A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants
Abstract
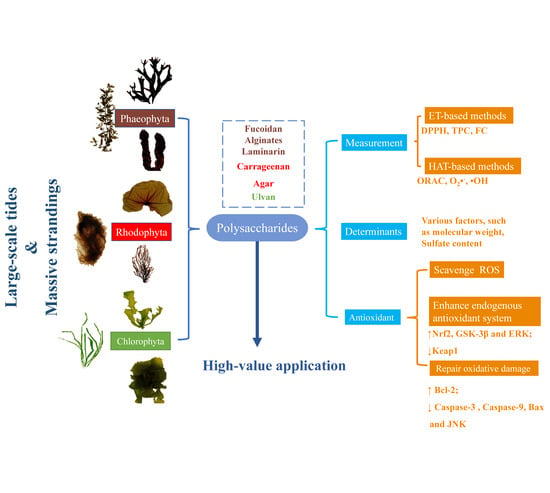
:1. Introduction
2. Polysaccharides from Seaweeds
2.1. Brown Seaweed Polysaccharides
2.2. Red Seaweed Polysaccharides
2.3. Green Seaweed Polysaccharides
3. Antioxidant Ability of Polysaccharides
3.1. Radical Scavenging Capacity
3.2. Endogenous Antioxidant Ability
4. Determinants of Antioxidant Activity
5. Molecular Mechanism of Polysaccharide-Induced Antioxidant Ability
5.1. Endogenous Antioxidant System
5.2. Apoptotic Pathway
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative Stress and Antioxidants in Disease and Cancer: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Faria, A.; Persaud, S.J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 2017, 172, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Li, H.; Weir, E.K.; Xu, Y.; Xu, D.; Chen, Y. Dimethylarginine dimethylaminohydrolase 1 deficiency aggravates monocrotaline-induced pulmonary oxidative stress, pulmonary arterial hypertension and right heart failure in rats. Int. J. Cardiol. 2019, 295, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.S.; Carvalho, M.d.G.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Cho, S. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.C.; Hyun, Y.J.; Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Hyun, J.W. 3-Bromo-4,5-dihydroxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Abdollahi, M.; Moridani, M.Y.; Aruoma, O.I.; Mostafalou, S. Oxidative Stress in Aging. Oxid. Med. Cell. Longev. 2014, 2014, 876834. [Google Scholar] [CrossRef] [Green Version]
- Tierney, M.S.; Croft, A.K.; Hayes, M. A review of antihypertensive and antioxidant activities in macroalgae. Bot. Mar. 2010, 53. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467. [Google Scholar] [CrossRef]
- Espinosa-Diez, C. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 15, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.K.; Singh, N. Morinda citrifolia (Noni) Alters Oxidative Stress Marker and Antioxidant Activity in Cervical Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2013, 14, 4603–4606. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chai, Z.; Wang, Q.; Chen, W.; He, Z.; Jiang, S. Cultivation of seaweed Gracilaria in Chinese coastal waters and its contribution to environmental improvements. Algal Res. 2015, 9, 236–244. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Q.; Nie, X.; Ren, H.; Shen, Z.; Xie, X.; Yang, Y. Heavy Metal Contamination in the Cultivated Oyster Crassostrea rivularis and Associated Health Risks from a Typical Mariculture Zone in the South China Sea. Bull. Environ. Contam. Toxicol. 2018, 101, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Jiang, Q.; Yang, Y. Concentrations of various elements in seaweed and seawater from Shen’ao Bay, Nan’ao Island, Guangdong coast, China: Environmental monitoring and the bioremediation potential of the seaweed. Sci. Total Environ. 2019, 659, 632–639. [Google Scholar] [CrossRef]
- Chen, B.; Xia, J.; Zou, D.; Zhang, X. Responses to ocean acidification and diurnal temperature variation in a commercially farmed seaweed, Pyropia haitanensis (Rhodophyta). Eur. J. Phycol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Chen, B.; Lin, L.; Ma, Z.; Zhang, T.; Chen, W.; Zou, D. Carbon and nitrogen accumulation and interspecific competition in two algae species, Pyropia haitanensis and Ulva lactuca, under ocean acidification conditions. Aquac. Int. 2019, 27, 721–733. [Google Scholar] [CrossRef]
- Zou, D.; Gao, K. Acquisition of inorganic carbon by Endarachne binghamiae (Scytosiphonales, Phaeophyceae). Eur. J. Phycol. 2010, 45, 117–126. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J.; Luo, Y. Removal of phenanthrene from coastal waters by green tide algae Ulva prolifera. Sci. Total Environ. 2017, 609, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-M.; Kim, G.; Shin, K.-H. Tracing nitrogen sources fueling coastal green tides off a volcanic island using radon and nitrogen isotopic tracers. Sci. Total Environ. 2019, 665, 913–919. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Wang, C.; Wei, W.; Liu, Z.; Tian, Y.; Zong, P.; Qiao, Y.; Qin, S. Golden seaweed tides from beach inundations as a valuable sustainable fuel resource: Fast pyrolysis characteristics, product distribution and pathway study on Sargassum horneri based on model compounds. Algal Res. Biomass Biofuels Bioprod. 2020, 48, 101888. [Google Scholar] [CrossRef]
