, G.C.; Sutton, J.R. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.L.; Lehti, M.; Hulmi, J.J. Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Camus, G.; Deby-Dupont, G.; Deby, C.; Juchmes-Ferir, A.; Pincemail, J.; Lamy, M. Inflammatory response to strenuous muscular exercise in man. Mediat. Inflamm. 1993, 2, 335–342. [Google Scholar] [CrossRef]
- Camus, G.; Pincemail, J.; Deby-Dupont, G.; Deby, C.; Juchmes-Ferir, A.; Lamy, M. Effects of methylprednisolone on exercise-induced increases of plasma levels of polymorphonuclear elastase and myeloperoxidase in man. Preliminary results. Mediat. Inflamm. 1993, 2, 323–326. [Google Scholar] [CrossRef] [Green Version]
- Round, J.M.; Jones, D.A.; Cambridge, G. Cellular infiltrates in human skeletal muscle: Exercise induced damage as a model for inflammatory muscle disease? J. Neurol. Sci. 1987, 82, 1–11. [Google Scholar] [CrossRef]
- Jones, D.A.; Newham, D.J.; Round, J.M.; Tolfree, S.E. Experimental human muscle damage: Morphological changes in relation to other indices of damage. J. Physiol. 1986, 375, 435–448. [Google Scholar] [CrossRef]
- Ergun, Y.; Uremis, M.; Kilinc, M.; Alici, T. Antioxidant effect of Legalon(r) SIL in ischemia-reperfusion injury of rat skeletal muscle. Acta Cir. Bras. 2016, 31, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Vaadala, S.; Ponneri, N.; Karnam, V.S.; Pamuru, R.R. Baicalein, a flavonoid, causes prolonged estrus and suppressed fertility output upon prenatal exposure in female mice. Iran. J. Basic Med. Sci. 2019, 22, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Barari, A.R.; Alavi, S.H.; Shirali, S.; Ghazalian, F. Effec of short-term endurance training and silymarin consumption on some or preinflammatory citokines, growth mediators and immune system performance. Ann. Biol. Res. 2012, 3, 2933–2937. [Google Scholar]
- Natali, A.J.; Turner, D.L.; Harrison, S.M.; White, E. Regional effects of voluntary exercise on cell size and contraction-frequency responses in rat cardiac myocytes. J. Exp. Biol. 2001, 204, 1191–1199. [Google Scholar] [PubMed]
- Russell, B.; Motlagh, D.; Ashley, W.W. Form follows function: How muscle shape is regulated by work. J. Appl. Physiol. 2000, 88, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef]
- Itoh, Y.; Hayakawa, K.; Mori, T.; Agata, N.; Inoue-Miyazu, M.; Murakami, T.; Sokabe, M.; Kawakami, K. Stand-up exercise training facilitates muscle recovery from disuse atrophy by stimulating myogenic satellite cell proliferation in mice. Physiol. Rep. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Itoh, Y.; Murakami, T.; Mori, T.; Agata, N.; Kimura, N.; Inoue-Miyazu, M.; Hayakawa, K.; Hirano, T.; Sokabe, M.; Kawakami, K. Training at non-damaging intensities facilitates recovery from muscle atrophy. Muscle Nerve 2017, 55, 243–253. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Smith, J.L.; Simpson, D.R. Muscle fibre type populations of human leg muscles. Histochem. J. 1975, 7, 259–266. [Google Scholar] [CrossRef]
- Klover, P.; Chen, W.; Zhu, B.M.; Hennighausen, L. Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: Linking growth hormone to the androgen receptor. FASEB J. 2009, 23, 3140–3148. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, P.M.; Kroll, W.; McBride, T.C. Maximal isometric strength and fiber type composition in power and endurance athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1980, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tesch, P.A.; Karlsson, J. Muscle fiber types and size in trained and untrained muscles of elite athletes. J. Appl. Physiol. 1985, 59, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Baguet, A.; Everaert, I.; Hespel, P.; Petrovic, M.; Achten, E.; Derave, W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS ONE 2011, 6, e21956. [Google Scholar] [CrossRef] [Green Version]
