Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020
Abstract
1. Introduction
2. The Rationale for Clinical Platelet-Rich Plasma Therapies
3. PRP Terminology and Classification
3.1. “Generic” PRP to Mimic the Onset of Healing
3.2. Confusing PRP Terminology and Synopsis of Proposed Classification Systems
3.3. PRP Preparation Methods Are a Work in Progress
3.4. The Current Status of PRP Classification Systems
4. Understanding In Vitro and In Vivo Platelet Dosing
The Significance of In Vitro Data on Platelet Concentrations
5. A Contemporary PRP Formulation: “Clinical PRP”
5.1. Clinical PRP Recipe
5.1.1. Platelet Granules
5.1.2. Platelet Concentration
5.1.3. Deleterious Red Blood Cells
5.1.4. Leukocytes in C-PRP
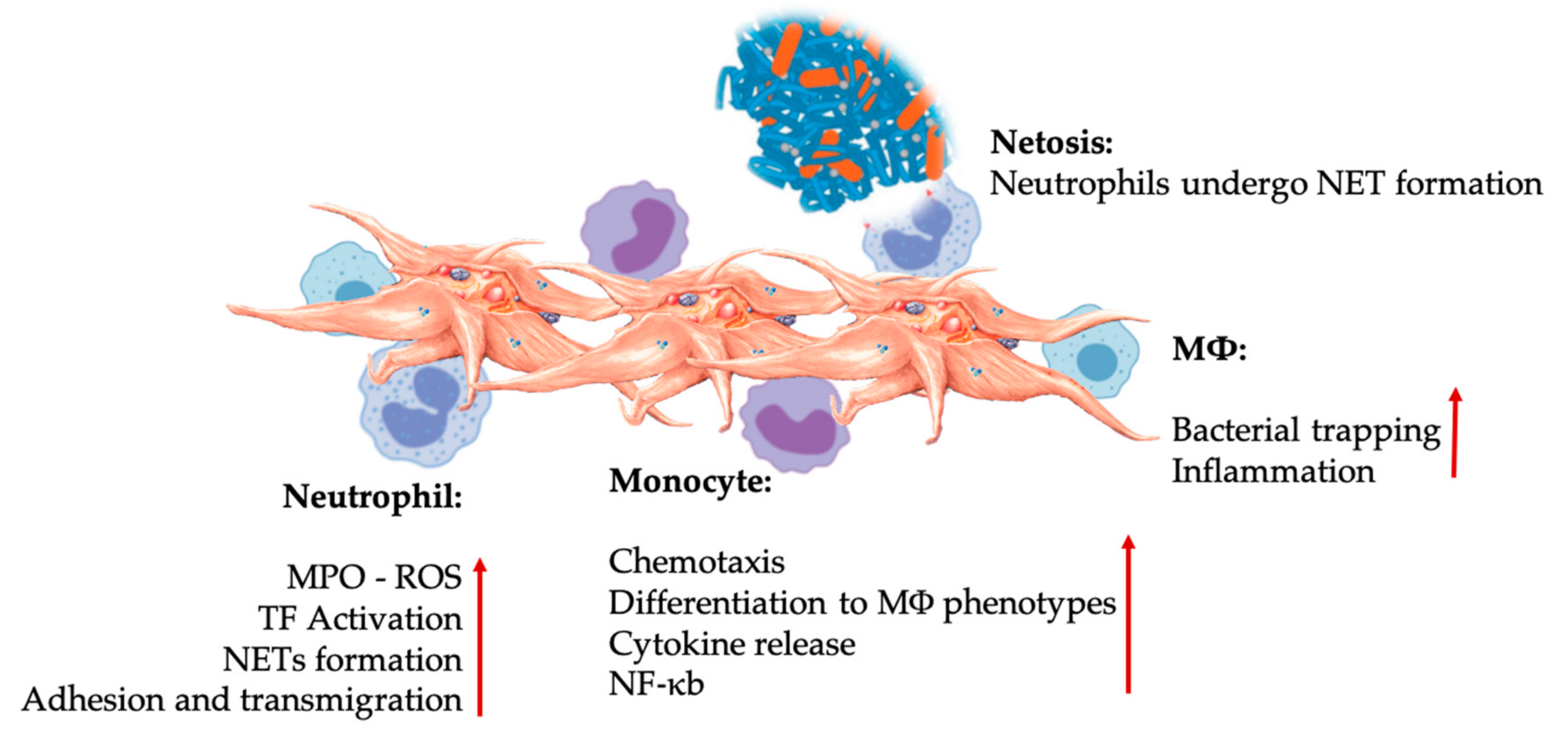
Neutrophils
Lymphocytes
Monocytes—Multipotential Repair Cells
5.2. Confusing Definitions for Leukocyte Fractions in PRP
6. Innate and Adaptive Immunomodulatory Capacities of PRP
6.1. Platelet Adhesion Molecules
6.2. Platelets and Leukocytes Play Pivotal Roles in Innate and Adaptive Immune Responses
6.2.1. Innate Immune System
Platelet-Leukocyte Interactions in Innate Immunity
6.2.2. Adaptive Immune System
Platelet-Leukocyte Interactions in Adaptive Immunity
6.3. Expanded Role of Platelet-Derived Serotonin in PRP
HT Paracrine and Autocrine Mechanisms
6.4. Immunomodulatory 5-HT Effects
7. PRP Analgesic Effects
8. PRP and Angiogenesis Effects
Pro- and Anti-Angiogenic Platelet Properties
9. Cell Senescence, Aging, and PRP
9.1. Effects of Aging and Cell Senescence
9.2. Cell Senescence and the Potential of PRP
10. The Role of Platelets in Bone Marrow Aspirate Concentrate
10.1. BMAC Repair Processes
10.2. Combining PRP and BMACs
10.3. PRP Growth Factors and BMAC Trophic Effects
11. Platelet Interactions with Anti-Platelet Medications and NSAIDs
12. Combining Platelet-Rich Plasma Applications with Rehabilitation
13. Future Prospect and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT | serotonin |
| 5HTR | serotonin receptors |
| Ang-1 | angiopoietin-1 |
| BMA | bone marrow aspirate |
| BMAC | bone marrow aspirate concentrate |
| C-PRP | clinical platelet-rich plasma |
| CD40L | cluster of differentiation 40 ligand |
| CTCG | connective tissue growth factor |
| CXCL | chemokine C-X-C motif ligand |
| DC | dendritic cell |
| EC | endothelial cells |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EPC | endothelial progenitor cell |
| FGF | fibroblast growth factor |
| GP | glycoprotein |
| HGF | hepatocyte growth factor |
| IFN | interferon |
| IGF | insulin-like growth factor |
| IL | interleukin |
| JAM | junctional adhesion molecule |
| KGF | keratinocyte growth factor |
| L-PRF | leucocyte platelet-rich plasma |
| LP-PRP | leukocyte-poor platelet-rich plasma |
| LR-PRP | leukocyte-rich platelet-rich plasma |
| Mac-1 | macrophage-1 antigen |
| MSC | mesenchymal stem cell |
| MMP | matrix metalloproteinase |
| MSK | musculoskeletal |
| MΦ | macrophage |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor kappa B |
| NSAID | non-steroidal anti-inflammatory drug |
| OA | osteoarthritis |
| PPP | platelet-poor plasma |
| P-PRF | pure platelet-rich fibrin |
| P-PRP | pure platelet-rich plasma |
| PDGF | platelet-derived growth factor |
| PF4 | platelet factor 4 |
| PFH | plasma-free hemoglobin |
| PGF | platelet growth factor |
| PPP | platelet-poor plasma |
| PRP | platelet-rich plasma |
| RANTES | regulated upon activation, normal T cell expressed and presumably secreted |
| RBC | red blood cell |
| SDF | stromal cell derived factor |
| SDF-1α | stromal cell-derived factor 1 alpha |
| SMC | smooth muscle cell |
| TGF | transforming growth factor |
| TLR | toll-like receptors |
| TNF | tumor necrosis factor |
| Treg | regular T lymphocyte |
| VEGF | vascular endothelial growth factor |
| vWF | von Willebrand factor |
| WBC | white blood cells |
References
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Di Matteo, B.; Kon, E.; Merli, G.; Marcacci, M. Platelet-rich plasma in tendon-related disorders: Results and indications. Knee Surg. Sports Traumatol. Arthrosc. 2016, 26, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Yu, W.; Dou, Y.; Wang, T. Efficacy of Platelet-rich Plasma for Low Back Pain: A Systematic Review and Meta-analysis. J. Neurol. Surg. Part A Central Eur. Neurosurg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Browning, S.R.; Weiser, A.M.; Woolf, N.; Golish, S.R.; SanGiovanni, T.P.; Scuderi, G.J.; Carballo, C.; Hanna, L.S. Platelet-Rich Plasma Increases Matrix Metalloproteinases in Cultures of Human Synovial Fibroblasts. J. Bone Jt. Surg. Am. Vol. 2012, 94, e172-1–e172-7. [Google Scholar] [CrossRef]
- Everts, P.A. Autologous Platelet-Rich Plasma and Mesenchymal Stem Cells for the Treatment of Chronic Wounds. In Wound Healing—Current Perspectives; Hakan Dogan, K., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth Factor and Catabolic Cytokine Concentrations Are Influenced by the Cellular Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Marrazzo, P.; Paduano, F.; Palmieri, F.; Marrelli, M.; Tatullo, M. Highly Efficient In Vitro Reparative Behaviour of Dental Pulp Stem Cells Cultured with Standardised Platelet Lysate Supplementation. Stem Cells Int. 2016, 2016, 7230987. [Google Scholar] [CrossRef]
- Everts, P.A.M.; Knape, J.T.A.; Weibrich, G.; Schönberger, J.P.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.M.; Van Zundert, A. Platelet-Rich Plasma and Platelet Gel: A Review. J. Extra Corpor. Technol. 2006, 38, 174–187. [Google Scholar]