- Resiere, D.; Valentino, R.; Nevière, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabié, A.; Lebrun, T.; Mégarbane, B.; Guerrier, G.; et al. Sargassum seaweed on Caribbean islands: An international public health concern. Lancet 2018, 392, 2691. [Google Scholar] [CrossRef] [Green Version]
- Cornish, M.L.; Critchley, A.T.; Mouritsen, O.G. A role for dietary macroalgae in the amelioration of certain risk factors associated with cardiovascular disease. Phycologia 2015, 54, 649–666. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Rathnayake, A.U.; Abuine, R.; Kim, Y.-J.; Byun, H.-G. Anti-Alzheimer’s Materials Isolated from Marine Bio-resources: A Review. Curr. Alzheimer Res. 2019, 16, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Pezzuto, J.M. Antioxidant Marine Products in Cancer Chemoprevention. Antioxid. Redox Signal. 2013, 19, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kang, N.; Ahn, G.; Jee, Y.; Kim, Y.-T.; Jeon, Y.-J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocoll. 2018, 81, 200–208. [Google Scholar] [CrossRef]
- Niu, T.; Fu, G.; Zhou, J.; Han, H.; Chen, J.; Wu, W.; Chen, H. Floridoside Exhibits Antioxidant Properties by Activating HO-1 Expression via p38/ERK MAPK Pathway. Mar. Drugs 2020, 18, 105. [Google Scholar] [CrossRef] [Green Version]
- Francisco, J.; Horta, A.; Pedrosa, R.; Afonso, C.; Cardoso, C.; Bandarra, N.M.; Gil, M.M. Bioaccessibility of antioxidants and fatty acids from Fucus spiralis. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Alves, C.; Neugebauer, A.; Silva, J.; Thomas, O.P.; Botana, L.M.; Gaspar, H.; Pedrosa, R. Marine invasive macroalgae: Turning a real threat into a major opportunity—The biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res. Biomass Biofuels Bioprod. 2018, 34, 217–234. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [Green Version]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef] [Green Version]
- Mtetwa, M.D.; Qian, L.S.; Zhu, H.A.; Cui, F.J.; Yang, Y. Ultrasound-assisted extraction and antioxidant activity of polysaccharides from Acanthus ilicifolius. J. Food Meas. Charact. 2020, 14. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the extracts and their bioactive potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Yin, J.; Shen, J.; Wang, H.; Wu, Y.; Jin, H. Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of A beta peptide in rats. Environ. Toxicol. Pharmacol. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.-K.; Lee, J.-C.; Yang, G.E.; Her, Y.; et al. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99–113. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.-Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.V.; Relleve, L.S.; Racadio, C.D.T.; Aranilla, C.T.; De la Rosa, A.M. Antioxidant activity potential of gamma irradiated carrageenan. Appl. Radiat. Isot. 2013, 79, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.J.; Mustapha, W.A.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O. Chemical properties and toxicology studies of fucoidan extracted from Malaysian Sargassum binderi. Food Sci. Biotechnol. 2016, 25, 23–29. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, physicochemical and antioxidant properties of sodium alginate isolated from a Tunisian brown seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. Biopolym. Online 2005, 6. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Seal, C.J.; Wilcox, M.; Dettmar, P.W.; Pearson, J.P. Applications of Alginates in Food. In Alginates: Biology and Applications; Springer-Verlag: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Graiff, A.; Ruth, W.; Kragl, U.; Karsten, U. Chemical characterization and quantification of the brown algal storage compound laminarin—A new methodological approach. J. Appl. Phycol. 