- Vega, R.B.; Konhilas, J.P.; Kelly, D.P.; Leinwand, L.A. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell Metab. 2017, 25, 1012–1026. [Google Scholar] [CrossRef]
- Fagard, R. Athlete’s heart. Heart 2003, 89, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Olver, T.D.; Ferguson, B.S.; Laughlin, M.H. Molecular Mechanisms for Exercise Training-Induced Changes in Vascular Structure and Function: Skeletal Muscle, Cardiac Muscle, and the Brain. Prog. Mol. Biol. Transl. Sci. 2015, 135, 227–257. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Holloway, T.M.; Van Kranenburg, J.; Van Loon, L.J. Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med. Sci. Sports Exerc. 2016, 48, 2157–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joanisse, S.; Nederveen, J.P.; Snijders, T.; McKay, B.R.; Parise, G. Skeletal Muscle Regeneration, Repair and Remodelling in Aging: The Importance of Muscle Stem Cells and Vascularization. Gerontology 2017, 63, 91–100. [Google Scholar] [CrossRef]
- Akbari-Kordkheyli, V.; Abbaszadeh-Goudarzi, K.; Nejati-Laskokalayeh, M.; Zarpou, S.; Khonakdar-Tarsi, A. The protective effects of silymarin on ischemia-reperfusion injuries: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 968–976. [Google Scholar] [CrossRef]
- Akbari-Kordkheyli, V.; Azizi, S.; Khonakdar-Tarsi, A. Effects of silibinin on hepatic warm ischemia-reperfusion injury in the rat model. Iran. J. Basic Med. Sci. 2019, 22, 789–796. [Google Scholar] [CrossRef]
- Zholobenko, A.; Modriansky, M. Silymarin and its constituents in cardiac preconditioning. Fitoterapia 2014, 97, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Alozhy, M.A.A.; Al-Sa’aidi, J.A.A.; AlKalby, J.M. Hepatoprotective effect of silymarin in cyclosporine-induced oxidative stressed male rats. Basrah J. Vet. Res. 2019, 18, 392–409. [Google Scholar]
- Lane, M.T.; Herda, T.J.; Fry, A.C.; Cooper, M.A.; Andre, M.J.; Gallagher, P.M. Endocrine responses and acute mTOR pathway phosphorylation to resistance exercise with leucine and whey. Biol. Sport 2017, 34, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soonnenbichler, J.; Zetl, I. Biochemical effect of the flavonolignane silibinin on RNA, protein and DNA synthesis in rat livers. Prog. Clin. Biol. Res. 1986, 213, 319–331. [Google Scholar]
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute exercise induced mitochondrial H(2)O(2) production in mouse skeletal muscle: Association with p(66Shc) and FOXO3a signaling and antioxidant enzymes. Oxidative Med. Cell. Longev. 2015, 2015, 536456. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Heffner, R.R., Jr.; Balos, L.L. Skeletal Muscle. In Histology for Pathologists; Mills, S.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; Chapter 8. [Google Scholar]
- Goldblum, J.R. Rosai and Ackerman’s Surgical Pathology, 11th ed.; Goldblum, J.R., Ed.; Elsevier Health: Amsterdam, The Netherlands, 2018. [Google Scholar]


| Group | Initial Weight (g) | Final Weight (g) | Bodyweight Gain (g) | Percentage of Weight Gain (%) |
|---|---|---|---|---|
| CON | 231.8 ± 7.06 | 372.8 ± 15.22 | 141 ±9.82 | 60.72 ± 3.42 |
| ET | 239.0 ± 2.76 | 352.4 ± 7.6 | 113.4 ± 7.03 a,c | 47.47 ± 2.93 a,c |
| ET + VC | 233.4 ± 8.37 | 233.4 ± 11.71 | 110.4 ± 7.49 a,c | 47.49 ± 3.45 a,c |
| ET + SM | 230.8 ± 4.37 | 373.6 ± 12.07 | 142.8 ± 11.29 b | 61.96 ± 4.92 b |
| Group | Food Intake (g) | Energy (Kcals) | Protein (g) | Fat (g) |
|---|---|---|---|---|
| CON | 232.75 ± 17.77 | 387.53 ± 29.59 | 53.53 ± 4.09 | 15.12 ± 1.16 |
| ET | 257.5 ± 20.69 | 428.73 ± 34.46 | 59.22 ± 4.76 | 16.73 ± 1.34 |
| ET + VC | 239.62 ± 14.51 | 398.97 ± 24.16 | 55.11 ± 3.38 | 15.63 ± 0.96 |
| ET + SM | 258.62 ±19.95 | 430.61 ± 33.22 | 59.48 ± 4.59 | 16.81 ± 1.29 |
| Week | CON Time (min) | ET | ET + VC | ET + SM |
|---|---|---|---|---|
| 1 | 0.58 ± 0.06 | 0.51 ± 0.09 | 0.60 ± 0.05 | 0.62 ± 0.07 |