- Everts, P.; Flanagan, G.F.; Rothenberg, J.; Mautner, K. The Rationale of Autologously Prepared Bone Marrow Aspirate Concentrate for use in Regenerative Medicine Applications. In Regenerative Medicine; Mahmood, C., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Hersant, B.; Sid-Ahmed, M.; Braud, L.; Jourdan, M.; Baba-Amer, Y.; Meningaud, J.-P.; Rodriguez, A.-M. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int. 2019, 2019, 1234263. [Google Scholar] [CrossRef]
- Johal, H.; Khan, M.; Yung, S.P.; Dhillon, M.S.; Fu, F.H.; Bedi, A.; Bhandari, M. Impact of Platelet-Rich Plasma Use on Pain in Orthopaedic Surgery: A Systematic Review and Meta-analysis. Sports Health Multidiscip. Approach 2019, 11, 355–366. [Google Scholar] [CrossRef]
- Giusti, I.; D’Ascenzo, S.; Macchiarelli, G.; Dolo, V. In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfus. 2020. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. A contemporary view of platelet-rich plasma therapies: Moving toward refined clinical protocols and precise indications. Regen. Med. 2018, 13, 717–728. [Google Scholar] [CrossRef]
- Puzzitiello, R.N.; Patel, B.H.; Forlenza, E.M.; Nwachukwu, B.U.; Allen, A.A.; Forsythe, B.; Salzler, M.J. Adverse Impact of Corticosteroids on Rotator Cuff Tendon Health and Repair: A Systematic Review of Basic Science Studies. Arthrosc. Sports Med. Rehabil. 2020, 2, e161–e169. [Google Scholar] [CrossRef]
- Beitzel, K.; Allen, D.; Apostolakos, J.; Russell, R.; McCarthy, M.; Gallo, G.; Coté, M.; Mazzocca, A. US Definitions, Current Use, and FDA Stance on Use of Platelet-Rich Plasma in Sports Medicine. J. Knee Surg. 2014, 28, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.M.; Hoffmann, J.; Weibrich, G.; Mahoney, C.B.; Schönberger, J.P.A.M.; Van Zundert, A.; Knape, J.T.A. Differences in platelet growth factor release and leucocyte kinetics during autologous platelet gel formation. Transfus. Med. 2006, 16, 363–368. [Google Scholar] [CrossRef]
- Mazzucco, L.; Balbo, V.; Cattana, E.; Guaschino, R.; Borzini, P. Not every PRP-gel is born equal Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet®, RegenPRP-Kit®, Plateltex® and one manual procedure. Vox Sang. 2009, 97, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The Structure and Biological Features of Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2006, 936, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, G.D.; Harbury, C.B.; Rubenstein, E. A physiological sealant for cerebrospinal fluid leaks. J. Neurosurg. 1977, 46, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; Laprade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting. J. Bone Jt. Surg. Am. Vol. 2017, 99, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.M.; Van Zundert, A.; Schönberger, J.P.A.M.; Devilee, R.J.J.; Knape, J.T.A. What do we use: Platelet-rich plasma or platelet-leukocyte gel? J. Biomed. Mater. Res. Part A 2008, 85, 1135–1136. [Google Scholar] [CrossRef]
- Kingsley, C. Blood Coagulation: Evidence of an Antagonist to Factor VI in Platelet-Rich Human Plasma. Nature 1954, 173, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Delong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-Rich Plasma: The PAW Classification System. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Harmon, K.; Woodall, J.; Vieira, A. Sports Medicine Applications of Platelet Rich Plasma. Curr. Pharm. Biotechnol. 2012, 13, 1185–1195. [Google Scholar] [CrossRef]
- Mautner, K.; Malanga, G.A.; Smith, J.; Shiple, B.; Ibrahim, V.; Sampson, S.; Bowen, J.E. A Call for a Standard Classification System for Future Biologic Research: The Rationale for New PRP Nomenclature. PM&R 2015, 7, S53–S59. [Google Scholar]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L., Jr.; Luzo Ângela, C.M.; Belangero, W.D.; et al. Contributions for classification of platelet rich plasma—Proposal of a new classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef]
- Harrison, P.; The Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef]
- Rossi, L.A.; Murray, I.R.; Chu, C.R.; Muschler, G.F.; Rodeo, S.A.; Piuzzi, N.S. Classification systems for platelet-rich plasma. Bone Jt. J. 2019, 891–896. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Everts, P.A. In Search of a Consensus Terminology in the Field of Platelet Concentrates for Surgical Use: Platelet-Rich Plasma (PRP), Platelet-Rich Fibrin (PRF), Fibrin Gel Polymerization and Leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar] [CrossRef]
- The GRIP (Groupe de Recherche sur les Injections de PRP, PRP Injection Research Group); Eymard, P.A.; Ornetti, P.; Maillet, J.; Noel, É.; Adam, P.; Legré-Boyer, V.; Boyer, T.; Allali, F.; Gremeaux, V.; et al. Intra-articular injections of platelet-rich plasma in symptomatic knee osteoarthritis: A consensus statement from French-speaking experts. Knee Surg. Sports Traumatol. Arthrosc. 2020. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-rich PRP for knee osteoarthritis: Current concepts. J. Clin. Orthop. Trauma 2019, 10, S179–S182. [Google Scholar] [CrossRef] [PubMed]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth. Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, Í.; Corrêa do Amaral, R.J.; Granjeiro, J.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Dionisi, L.; Pizzicanella, J.; Kothari, A.; De Fazio, D.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Autologous Non-Activated Platelet-Rich Plasma (A-PRP) and Activated Platelet-Rich Plasma (AA-PRP) in Plastic Surgery: Wound Healing and Hair Regrowth Evaluation. Int. J. Mol. Sci. 2020, 21, 431. [Google Scholar] [CrossRef] [PubMed]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2018, 43, 803–814. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Pham, T.A.V. Effects of platelet-rich plasma on human gingival fibroblast proliferation and migration in vitro. J. Appl. Oral Sci. 2018, 26. [Google Scholar] [CrossRef]