2016. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, H.J.; Lee, J.W. Structural feature and antioxidant activity of low molecular weight laminarin degraded by gamma irradiation. Food Chem. 2011, 129, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Read, S.M.; Currie, G.; Bacic, A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 1996, 281, 187–201. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, C.; Yang, H.; Huang, X.; Ma, H.; Qin, X.; Hu, J. Effect of ultrasonic treatment on the degradation and inhibition cancer cell lines of polysaccharides from Porphyra yezoensis. Carbohydr. Polym. 2015, 117, 650–656. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Veterinární Medicína 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, B.; Mansouri, M.; Hemmati, A.; Naghizadeh, B.; Mard, S.; Rezaie, A. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292. [Google Scholar] [CrossRef] [Green Version]
- Venkatranganna, M.V.; Bhonde, R.R.; Shree, N.; Venkategowda, S. Treatment with adipose derived mesenchymal stem cells and their conditioned media reverse carrageenan induced paw oedema in db/db mice. Biomed. Pharmacother. 2017, 90, 350–353. [Google Scholar]
- Arslan, R.; Bektas, N.; Bor, Z.; Sener, E. Evaluation of the antithrombotic effects of Crataegus monogyna and Crataegus davisii in the carrageenan-induced tail thrombosis model. Pharm. Biol. 2015, 53, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Xi, M.-Z.; Choi, Y.-B.; Lee, B.-H. Antithrombotic Effect of Fermented Ophiopogon japonicus in Thrombosis-Induced Rat Models. J. Med. Food 2017, 20, 637–645. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Factors affecting yield and gelling properties of agar. J. Appl. Phycol. 2017, 29, 1527–1540. [Google Scholar] [CrossRef]
- Lee, W.-K.; Lim, Y.-Y.; Leow, A.T.-C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.-L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef]
- María, P.; Elena, F.; Herminia, D. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011, 23, 321–335. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Díaz, C.; Coste, O.; Malta, E.-J. Polymer chitosan nanoparticles functionalized with Ulva ohnoi extracts boost in vitro ulvan immunostimulant effect in Solea senegalensis macrophages. Algal Res. 2017, 26, 135–142. [Google Scholar] [CrossRef]
- Ponce, M.; Zuasti, E.; Anguís, V.; Fernández-Díaz, C. Effects of the sulfated polysaccharide ulvan from Ulva ohnoi on the modulation of the immune response in Senegalese sole (Solea senegalensis). Fish Shellfish Immunol. 2020, 100, 27–40. [Google Scholar] [CrossRef]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314. [Google Scholar] [CrossRef]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697. [Google Scholar] [CrossRef]
- Peso-Echarri, P.; Frontela-Saseta, C.; Gonzalez-Bermudez, C.A. Polysaccharides from seaweed as ingredients in marine aquaculture feeding: Alginate, carrageenan and ulvan. Rev. Biol. Mar. Oceanogr. 2012. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Liu, X.; Wang, K.; Liu, D.; Huang, L.; Liu, S.; Zhang, Q. Subchronic toxicity study of ulvan from Ulva pertusa (Chlorophyta) in Wistar rats. Food Chem. Toxicol. 2013, 62, 573–578. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables–evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Alonso de Linaje, A.; Herrero, A.; Asensio-Vegas, C.; Miranda, J.; Martínez-Villaluenga, C.; de Luis, D.A.; Martin-Diana, A.B. Carob by-products and seaweeds for the development of functional bread. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of Polyphenols from Chilean Brown Seaweeds Extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Belda, M.; Sanchez, D.; Bover, E.; Prieto, B.; Padron, C.; Cejalvo, D.; Miguel Lloris, J. Extraction of polyphenols in Himanthalia elongata and determination by high performance liquid chromatography with diode array detector prior to its potential use against oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033, 334–341. [Google Scholar] [CrossRef]