| 8 | 0.50 ± 0.04 | 2.06 ± 0.19 a | 2.89 ± 0.13 a,b,c | 3.70 ± 0.33 a,b |
| Distance (m) | ||||
| 1 | 9.70 ± 0.98 | 8.47 ± 1.46 | 9.99 ± 0.88 | 10.29 ± 1.14 |
| 8 | 8.42 ± 1.06 | 46.11 ± 3.10 a | 71.17 ± 8.48 a,b,c | 105.23 ± 14.42 a,b |
| Group | Hypertrophy | Polygonal Fiber Shape | Striated Appearance | Endomysium | Epimysium | Perimysium | Vascularization | Splitting | Inflammation | Lipid Content | Cyanophile Sarcoplasm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | 0 | 0 | + | ++ | ++ | +++ | 0 a + | 0 | + | ++ | 0 |
| ET | ++ | + | ++ | + | + | ++ | + | +++ | ++ | + | ++ |
| ET + VC | +++ | ++ | ++ | + | + | ++ | +++ | ++ | 0/+ | 0/+ | 0 |
| ET + SM | ++++ | ++++ | +++ | + | ++ | + | ++++ | + | 0 | 0/+ | 0 |
| Group | Hypertrophy | Polygonal Fiber Shape | Striated Appearance | Endomysium | Epimysium | Perimysium | Vascularization | Splitting | Inflammation | Lipid Content | Cyanophile Sarcoplasm | Satellite Cells | White Fibers | Red Fibers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | 0 | 0 | ++ | ++ | +++ | ++ | + | 0 a + | 0 | ++ | 0 | 0 a + | + | +++ |
| ET | +++ | +++ | +++ | + | ++ | ++ | +++ | ++++ | 0 a + | + | ++ | 0 a + | + | +++ |
| ET+ VC | ++++ | +++ | +++ | 0 | + | ++ | +++ | ++ | 0 | 0 | 0 | 0 | +++ | + |
| ET + SM | ++++ | ++++ | ++++ | 0 | + | ++ | +++ | + | 0 | 0 | 0 | 0 a + | ++ | +++ |
| Group | Hypertrophy | Endocardium | Pericardium | Vascularization | Splitting | Eosinophilia |
|---|---|---|---|---|---|---|
| CON | 0 | 0/+ | +++ | + | 0/+ | + |
| ET | +++ | + | + | +++ | ++++ | +++ |
| ET + VC | +++ | + | + | +++ | 0/+ | + |
| ET + SM | +++ | + | + | +++ | 0/+ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Mendoza, N.; Ángeles-Valencia, M.; Madrigal-Santillán, E.O.; Morales-Martínez, M.; Tirado-Lule, J.M.; Solano-Urrusquieta, A.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Fregoso-Aguilar, T.; Morales-González, Á.; et al. Effect of Silymarin Supplementation on Physical Performance, Muscle and Myocardium Histological Changes, Bodyweight, and Food Consumption in Rats Subjected to Regular Exercise Training. Int. J. Mol. Sci. 2020, 21, 7724. https://doi.org/10.3390/ijms21207724
Vargas-Mendoza N, Ángeles-Valencia M, Madrigal-Santillán EO, Morales-Martínez M, Tirado-Lule JM, Solano-Urrusquieta A, Madrigal-Bujaidar E, Álvarez-González I, Fregoso-Aguilar T, Morales-González Á, et al. Effect of Silymarin Supplementation on Physical Performance, Muscle and Myocardium Histological Changes, Bodyweight, and Food Consumption in Rats Subjected to Regular Exercise Training. International Journal of Molecular Sciences. 2020; 21(20):7724. https://doi.org/10.3390/ijms21207724
Chicago/Turabian StyleVargas-Mendoza, Nancy, Marcelo Ángeles-Valencia, Eduardo Osiris Madrigal-Santillán, Mauricio Morales-Martínez, Judith Margarita Tirado-Lule, Arturo Solano-Urrusquieta, Eduardo Madrigal-Bujaidar, Isela Álvarez-González, Tomás Fregoso-Aguilar, Ángel Morales-González, and et al. 2020. "Effect of Silymarin Supplementation on Physical Performance, Muscle and Myocardium Histological Changes, Bodyweight, and Food Consumption in Rats Subjected to Regular Exercise Training" International Journal of Molecular Sciences 21, no. 20: 7724. https://doi.org/10.3390/ijms21207724
APA StyleVargas-Mendoza, N., Ángeles-Valencia, M., Madrigal-Santillán, E. O., Morales-Martínez, M., Tirado-Lule, J. M., Solano-Urrusquieta, A., Madrigal-Bujaidar, E., Álvarez-González, I., Fregoso-Aguilar, T., Morales-González, Á., & Morales-González, J. A. (2020). Effect of Silymarin Supplementation on Physical Performance, Muscle and Myocardium Histological Changes, Bodyweight, and Food Consumption in Rats Subjected to Regular Exercise Training. International Journal of Molecular Sciences, 21(20), 7724. https://doi.org/10.3390/ijms21207724