- Vahabi, S.; Yadegari, Z.; Mohammad-Rahimi, H. Comparison of the effect of activated or non-activated PRP in various concentrations on osteoblast and fibroblast cell line proliferation. Cell Tissue Bank. 2017, 18, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implant. Res. 2006, 17, 212–219. [Google Scholar] [CrossRef]
- Park, M.S.; Moon, S.-H.; Kim, T.-H.; Oh, J.K.; Yoon, W.Y.; Chang, H.G. Platelet-rich plasma for the spinal fusion. J. Orthop. Surg. 2018, 26. [Google Scholar] [CrossRef]
- De Mos, M.; Van Der Windt, A.E.; Jahr, H.; Van Schie, H.T.M.; Weinans, H.; Verhaar, J.A.N.; Van Osch, G.J.V.M. Can Platelet-Rich Plasma Enhance Tendon Repair? Am. J. Sports Med. 2008, 36, 1171–1178. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, C.-Q.; Wang, H.-C. Augmenting tendon and ligament repair with platelet-rich plasma (PRP). Muscle Ligaments Tendons J. 2019, 3, 139. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cole, J.; Deutsch, D.P.; Everts, P.A.; Niedbalski, R.P.; Panchaprateep, R.; Rinaldi, F.; Rose, P.T.; Sinclair, R.; Vogel, J.E.; et al. Platelet-Rich Plasma as a Treatment for Androgenetic Alopecia. Dermatol. Surg. 2019, 45, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, B.H.; Jones, S.G.; Datta, S.; McLaughlin, E.K.; Hoschtitzky, A.J. A meta-analysis of platelet gel for prevention of sternal wound infections following cardiac surgery. Blood Transfus. 2016. [Google Scholar] [CrossRef]
- Willemsen, J.C.N.; Van Dongen, J.; Spiekman, M.; Vermeulen, K.M.; Harmsen, M.C.; Van Der Lei, B.; Stevens, H.P.J. The addition of PRP to facial lipofilling. Plast. Reconstr. Surg. 2017, 141, 331–343. [Google Scholar] [CrossRef]
- Muchedzi, T.A.; Roberts, S.B. A systematic review of the effects of platelet rich plasma on outcomes for patients with knee osteoarthritis and following total knee arthroplasty. Surgeon 2018, 16, 250–258. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Pereira, H.; Espregueira-Mendes, J.; Reis, R.L.; Oliveira, J.M. The Clinical Use of Biologics in the Knee Lesions: Does the Patient Benefit? Curr. Rev. Musculoskelet. Med. 2019, 12, 406–414. [Google Scholar] [CrossRef]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM&R 2015, 8, 1–10. [Google Scholar]
- Mariani, E.; Pulsatelli, L. Platelet Concentrates in Musculoskeletal Medicine. Int. J. Mol. Sci. 2020, 21, 1328. [Google Scholar] [CrossRef]
- Manini, D.R.; Shega, F.D.; Guo, C.; Wang, Y. Role of Platelet-Rich Plasma in Spinal Fusion Surgery: Systematic Review and Meta-Analysis. Adv. Orthop. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Haunschild, E.D.; Huddleston, H.P.; Chahla, J.; Gilat, R.; Cole, B.J.; Yanke, A.B. Platelet-Rich Plasma Augmentation in Meniscal Repair Surgery: A Systematic Review of Comparative Studies. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 1765–1774. [Google Scholar] [CrossRef]
- Kushida, S.; Kakudo, N.; Morimoto, N.; Hara, T.; Ogawa, T.; Mitsui, T.; Kusumoto, K. Platelet and growth factor concentrations in activated platelet-rich plasma: A comparison of seven commercial separation systems. J. Artif. Organs 2014, 17, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Senzel, L.; Gnatenko, D.V.; Bahou, W.F. The platelet proteome. Curr. Opin. Hematol. 2009, 16, 329–333. [Google Scholar] [CrossRef]
- Garcia, A.; Senis, Y. Platelet Proteomics: Principles, Analysis, and Applications; Wiley-Interscience Series on Mass, Spectrometry; García, Á., Senis, Y., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 403. [Google Scholar]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Iberg, C.A.; Hawiger, D. Natural and Induced Tolerogenic Dendritic Cells. J. Immunol. 2020, 204, 733–744. [Google Scholar] [CrossRef]
- Younas, M.; Hue, S.; Lacabaratz, C.; Guguin, A.; Wiedemann, A.; Surenaud, M.; Beq, S.; Croughs, T.; Lelièvre, J.-D.; Lévy, Y. IL-7 Modulates In Vitro and In Vivo Human Memory T Regulatory Cell Functions through the CD39/ATP Axis. J. Immunol. 2013, 191, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Ganor, Y.; Besser, M.; Ben-Zakay, N.; Unger, T.; Levite, M. Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin-mediated adhesion to laminin and fibronectin and chemotactic migration. J. Immunol. 2003, 170, 4362–4372. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Jakimowicz, J.J.; Van Beek, M.; Schönberger, J.P.A.M.; Devilee, R.J.J.; Overdevest, E.P.; Knape, J.; Van Zundert, A. Reviewing the Structural Features of Autologous Platelet-Leukocyte Gel and Suggestions for Use in Surgery. Eur. Surg. Res. 2007, 39, 199–207. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Zheng, C.; Zhu, Q.; Liu, X.; Huang, X.; He, C.; Jiang, L.; Quan, D.; Zhou, X.; Zhu, Z. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J. Tissue Eng. Regen. Med. 2016, 10, 428–436. [Google Scholar] [CrossRef]
- Hee, H.T.; Majd, M.E.; Holt, R.T.; Myers, L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur. Spine J. 2003, 12, 400–407. [Google Scholar] [CrossRef]
- Giusti, I.; Rughetti, A.; D’Ascenzo, S.; Millimaggi, D.; Pavan, A.; Dell’Orso, L.; Dolo, V. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion (Paris) 2009, 49, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Creeper, F.; Lichanska, A.M.; Marshall, R.I.; Seymour, G.J.; Ivanovski, S. The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. J. Periodontal Res. 2009, 44, 258–265. [Google Scholar] [CrossRef]