- Benitez Garcia, I.; Duenas Ledezma, A.K.; Martinez Montano, E.; Salazar Leyva, J.A.; Carrera, E.; Osuna Ruiz, I. Identification and Quantification of Plant Growth Regulators and Antioxidant Compounds in Aqueous Extracts of Padina durvillaei and Ulva lactuca. Agronomy 2020, 10. [Google Scholar] [CrossRef]
- Barahona, T.; Chandia, N.P.; Encinas, M.V.; Matsuhiro, B.; Zuniga, E.A. Antioxidant capacity of sulfated polysaccharides from seaweeds. A kinetic approach. Food Hydrocoll. 2011, 25, 529–535. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT Food Sci. Technol. 2016, 73, 602–608. [Google Scholar] [CrossRef]
- Lorbeer, A.J.; Charoensiddhi, S.; Lahnstein, J.; Lars, C.; Franco, C.M.M.; Bulone, V.; Zhang, W. Sequential extraction and characterization of fucoidans and alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 2017, 29, 1515–1526. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; López, J.; Goñi, G. Effect of different drying methods on phytochemical content and amino acid and fatty acid profiles of the green seaweed, Ulva spp. J. Appl. Phycol. 2019, 31, 1967–1979. [Google Scholar] [CrossRef]
- Agregán, R.; Lorenzo, J.M.; Munekata, P.E.S.; Dominguez, R.; Carballo, J.; Franco, D. Assessment of the antioxidant activity of Bifurcaria bifurcata aqueous extract on canola oil. Effect of extract concentration on the oxidation stability and volatile compound generation during oil storage. Food Res. Int. 2017, 99, 1095–1102. [Google Scholar] [CrossRef]
- Oliveira, L.C.B.P.; Queiroz, M.F.; Fidelis, G.P.; Melo, K.R.T.; Rocha, H.A.O. Antioxidant Sulfated Polysaccharide from Edible Red Seaweed Gracilaria birdiae Is an Inhibitor of Calcium Oxalate Crystal Formation. Molecules 2020, 25, 2055. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senanayake, S.P.J.N.; Wanasundara, P.K.J.P.D.; Shahidi, F. Antioxidants: Science, Technology, and Applications. In Bailey’s Industrial Oil and Fat Products; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar] [CrossRef]
- Daramola, B.; Adegoke, G. Bitter kola (Garcinia kola) seeds and health management potential. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 213–220. [Google Scholar]
- Berdahl, D.; Nahas, R.; Barren, J. Synthetic and natural antioxidant additives in food stabilization: Current applications and future research. In Oxidation in Foods and Beverages and Antioxidant Applications; Elsevier: Amsterdam, The Netherlands, 2010; pp. 272–320. [Google Scholar]
- Mishra, R.; Bisht, S.S. Antioxidants and their characterization. J. Pharm. Res. 2011, 4, 2744–2746. [Google Scholar]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Barabanova, A.O.; Bogdanovich, R.N.; Khomenko, V.A.; Solov’eva, T.F.; Yermak, I.M. In vitro antioxidant properties of red algal polysaccharides. Biomed. Prev. Nutr. 2011, 1, 161–167. [Google Scholar] [CrossRef]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocoll. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Chandran, M.N.; Suganya, A.M.; Immanuel, G. Investigation on bio-properties and in-vivo antioxidant potential of carrageenans against alloxan induced oxidative stress in Wistar albino rats. Int. J. Biol. Macromol. 2020, 151, 650–662. [Google Scholar] [CrossRef]
- Dai, M.; Zhou, Y.-L.; Jiang, T.; Luo, C.-D.; Wang, H.; Du, W.; Wang, M. Characterization of Polysaccharides Extracted from Sargassum fusiforme and Its Effective Prevention of Contrast-Induced Nephropathy via Enhancing Antioxidant Capacity. Int. J. Polym. Sci. 2019, 2019, 9035818. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.; Zhai, Y.; Li, Y.; Zhu, X.; Zhang, W. Laminarin protects against hydrogen peroxide-induced oxidative damage in MRC-5 cells possibly via regulating NRF2. PeerJ 2017, 5, e3642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Yang, D.; Huang, Y.; Zhao, Y.; Ye, J.; Xiao, M. The Potential of Neoagaro-Oligosaccharides as a Treatment of Type II Diabetes in Mice. Mar. Drugs 2019, 17, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.M.; Yan, X.J. Antioxidant activities of agaro-oligosaccharides with different degrees of polymerization in cell-based system. Biochim. Biophys. Acta 2005, 1722, 103–111. [Google Scholar] [CrossRef]