- Dale, D.C.; Boxer, L.; Liles, W.C. The phagocytes: Neutrophils and monocytes. Blood 2008, 112, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, A.; Maier, C.; Gerdes, N.; Macfarlane, L.; Soosairajah, J.; Bavendiek, U.; Ahrens, I.; Ernst, S.; Bassler, N.; Missiou, A.; et al. CD40 Ligand Mediates Inflammation Independently of CD40 by Interaction with Mac-1. Circ. 2007, 115, 1571–1580. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Hara, G.R.; Basu, T. Platelet-rich plasma in regenerative medicine. Biomed. Res. Ther. 2014, 1, 25–31. [Google Scholar] [CrossRef]
- Van Gils, J.M.; Zwaginga, J.J.; Hordijk, P.L. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J. Leukoc. Biol. 2008, 85, 195–204. [Google Scholar] [CrossRef]
- Bennett, J.S. Structure and function of the platelet integrin alphaIIbbeta3. J. Clin. Investig. 2005, 115, 3363–3369. [Google Scholar] [CrossRef]
- Soffer, E.; Ouhayoun, J.-P.; Dosquet, C.; Meunier, A.; Anagnostou, F. Effects of platelet lysates on select bone cell functions. Clin. Oral Implant. Res. 2004, 15, 581–588. [Google Scholar] [CrossRef]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and Vascular Function: Oxygen and Nitric Oxide. Front. Physiol. 2018, 9, 125. [Google Scholar] [CrossRef]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 121, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Repsold, L.; Joubert, A.M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Bulsara, M.K.; McCrory, P.R.; Richardson, M.D.; Zheng, M.H. Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components between 4 Common Commercial Kits. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- De Melo, B.A.; Luzo, A.C.M.; Lana, J.F.; Santana, M.H.A. Centrifugation Conditions in the L-PRP Preparation Affect Soluble Factors Release and Mesenchymal Stem Cell Proliferation in Fibrin Nanofibers. Molecules 2019, 24, 2729. [Google Scholar] [CrossRef]
- Moojen, D.J.F.; Everts, P.A.; Schure, R.-M.; Overdevest, E.P.; Van Zundert, A.; Knape, J.T.A.; Castelein, R.M.; Creemers, L.B.; Dhert, W.J. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J. Orthop. Res. 2008, 26, 404–410. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Yeaman, M.R.; Selsted, M.E. Antimicrobial Peptides from Human Platelets. Infect. Immun. 2002, 70, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Bergdolt, S.; Wiegner, R.; Radermacher, P.; Huber-Lang, M.; Ignatius, A. The crucial role of neutrophil granulocytes in bone fracture healing. eCells Mater. 2016, 32, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.H.-C. PRP Treatment Efficacy for Tendinopathy: A Review of Basic Science Studies. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Fedorova, N.V.; Ksenofontov, A.L.; Serebryakova, M.V.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’Ina, G.F.; Galkina, S.I. Neutrophils Release Metalloproteinases during Adhesion in the Presence of Insulin, but Cathepsin G in the Presence of Glucagon. Mediat. Inflamm. 2018, 2018, 1574928. [Google Scholar] [CrossRef]
- Ubezio, G.; Ghio, M. Bio-modulators in platelet-rich plasma: A comparison of the amounts in products from healthy donors and patients produced with three different techniques. Blood Transfus. 2014. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Weirather, J.; Hofmann, U.D.W.; Beyersdorf, N.; Ramos, G.C.; Vogel, B.; Frey, A.; Ertl, G.; Kerkau, T.; Frantz, S. Foxp3 + CD4 + T Cells Improve Healing After Myocardial Infarction by Modulating Monocyte/Macrophage Differentiation. Circ. Res. 2014, 115, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Riboh, J.C.; Saltzman, B.M.; Yanke, A.B.; Fortier, L.; Cole, B.J. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2015, 44, 792–800. [Google Scholar] [CrossRef]
- Mariani, E.; Canella, V.; Cattini, L.; Kon, E.; Marcacci, M.; Di Matteo, B.; Pulsatelli, L.; Filardo, G. Leukocyte-Rich Platelet-Rich Plasma Injections Do Not Up-Modulate Intra-Articular Pro-Inflammatory Cytokines in the Osteoarthritic Knee. PLoS ONE 2016, 11, e0156137. [Google Scholar] [CrossRef] [PubMed]
- Parrish, W.R. Physiology of Blood Components in Wound Healing: An Appreciation of Cellular Co-Operativity in Platelet Rich Plasma Action. J. Exerc. Sports Orthop. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Seta, N.; Kuwana, M. Human circulating monocytes as multipotential progenitors. Keio J. Med. 2007, 56, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Filardo, G.; Mariani, E.; Cenacchi, A.; Pratelli, L.; Devescovi, V.; Kon, E.; Marcacci, M.; Facchini, A.; Ebaldini, N.; et al. Preparation method and growth factor content of platelet concentrate influence the osteogenic differentiation of bone marrow stromal cells. Cytotherapy 2013, 15, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Li, Y.; Lang, S.; Yougbare, I.; Zhu, G.; Chen, P.; Ni, H. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv. Hematol. 2012, 2012, 384685. [Google Scholar] [CrossRef]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef]
- Thon, J.N.; Peters, C.G.; Machlus, K.R.; Aslam, R.; Rowley, J.; MacLeod, H.; Devine, M.T.; Fuchs, T.A.; Weyrich, A.S.; Semple, J.W.; et al. T granules in human platelets function in TLR9 organization and signaling. J. Cell Biol. 2012, 198, 561–574. [Google Scholar] [CrossRef]
- Rossaint, J.; Zarbock, A. Platelets in leucocyte recruitment and function. Cardiovasc. Res. 2015, 107, 386–395. [Google Scholar] [CrossRef]
- Cruz, M.; Diacovo, T.; Emsley, J.; Liddington, R.; Handin, R. Mapping the Glycoprotein Ib-binding Site in the von Willebrand Factor A1 Domain. J. Biol. Chem. 2000, 275, 19098–19105. [Google Scholar] [CrossRef]
- Zarbock, A.; Ley, K.; McEver, R.P.; Hidalgo, A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011, 118, 6743–6751. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.; Yang, J. The Biology of P-Selectin Glycoprotein Ligand-1: Its Role as a Selectin Counterreceptor in Leukocyte-Endothelial and Leukocyte-Platelet Interaction. Thromb. Haemost. 1999, 81, 1–7. [Google Scholar] [CrossRef]
- Weber, C.; Springer, T. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J. Clin. Investig. 1997, 100, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Diacovo, T.; Defougerolles, A.; Bainton, D.; Springer, T. A functional integrin ligand on the surface of platelets: Intercellular adhesion molecule-2. J. Clin. Investig. 1994, 94, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Cognasse, F.; Laradi, S.; Berthelot, P.; Bourlet, T.; Marotte, H.; Mismetti, P.; Garraud, O.; Hamzeh-Cognasse, H. Platelet Inflammatory Response to Stress. Front. Immunol. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.; Clemetson, J.; Proudfoot, A.; Power, C.; Baggiolini, M.; Wells, T. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 2000, 96, 4046–4054. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Alberts, B., Ed.; Garland Science: New York, NY, USA, 2002; p. 1548. [Google Scholar]
- Vasina, E.M.; Cauwenberghs, S.; Feijge, M.A.H.; Heemskerk, J.W.M.; Weber, C.; Koenen, R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011, 2, e211. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Syvannarath, V.; Lamrani, L.; Ollivier, V.; Loyau, S.; Goerge, T.; Nieswandt, B.; Jandrot-Perrus, M.; Ho-Tin-Noé, B. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex–mediated inflammation in mice. Blood 2015, 126, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Kapur, R.; Zufferey, A.; Boilard, E.; Semple, J.W. Nouvelle Cuisine: Platelets Served with Inflammation. J. Immunol. 2015, 194, 5579–5587. [Google Scholar] [CrossRef]
- Scheuerer, B.; Ernst, M.; Dürrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.-D.; Petersen, F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000, 95, 1158–1166. [Google Scholar] [CrossRef]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17–producing human T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.J.; Kumar, P. Cross-Talk between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, X.; Gong, R.; Zhu, J.; Wei, L.; Yao, J. Recent advances in CD8+ regulatory T cell research (Review). Oncol. Lett. 2018, 15, 8187–8194. [Google Scholar] [CrossRef]
- Renshaw, B.; Fanslow, W.; Armitage, R.; Campbell, K.A.; Liggitt, D.; Wright, B.; Davison, B.L.; Maliszewski, C.R. Humoral immune responses in CD40 ligand-deficient mice. J. Exp. Med. 1994, 180, 1889–1900. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garcés-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavón, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 1–21. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Cloëz-Tayarani, I.; Petit-Bertron, A.; Venters, H.D.; Cavaillon, J. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: Involvement of 5-hydroxytryptamine2A receptors. Int. Immunol. 2003, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.-Y.; Wang, P.-X.; Wei, S.-Q.; Traub, R.J.; Li, J.-F.; Cao, D.-Y. The Role of Descending Pain Modulation in Chronic Primary Pain: Potential Application of Drugs Targeting Serotonergic System. Neural Plast. 2019, 2019, 1389296. [Google Scholar] [CrossRef] [PubMed]
- Pakala, R.; Willerson, J.T.; Benedict, C.R. Mitogenic effect of serotonin on vascular endothelial cells. Circulation 1994, 90, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Mauler, M.; Braun, A.; Duerschmied, D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets 2018, 29, 541–548. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Duerschmied, D.; Suidan, G.L.; Demers, M.; Herr, N.; Carbo, C.; Brill, A.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef]
- Freire-Garabal, M.; Núñez, M.J.; Balboa, J.; López-Delgado, P.; Gallego, R.; García-Caballero, T.; Fernández-Roel, M.D.; Brenlla, J.; Rey-Méndez, M. Serotonin upregulates the activity of phagocytosis through 5-HT1Areceptors. Br. J. Pharmacol. 2003, 139, 457–463. [Google Scholar] [CrossRef]
- Everts, P.A.; Devilee, R.J.J.; Mahoney, C.; Van Erp, A.; Oosterbos, C.J.M.; Stellenboom, M.; Knape, J.; Van Zundert, A. Exogenous Application of Platelet-Leukocyte Gel during Open Subacromial Decompression Contributes to Improved Patient Outcome. Eur. Surg. Res. 2007, 40, 203–210. [Google Scholar] [CrossRef]
- Odem, M.A.; Bavencoffe, A.G.; Cassidy, R.M.; Lopez, E.R.; Tian, J.; Dessauer, C.; Walters, E.T. Isolated nociceptors reveal multiple specializations for generating irregular ongoing activity associated with ongoing pain. Pain 2018, 159, 2347–2362. [Google Scholar] [CrossRef]
- Sprott, H.; Franke, S.; Kluge, H.; Hein, G. Pain treatment of fibromyalgia by acupuncture. Rheumatol. Int. 1998, 18, 35–36. [Google Scholar] [CrossRef]
- Sommer, C. Serotonin in Pain and Analgesia: Actions in the Periphery. Mol. Neurobiol. 2004, 30, 117–126. [Google Scholar] [CrossRef]
- Nicholson, R.; Small, J.; Dixon, A.; Spanswick, D.; Lee, K. Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci. Lett. 2003, 337, 119–122. [Google Scholar] [CrossRef]
- Wu, W.-P.; Hao, J.-X.; Xu, X.-J.; Wiesenfeld-Hallin, Z.; Koek, W.; Colpaert, F.C. The very-high-efficacy 5-HT1A receptor agonist, F 13640, preempts the development of allodynia-like behaviors in rats with spinal cord injury. Eur. J. Pharmacol. 2003, 478, 131–137. [Google Scholar] [CrossRef]
- Rosenthal, N.; Mazzanti, C.; Barnett, R.; Hardin, T.; Turner, E.; Lam, G.; Ozaki, N.; Goldman, D. Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol. Psychiatry 1998, 3, 175–177. [Google Scholar] [CrossRef]
- Patetsos, E.; Horjales-Araujo, E. Treating Chronic Pain with SSRIs: What Do We Know? Pain Res. Manag. 2016, 2016, 2020915. [Google Scholar] [CrossRef]