- Cavalcante Alencar, P.O.; Lima, G.C.; Barros, F.C.N.; Costa, L.E.C.; Ribeiro, C.V.P.E.; Sousa, W.M.; Sombra, V.G.; Abreu, C.M.W.S.; Abreu, E.S.; Pontes, E.O.B.; et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: In vitro and in vivo activities. Food Hydrocoll. 2019, 90, 28–34. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Khodagholi, F.; Abdi, A.; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr. Polym. 2010, 79, 1063–1072. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wei, J.-G.; Tu, M.-J.; Gu, J.-G.; Zhang, W. Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.-W.; Lee, H.-S.; Jung, K.H.; Lee, H.; Hong, S.-S. Protective effect of fucoidan against acetaminophen-induced liver injury. Arch. Pharm. Res. 2012, 35, 1099–1105. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, R.; Li, M.; Zhou, Q.; Liang, X.; Elmada, Z.C. Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2014, 41, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; He, J. Alginic acid oligosaccharide accelerates weaned pig growth through regulating antioxidant capacity, immunity and intestinal development. RSC Adv. 2016, 6, 87026–87035. [Google Scholar] [CrossRef]
- Sathivel, A.; Balavinayagamani; Rao, B.R.H.; Devaki, T. Sulfated polysaccharide isolated from Ulva lactuca attenuates D-galactosamine induced DNA fragmentation and necrosis during liver damage in rats. Pharm. Biol. 2014, 52, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, G.P.; Ferreira Silva, C.H.; Duarte Barreto Nobre, L.T.; Medeiros, V.P.; Oliveira Rocha, H.A.; Costa, L.S. Antioxidant Fucoidans Obtained from Tropical Seaweed Protect Pre-Osteoblastic Cells from Hydrogen Peroxide-Induced Damage. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.-Y.; Hwang, H.-J.; Nam, T.-J. Protective effect of a polysaccharide from Hizikia fusiformis against ethanol-induced cytotoxicity in IEC-6 cells. Toxicol. In Vitro 2010, 24, 79–84. [Google Scholar] [CrossRef]
- Hoang, M.H.; Kim, J.-Y.; Lee, J.H.; You, S.G.; Lee, S.-J. Antioxidative, hypolipidemic, and anti-inflammatory activities of sulfated polysaccharides from Monostroma nitidum. Food Sci. Biotechnol. 2015, 24, 199–205. [Google Scholar] [CrossRef]
- Presa, F.B.; Mendes Marques, M.L.; Silva Viana, R.L.; Duarte Barreto Nobre, L.T.; Costa, L.S.; Oliveira Rocha, H.A. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum in Cells Exposed to Oxidative Damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Choi, J.; Park, H. Antioxidant activities of fucoidan degraded by gamma irradiation and acidic hydrolysis. Radiat. Phys. Chem. 2015, 109, 23–26. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jiang, X.; Mou, H.; Guan, H. Anti-oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J. Appl. Phycol. 2004, 16, 333–340. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.C.-T.; Chang, C.A.; Chiu, K.-H.; Tsay, P.-K.; Jen, J.-F. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of kappa-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Ahmed, R.; Lee, J.M.; Noh, G.; Jo, G.; Kong, I.-S. Improved methods for isolation of carrageenan from Hypnea musciformis and its antioxidant activity. J. Appl. Phycol. 2016, 28, 1265–1274. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Kensler, T.W. The Role of Keap1 in Cellular Protective Responses. Chem. Res. Toxicol. 2005, 18, 1779–1791. [Google Scholar] [CrossRef]