- Yoshida, M.; Funasaki, H.; Marumo, K. Efficacy of autologous leukocyte-reduced platelet-rich plasma therapy for patellar tendinopathy in a rat treadmill model. Muscle Ligaments Tendons J. 2016, 6, 205–215. [Google Scholar] [CrossRef]
- Fu, C.-J.; Sun, J.-B.; Bi, Z.-G.; Wang, X.-M.; Yang, C.-L. Evaluation of platelet-rich plasma and fibrin matrix to assist in healing and repair of rotator cuff injuries: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 158–172. [Google Scholar] [CrossRef]
- Verhaegen, F.; Brys, P.; Debeer, P. Rotator cuff healing after needling of a calcific deposit using platelet-rich plasma augmentation: A randomized, prospective clinical trial. J. Shoulder Elb. Surg. 2016, 25, 169–173. [Google Scholar] [CrossRef]
- Lin, M.-T.; Wei, K.-C.; Wu, C.-H. Effectiveness of Platelet-Rich Plasma Injection in Rotator Cuff Tendinopathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diagnostics 2020, 10, 189. [Google Scholar] [CrossRef]
- Urits, I.; Smoots, D.; Franscioni, H.; Patel, A.; Fackler, N.; Wiley, S.; Berger, A.A.; Kassem, H.; Urman, R.D.; Manchikanti, L.; et al. Injection Techniques for Common Chronic Pain Conditions of the Foot: A Comprehensive Review. Pain Ther. 2020, 9, 145–160. [Google Scholar] [CrossRef]
- Kuffler, D.P. Platelet-Rich Plasma Promotes Axon Regeneration, Wound Healing, and Pain Reduction: Fact or Fiction. Mol. Neurobiol. 2015, 52, 990–1014. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nasiri, S.; Mohammadi, M.H.; Mohammadi, A.M.; Nikbakht, M.; Panah, M.Z.; Safar, H.; Mostafaei, S.; Norooznezhad, A.H.; Soroosh, A.R.; et al. Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: A randomized controlled parallel clinical trial. Transfus. Apher. Sci. 2017, 56, 226–232. [Google Scholar] [CrossRef]
- Deppermann, C.; Kubes, P. Start a fire, kill the bug: The role of platelets in inflammation and infection. Innate Immun. 2018, 24, 335–348. [Google Scholar] [CrossRef]
- Lansdown, D.A.; Fortier, L.A. Platelet-Rich Plasma: Formulations, Preparations, Constituents, and Their Effects. Oper. Tech. Sports Med. 2017, 25, 7–12. [Google Scholar] [CrossRef][Green Version]
- Walsh, T.G.; Metharom, P.; Berndt, M.C. The functional role of platelets in the regulation of angiogenesis. Platelets 2014, 26, 199–211. [Google Scholar] [CrossRef]
- Baka, S.; Clamp, A.R.; Jayson, G.C. A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert Opin. Ther. Targets 2006, 10, 867–876. [Google Scholar] [CrossRef]
- Ferrara, N. The Role of the VEGF Signaling Pathway in Tumor Angiogenesis. In Tumor Angiogenesis: A Key Target for Cancer Therapy; Marmé, D., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 211–226. [Google Scholar] [CrossRef]
- Brill, A.; Elinav, H.; Varon, D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc. Res. 2004, 63, 226–235. [Google Scholar] [CrossRef]
- Bir, S.C.; Esaki, J.; Marui, A.; Sakaguchi, H.; Kevil, C.G.; Ikeda, T.; Komeda, M.; Tabata, Y.; Sakata, R. Therapeutic treatment with sustained-release platelet-rich plasma restores blood perfusion by augmenting ischemia-induced angiogenesis and arteriogenesis in diabetic mice. J. Vasc. Res. 2011, 48, 195–205. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef]
- Marushima, A.; Nieminen, M.; Kremenetskaia, I.; Gianni-Barrera, R.; Woitzik, J.; von Degenfeld, G.; Banfi, A.; Vajkoczy, P.; Hecht, N. Balanced single-vector co-delivery of VEGF/PDGF-BB improves functional collateralization in chronic cerebral ischemia. J. Cereb. Blood Flow Metab. 2020, 40, 404–419. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; DeMaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Biran, A.; Zada, L.; Abou Karam, P.; Vadai, E.; Roitman, L.; Ovadya, Y.; Porat, Z.; Krizhanovsky, V. Quantitative identification of senescent cells in aging and disease. Aging Cell 2017, 16, 661–671. [Google Scholar] [CrossRef]
- Michaloglou, C.; Vredeveld, L.C.W.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; Van Der Horst, C.M.A.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Patil, P.; Dong, Q.; Wang, D.; Chang, J.; Wiley, C.; DeMaria, M.; Lee, J.; Kang, J.; Niedernhofer, L.J.; Robbins, P.D.; et al. Systemic clearance of p16 INK4a -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell 2019, 18, e12927. [Google Scholar] [CrossRef]
- Walters, H.E.; Yun, M.H. Rising from the ashes: Cellular senescence in regeneration. Curr. Opin. Genet. Dev. 2020, 64, 94–100. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Baar, M.P.; Perdiguero, E.; Muñoz-Cánoves, P.; De Keizer, P.L. Musculoskeletal senescence: A moving target ready to be eliminated. Curr. Opin. Pharmacol. 2018, 40, 147–155. [Google Scholar] [CrossRef]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Nirmala, X. Perspectives on Improving the Efficacy of PRP Treatment for Tendinopathy. J. Musculoskelet. Disord. Treat. 2016, 2. Available online: https://clinmedjournals.org/articles/jmdt/journal-of-musculoskeletal-disorders-and-treatment-jmdt-2-015.php?jid=jmdt (accessed on 17 October 2020). [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Huang, C.-F.; Lin, T.-C.; Tsai, C.-Y.; Chen, S.-Y.T.; Liu, A.; Chen, W.; Wei, H.-J.; Wang, M.-F.; Williams, D.F.; et al. Delayed animal aging through the recovery of stem cell senescence by platelet rich plasma. Biomaterials 2014, 35, 9767–9776. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C. Can PRP effectively treat injured tendons? Muscle Ligaments Tendons J. 2014, 4, 35–37. [Google Scholar] [CrossRef]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef]