- Juarez-Portilla, C.; Olivares-Banuelos, T.; Molina-Jimenez, T.; Armando Sanchez-Salcedo, J.; Del Moral, D.I.; Meza-Menchaca, T.; Flores-Munoz, M.; Lopez-Franco, O.; Roldan-Roldan, G.; Ortega, A.; et al. Seaweeds-derived compounds modulating effects on signal transduction pathways: A systematic review. Phytomedicine 2019, 63. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Park, J.H.; Kim, M.J.; Kim, W.J.; Ha, K.-T.; Choi, B.T.; Lee, S.-Y.; Shin, H.K. Isolinderalactone regulates the BCL-2/caspase-3/PARP pathway and suppresses tumor growth in a human glioblastoma multiforme xenograft mouse model. Cancer Lett. 2019, 443, 25–33. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.-J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef] [Green Version]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 32, 241–272. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Barber, A.J.; Spagnuolo, C.; Russo, G.L.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sánchez, E. Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 293–312. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-Y.; Choi, Y.H.; Nam, T.-J. Identification and antioxidant activity of synthetic peptides from phycobiliproteins of Pyropia yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Paul, V.J.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, M.J.; Chung, H.S. Fucoidan reduces oxidative stress by regulating the gene expression of HO-1 and SOD-1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol. Med. Rep. 2016, 14, 3255–3260. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M.; et al. Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 8918914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.Y.; He, F.; Xu, J.; Wang, H.Q.; Ken, A. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS ONE 2016, 11, e0152371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Darzynkiewicz, Z. Cleavage of Poly (ADP-ribose) polymerase measured in situ in individual cells: Relationship to DNA fragmentation and cell cycle position during apoptosis. Exp. Cell Res. 2000, 255, 125–132. [Google Scholar] [CrossRef]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N.T. Activation of Mitogen-activated Protein Kinase Pathways Induces Antioxidant Response Element-mediated Gene Expression via a Nrf2-dependent Mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.-Z.; Wang, Y.-T.; Zhuo, Y.-L.; Zhu, K.-J.; Wang, X.-Z.; Liu, A.-J. Fucoidan inhibits LPS-induced acute lung injury in mice through regulating GSK-3 beta-Nrf2 signaling pathway. Arch. Pharm. Res. 2020, 43, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Ge, B.; Wang, M.; Zhou, H.; Sang, R.; Yu, Y.; Xu, L.; Zhang, X. Inonotus obliquus polysaccharide ameliorates impaired reproductive function caused by Toxoplasma gondii infection in male mice via regulating Nrf2-PI3K/AKT pathway. Int. J. Biol. Macromol. 2020, 151, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Srivastava, S.K.; Kim, S.-H. Caspase-9 as a therapeutic target for treating cancer. Expert Opin. Ther. Targets 2015, 19, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Faucher, K.; Rabinovitch-Chable, H.; Cook-Moreau, J.; Barriere, G.; Sturtz, F.; Rigaud, M. Overexpression of human GPX1 modifies Bax to Bcl-2 apoptotic ratio in human endothelial cells. Mol. Cell. Biochem. 2005, 277, 81–87. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sorenson, C.M.; Sheibani, N. Attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy in Bcl-2−/− mice. Dev. Biol. 2005, 279, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wang, S.; Xie, Y.; Sun, J.; Wang, J. HPLC analysis of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes activity and Bax, Bcl-2 expression. Int. J. Biol. Macromol. 2010, 46, 167–172. [Google Scholar] [CrossRef]
- Gunawan, B.K.; Liu, Z.-X.; Han, D.; Hanawa, N.; Gaarde, W.A.; Kaplowitz, N. c-Jun N-Terminal Kinase Plays a Major Role in Murine Acetaminophen Hepatotoxicity. Gastroenterology 2006, 131, 165–178. [Google Scholar] [CrossRef]


| Type | Main Backbone | Source | Antioxidant Ability | Reference |
|---|---|---|---|---|
| Fucoidan | α-1,3-L-fucop yranose and the other alternating 1,3-and 1,4-linked α-L-fucopyranose | Undaria pinnatifida from New Zealand | At 2.0 mg/mL: DPPH (μg/mL TE): 7.43 ± 0.99; OH (%): 75.97 ± 1.69 | [99] |
| Undaria pinnatifida from Sigma-Aldrich | At 2.0 mg/mL: DPPH (μg/mL TE): 8.05 ± 1.49; ·OH (%): 75.32 ± 1.08 | |||
| Sargassum binderi from Malaysia | At 2.0 mg/mL: TPC (mg GAE/100 g), 3.69 ± 0.15; DPPH (IC50 (mg/ml), 2.01 ± 0.29; O2− (%), 26.78 ± 1.90; ·OH (%), 60.95 ± 0.69; FRAP (mg GAE/100 g), 0.60 ± 0.08 | [100] | ||