- Jia, C.; Lu, Y.; Bi, B.; Chen, L.; Yang, Q.; Yang, P.; Guo, Y.; Zhu, J.; Zhu, N.; Tianyi, L. Platelet-rich plasma ameliorates senescence-like phenotypes in a cellular photoaging model. RSC Adv. 2017, 7, 3152–3160. [Google Scholar] [CrossRef]
- Oberlohr, V.; Lengel, H.; Hambright, W.S.; Whitney, K.E.; Evans, T.A.; Huard, J. Biologics for Skeletal Muscle Healing: The Role of Senescence and Platelet-Based Treatment Modalities. Oper. Tech. Sports Med. 2020, 28, 150754. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, E.K.; Kim, S.J.; Song, D.H. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J. Orthop. Surg. Res. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Zhao, T.; Yan, W.; Xu, K.; Qi, Y.; Dai, X.; Shi, Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy 2013, 15, 792–804. [Google Scholar] [CrossRef]
- Hede, K.; Christensen, B.B.; Jensen, J.; Foldager, C.B.; Lind, M. Combined Bone Marrow Aspirate and Platelet-Rich Plasma for Cartilage Repair: Two-Year Clinical Results. Cartillage 2019. [Google Scholar] [CrossRef]
- Lian, Z.; Yin, X.; Li, H.; Jia, L.; He, X.; Yan, Y.; Liu, N.; Wan, K.; Li, X.; Lin, S. Synergistic Effect of Bone Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma in Streptozotocin-Induced Diabetic Rats. Ann. Dermatol. 2014, 26, 1–10. [Google Scholar] [CrossRef]
- Betsch, M.; Schneppendahl, J.; Thuns, S.; Herten, M.; Sager, M.; Jungbluth, P.; Hakimi, M.; Wild, M. Bone Marrow Aspiration Concentrate and Platelet Rich Plasma for Osteochondral Repair in a Porcine Osteochondral Defect Model. PLoS ONE 2013, 8, e71602. [Google Scholar] [CrossRef]
- Kim, G.B.; Seo, M.-S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. [Google Scholar] [CrossRef]
- Cassano, J.M.; Kennedy, J.G.; Ross, K.A.; Fraser, E.J.; Goodale, M.B.; Sundman, E.A. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg. Sports Traumatol. Arthrosc. 2016, 26, 333–342. [Google Scholar] [CrossRef]
- Gharibi, B.; Hughes, F.J. Effects of Medium Supplements on Proliferation, Differentiation Potential, and In Vitro Expansion of Mesenchymal Stem Cells. Stem Cells Transl. Med. 2012, 1, 771–782. [Google Scholar] [CrossRef]
- Muiños-López, E.; Delgado, D.; Sánchez, P.; Paiva, B.; Anitua, E.; Fiz, N.; Aizpurua, B.; Guadilla, J.; Padilla, S.; Granero-Moltó, F.; et al. Modulation of Synovial Fluid-Derived Mesenchymal Stem Cells by Intra-Articular and Intraosseous Platelet Rich Plasma Administration. Stem Cells Int. 2016, 2016, 1247950. [Google Scholar] [CrossRef]
- Sassoli, C.; Vallone, L.; Tani, A.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S. Combined use of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and platelet rich plasma (PRP) stimulates proliferation and differentiation of myoblasts in vitro: New therapeutic perspectives for skeletal muscle repair/regeneration. Cell Tissue Res. 2018, 372, 549–570. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Jayaram, P.; Yeh, P.; Patel, S.J.; Cela, R.; Shybut, T.B.; Grol, M.W.; Lee, B.H. Effects of Aspirin on Growth Factor Release From Freshly Isolated Leukocyte-Rich Platelet-Rich Plasma in Healthy Men: A Prospective Fixed-Sequence Controlled Laboratory Study. Am. J. Sports Med. 2019, 47, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Frey, C.; Yeh, P.C.; Jayaram, P. Effects of Antiplatelet and Nonsteroidal Anti-inflammatory Medications on Platelet-Rich Plasma: A Systematic Review. Orthop. J. Sports Med. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Schippinger, G.; Prüller, F.; Divjak, M.; Mahla, E.; Fankhauser, F.; Rackemann, S.; Raggam, R.B. Autologous Platelet-Rich Plasma Preparations. Orthop. J. Sports Med. 2015, 3. [Google Scholar] [CrossRef]
- Reed, G.L.; Fitzgerald, M.L.; Polgár, J. Molecular mechanisms of platelet exocytosis: Insights into the “secrete” life of thrombocytes. Blood 2000, 96, 10. [Google Scholar]
- Castaño, E.; Bartrons, R.; Gil, J. Inhibition of cyclooxygenase-2 decreases DNA synthesis induced by platelet-derived growth factor in Swiss 3T3 fibroblasts. J. Pharmacol. Exp. Ther. 2000, 293, 509–513. [Google Scholar]
- Tarnawski, A.S.; Jones, M.K. Inhibition of angiogenesis by NSAIDs: Molecular mechanisms and clinical implications. J. Mol. Med. 2003, 81, 627–636. [Google Scholar] [CrossRef]
- Mannava, S.; Whitney, K.E.; Kennedy, M.I.; King, J.; Dornan, G.J.; Klett, K.; Chahla, J.; Evans, T.A.; Huard, J.; Laprade, R.F. The Influence of Naproxen on Biological Factors in Leukocyte-Rich Platelet-Rich Plasma: A Prospective Comparative Study. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 201–210. [Google Scholar] [CrossRef]
- Neph, A.; Schroeder, A.; Enseki, K.R.; Everts, P.A.; Wang, J.H.-C.; Onishi, K. Role of Mechanical Loading for Platelet-Rich Plasma-Treated Achilles Tendinopathy. Curr. Sports Med. Rep. 2020, 19, 209–216. [Google Scholar] [CrossRef]
- Andia, I.; Rubio-Azpeitia, E.; Martin, J.; Abate, M. Current Concepts and Translational Uses of Platelet Rich Plasma Biotechnology. In Biotechnology; Ekinci, D., Ed.; InTech: London, UK, 2015. [Google Scholar]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2020. [Google Scholar] [CrossRef]
- Virchenko, O.; Aspenberg, P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006, 77, 806–812. [Google Scholar] [CrossRef]
- Harshwardhan, H.; Saini, H.K.; Gupta, P. Assessment of clinical outcomes of PRP therapy in OA knee. Int. J. Orthop. Sci. 2020, 6, 201–203. [Google Scholar] [CrossRef]
- Bek, N.; Simşek, I.E.; Erel, S.; Yakut, Y.; Uygur, F. Home-based general versus center-based selective rehabilitation in patients with posterior tibial tendon dysfunction. Acta Orthop. Traumatol. Turc. 2012, 46, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lisiński, P.; Huber, J.; Wilkosz, P.; Witkowska, A.; Wytrazek, M.; Samborski, W.; Zagloba, A. Supervised versus Uncontrolled Rehabilitation of Patients after Rotator Cuff Repair-Clinical and Neurophysiological Comparative Study. Int. J. Artif. Organs 2012, 35, 45–54. [Google Scholar] [CrossRef]