| Alginates | β-1,4-D-mannuronic acid (M) and α-1,4-L-guluronic acid (G) | Cystoseira barbata from Tunisia | At 0.5 mg/L: DPPH (%), 74%; At 4 and 5 mg/ml, ·OH: 80 and 82% | [54] |
| Laminaria japonica from China | MW of 1–6 KDa and 6–10 KDa: O2− (I50): 8 μg mL−1 and 18 μg mL−1; OH (I50): 0.01 mg mL−1 and 0.03 mg mL−1 | [57] | ||
| Laminarin | β-1,3-d-glucopyranose, with the β-1,6-linked d-glucopyranose units as branch-points or interchain residues | Laminaria digitata/Ascophyllum nodosum from SigmaeAldrich, USA | DPPH: reach 93.23%/87.57%; TPC (mg PGE/g): 0.343–0.365/0.110–0.166 | [101] |
| Carrageenan | d-galactopyranosyl with one or two sulfate groups, linked via alternated (1→3)-β-d-and (1→4)-β-d-glucoside | Chondrus armatus and C. pinnulatus from Russian | At 1 mg/mL, 0.25–0.50 wt% substrate and 1.0–5.0 wt% enzyme: FRAP (mM AAE/g), 58.50–98.22; O2− (%), 45.95–54.82% | [102] |
| Agar | repeating D-galactose and 3,6-anhydro-L-galactose | SigmaeAldrich, USA | At 10 mg/ml, DPPH (%), 16.47–22.71%; ABTS (%), 61.95–81.26%; FRAP, 0.95–1.46 | [103] |
| Ulvan | repeating disaccharide units, α-and β-(1,4)-linked monosaccharides | Ulva pertusa from Korea | Among 0.025-0.800 mg/L: ABTS (%), 20.15–30.25; DPPH (%), 5.61–46.51; | [94] |
| Seaweed | Compound | Source | Administration | Dose (mg/kg) | Markers | Tissues | Model | Reference |
|---|---|---|---|---|---|---|---|---|
| Red | Carrageenan | Kappaphycus alvarezii from India/ Sigma-Aldrich, USA | 2 days after induction, daily treatment, lasts 45 d | 500, 750 and 1000 | ↑CAT, GPx, SOD, GST, and GSH; ↓LPO | Liver | Alloxan induced diabetic rats | [104] |
| Sulfated polysaccharide | Gracilaria Caudata from Brazilian Atlantic coast | Before 18 h induction; pretreatment lasts 30 min | 3 and 10 | ↑CAT and SOD | ABAP induced Female Wistar rats | [110] | ||
| Oligosaccharide | G. lemaneiformis | Pretreatment 2 h before induction, once daily, lasts 21 d; pretreatment daily, lasts 2 weeks, followed by induction daily for 3 weeks; pretreatment immediately followed by induction, once daily, lasts 2 weeks; induction daily, lasts 3 weeks, followed by treatment daily for 2 weeks | 50,150 and 250 | ↑GSH and SOD; ↓MDA | Alcohol induced male Kunming mice | [106] | ||
| Brown | Sulfated polysaccharide | Sargassum fusiforme from China | Pretreatment twice daily, lasts 5 days | 5670 | ↑SOD; ↓MDA | Kidney | Contrast-induced nephropathy rats | [105] |
| Pretreatment 2 h before induction, 3 times a week, lasts 9 weeks | 200, 400, 600 | ↑SOD and CAT; ↓ROS and MDA | Skin | UVB radiation induced hairless Kun Ming mice | [111] | |||
| Fucoidan | Cool Chemistry CO. Ltd., China | Pretreatment once daily, lasts 7 days | 100 or 200 | ↑SOD, GSH and CAT ↓ROS and MDA | Liver | Acetaminophen induced male ICR mice | [113] | |
| Laminaria Japonica from China | After induction, treatment once daily, lasts 14 days | 50, 100, 200 | ↑SDO and GPX; ↓MDA | Hippocampus | Aβ-induced Sprague–Dawley rats | [42] | ||
| Sigma-Aldrich, USA | Pretreatment 2 h before induction, lasts 2 days | 100 | ↑GSH, GPx and SOD; ↓MDA | liver | Acetaminophen induced Sprague–Dawleyrats | [114] | ||
| Saccharina japonica from Ciyuan Biotechnology/Sargassum horneri from laboratory of Ningbo university | Twice daily, lasts 12 weeks | 110, 220 and 440/30, 60, 120 | ↑CAT and SOD; ↓MDA | blood | yellow catfish (Pelteobagrus fulvidraco) | [115] | ||
| Alginic acid oligosaccharide | Dalian Institute of Chemical Physics, Chinese Academy of Sciences | Daily, lasts 21 days | 100 | ↑SOD, CAT, T-AOC and GPx; ↓MDA | weaned pigs | [116] | ||
| Green | Sulfated polysaccharide | Ulva lactuca from Mandapam region | After induction, treatment daily, lasts 4 weeks; pretreatment 4 weeks before induction | 100 | ↑CAT and SOD | liver | D-galactosamine induced Adult male Albino Wistar rats | [117] |
| Seaweed | Compound | Source | Administration | Dose (mg/mL) | Markers | Model | Reference |
|---|---|---|---|---|---|---|---|
| Brown | Alginate | Sigma Aldrich, USA | Pretreatment 1 h before induction; | 0.030 | ↑GSH | H2O2-induced NT2 neurons | [112] |
| Fucoidan | Cool Chemistry CO., China | Pretreatment 4 h before induction | 0.025. 0.050 and 0.100 | ↑GSH and SOD; ↓ROS and MDA | Acetaminophen induced HL-7702 cell line | [113] | |
| Dictyota mertensii from Brazil | Co-treatment with induction, lasts 6 h | 0.050-0.500 | ↑SOD; ↓ROS, | H2O2 induced pre- osteoblast-like cells (MC3T3-L1) | [118] | ||
| Laminarin | Sigma-Aldrich, USA | Treatment 1 h before or after induction | 0.020 | ↑SOD, GSH and CAT; ↓MDA | H2O2 induced Human lung fibroblasts MRC-5 cells | [107] | |
| Sulfated polysaccharide | Hizikia fusiformis from Korean | Pretreatment 24 h before induction | 0.500 | ↑GSH | Ethanol induced rat intestinal cell line IEC-6 | [119] | |
| Green | Ulvan | Ulva pertusa from Korea | Pretreatment 2 h before induction | 0.100 and 0.200 | ↑SOD and CAT | H2O2 induced RAW264.7 murine macrophage cell line | [94] |
| Sulfated Polysaccharides | Monostroma nitidum from Korea | Treatment after induction, lasts 24 h | 0.050, 0.100 and 0.200 | ↑SOD | Lipid-loaded HepG2 cells | [120] | |
| Udotea flabellum from Brazil | Co-treatment, with induction, lasts 1.5 h | 1.000 | ↑SOD and GSH; ↓MDA | FeSO4 or CuSO4 and ascorbate induced 3T3 fibroblasts | [121] |
| Seaweed | Compound | Source | Administration | Dose | Model | Possible Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Brown | Sulfated polysaccharide | Hizikia fusiformis | Pretreatment 24 h before induction | 0.5 mg/L | Ethanol induced rat intestinal cell line IEC-6 | ↓JNK phosphorylation | [119] |
| Fucoidan | Dictyota mertensii from Brazil | Co-treatment with induction, lasts for 6 h | 0.5 mg/L | H2O2 induced pre- osteoblast-like cells (MC3T3-L1) | ↓caspase-3 and caspase-9 | [118] | |
| Sargassum fusiforme from China | Co-treatment for 50 days | 400, 800 and 1600 mg/L | Heat Stress induced Drosophila melanogaster | ↑Nrf2; ↓keap1 | [141] | ||
| Laminaria Japonica from China | After induction, treatment once daily, lasts for 14 days | 100 and 200 mg/Kg | Aβ induced Sprague–Dawley rats | ↑Bcl-2/Bax; ↓caspase-3 | [42] | ||
| Sigma-Aldrich, USA | 24 h pretreatment | 0.1 and 1 μM | Aβ induced rat cholinergic basal forebrain neurons | ↓caspase-3 and caspase-9 | [148] | ||
| 24 h treatment | 30 mg/L | human keratinocyte cell line (HaCaT) | ↑HO-1, SOD-1, Nrf2 and ERK; ↓Keap1 | [140] | |||
| Pretreatment 1 h before induction | 20, 40, and 80 mg/kg | Lipopolysaccharide (LPS)-induced male BALB/c mice | ↑GSK-3β, Nrf2, and HO-1 | [145] | |||
| Pretreatment 2 h before induction, lasts 2 days | 100 mg/kg | Acetaminophen induced Sprague–Dawley rats | ↑Bcl-2; ↓Bax and caspse-3 | [114] | |||
| Alginate | Sigma Aldrich, USA | Pretreatment 1 h before induction | 30 mg/L | H2O2 induced NT2 neural cell line | ↑HO-1, γ-GCS, Hsp-70 and Nrf2; ↓caspase-3 and NF-κB | [112] | |
| Laminarin | Sigma-Aldrich, USA | Treatment 1 h before or after induction, lasts 24 h | 20 mg/L | H2O2 induced Human lung fibroblasts MRC-5 cells | ↑Nrf2, NQO1, GCLC and HO1; ↓KEAP1 | [107] | |
| Fucoidan | Cool Chemistry CO. | Pretreatment once daily, lasts 7 days | 100 and 200 mg/kg | APAP included human normal hepatocyte HL-7702 cell line | ↑Nrf2; ↓JNK Phosphorylation and ASK1 | [113] | |
| Green | sulfated polysaccharide | Udotea flabellum | Co-treatment, with induction, lasts 1.5 h | 1000 mg/L | FeSO4 or CuSO4 and ascorbate induced 3T3 fibroblasts | ↓caspase-3 and caspase-9 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. Int. J. Mol. Sci. 2020, 21, 7774. https://doi.org/10.3390/ijms21207774
Liu Z, Sun X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. International Journal of Molecular Sciences. 2020; 21(20):7774. https://doi.org/10.3390/ijms21207774
Chicago/Turabian StyleLiu, Zhiwei, and Xian Sun. 2020. "A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants" International Journal of Molecular Sciences 21, no. 20: 7774. https://doi.org/10.3390/ijms21207774
APA StyleLiu, Z., & Sun, X. (2020). A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. International Journal of Molecular Sciences, 21(20), 7774. https://doi.org/10.3390/ijms21207774