| PGF and Cytokines | Cell Sources | Function and Effects |
|---|---|---|
| PDGF (AA-BB-AB) | Platelets, endothelial cells, macrophages, smooth muscle cells | Mitogenic for mesenchymal cells and osteoblasts; stimulates chemotaxis and mitogenesis in fibroblast/ glial/smooth muscle cells; regulates collagenase secretion and collagen synthesis; stimulates macrophage and neutrophil chemotaxis |
| TGF (α–β) | Macrophages, T lymphocytes, keratinocytes | Stimulates undifferentiated mesenchymal cell proliferation; regulates endothelial, fibroblastic, and osteoblastic mitogenesis; regulates collagen synthesis and collagenase secretion; regulates mitogenic effects of other growth factors; stimulates endothelial chemotaxis and angiogenesis; inhibits macrophage and lymphocyte proliferation |
| VEGF | Platelets, macrophages, keratinocytes, endothelial cells | Increases angiogenesis and vessel permeability; stimulates mitogenesis for endothelial cells |
| EGF | Platelets, macrophages, monocytes | Proliferation of keratinocytes, fibroblasts, stimulates mitogenesis for endothelial cells |
| (a-b)-FGF | Platelets, macrophages, mesenchymal cells, chondrocytes, osteoblasts | Promotes growth and differentiation of chondrocytes and osteoblasts; mitogenic for mesenchymal cells, chondrocytes, and osteoblasts |
| CTGF | Platelets, fibroblasts | Promotes angiogenesis, cartilage regeneration, fibrosis, and platelet adhesion |
| IGF-1 | Platelets, plasma, epithelial cells, endothelial cells, fibroblasts, osteoblasts, bone matrix | Chemotactic for fibroblasts and stimulates protein synthesis. Enhances bone formation by proliferation and differentiation of osteoblasts |
| HGF | Platelets, mesenchymal cells | Regulates cell growth and motility in epithelial/endothelial cells, supporting epithelial repair and neovascularization during wound healing |
| KGF | Fibroblasts, mesenchymal cells | Regulates epithelial migration and proliferation |
| Ang-1 | Platelets, neutrophils | Induces angiogenesis stimulating migration and proliferation of endothelial cells. Supports and stabilizes blood vessel development via the recruitment of pericyte |
| PF4 | Platelets | Calls leucocytes and regulates their activation. Microbiocidal activities |
| SDF-1α | Platelets, endothelial cells, fibroblasts | Calls CD34+ cells, induces their homing, proliferation and differentiation into endothelial progenitor cells stimulating angiogenesis. Calls mesenchymal stem cells and leucocytes |
| TNF | Macrophages, mast cells, T lymphocytes | Regulates monocyte migration, fibroblast proliferation, macrophage activation, angiogenesis |
| A-PRF | Advanced Platelet-Rich Fibrin |
| ACP | Autologous Conditioned Plasma |
| AGF | Autologous Growth Factors |
| APG | Autologous Platelet Gel |
| C-PRP | Clinical Platelet-Rich Plasma |
| i-PRF | Injectable Platelet-Rich Fibrin |
| LP-PRP | Leukocyte-Poor Platelet-Rich Plasma |
| LR-PRP | Leukocyte-Rich Platelet-Rich Plasma |
| PFC | Platelet-derived Factor Concentrate |
| P-PRP | Pure Platelet Rich Plasma |
| PFS | Platelet Fibrin Sealant |
| PLG | Platelet-Leukocyte Gel |
| PRF | Platelet-Rich Fibrin |
| PRFM | Platelet-Rich Fibrin Matrix |
| PRGF | Preparation Rich in Growth Factors |
| Parameters | Differentials | Options |
|---|---|---|
| Biological Product Allocation | Autologous Allogeneic | Buffy Coat Partial Buffy Coat Fresh Frozen/Thawed Platelet Lysate Umbilical cord blood |
| Preparation Technology | Gravitational Centrifugation Blood Salvage Blood Separators Plasmapheresis | Preparation time Spin-Cycles G-Forces |
| Anticoagulation | ACD-A EDTA SC Heparin | |
| Platelet dosing | Concentration ranges | 0–500 × 106/mL 500–1000 × 106/mL 1000–1500 × 106/mL >1500 × 106/mL |
| Leukocytes Presence | Yes No | Neutrophils–Monocytes–Lymphocytes Poor–Poor |
| RBC | Yes No | Hematocrit (range) |
| Delivery Form | Liquid Coagulated | Partial Full |
| Fibrin Matrix | Yes No | Concentration levels Content specific |
| Activation | Yes No | CaCl Thrombin Collagen Electrical Freeze Sonication Light |
| Additives | Biodegradable Scaffolds Matrices Autologous Biologics Non-autologous | Dexamethasone—HA—cPPP—BMAC—Adipose—Bone-Exosomes—Amniotic -Wharton Jelly—A-Cell Protein Preparations— Antibiotics—Pain medication |
| Administration Routes | Topical IV Tissue structure Intraosseous | Soft tissue: Tendon—Ligament—Muscle—Scar Intradiscal—Epidural—Intrathecal—Intra-Articular |
| Pro-Angiogenetic | Anti-Angiogenetic |
|---|---|
| VEGF | TGF-β1 |
| PDGF | PAI |
| TGF-β1 | TSP |
| EGF | Angiostatin |
| Serotonin | Endostatin |
| SDF-1 | PF4 |
| Angiopoietin -1, -2 | CXCL4L |
| MMP -1, -2 | TIMPS |
| IL-8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. https://doi.org/10.3390/ijms21207794
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. International Journal of Molecular Sciences. 2020; 21(20):7794. https://doi.org/10.3390/ijms21207794
Chicago/Turabian StyleEverts, Peter, Kentaro Onishi, Prathap Jayaram, José Fábio Lana, and Kenneth Mautner. 2020. "Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020" International Journal of Molecular Sciences 21, no. 20: 7794. https://doi.org/10.3390/ijms21207794
APA StyleEverts, P., Onishi, K., Jayaram, P., Lana, J. F., & Mautner, K. (2020). Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. International Journal of Molecular Sciences, 21(20), 7794. https://doi.org/10.3390/ijms21207794







