Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration
Abstract
:1. Introduction
2. Results
2.1. Phytocannabinoid Characterization of CRCE
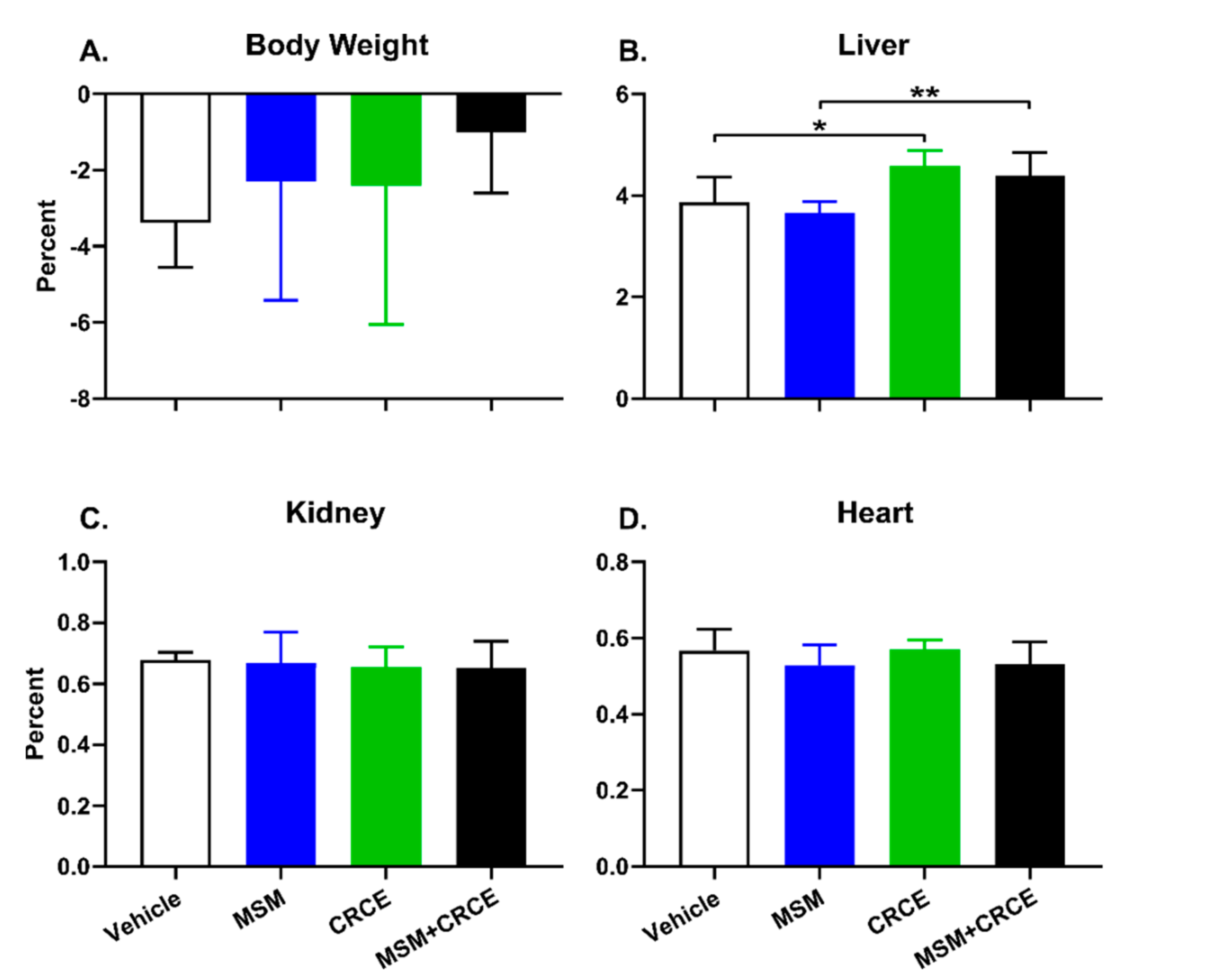
2.2. Anatomical Examination and Physiological Parameters
2.3. Histological Findings
2.4. Clinical Biochemistry
2.5. Cytochrome P450 Expression
2.6. Glutathione Measurement
3. Discussion
4. Materials and Methods
4.1. CRCE Extract Characterization, Dosing Solution, and Dose Calculations
4.2. Animals
4.3. Histopathological Assessment
4.4. Blood Sampling and Clinical Biochemistry
4.5. miR-122 Analysis
4.6. Gene Expression
4.7. Glutathione Measurement
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CBD | Cannabidiol |
| CRCE | Cannabidiol-rich cannabis extract |
| CYP | Cytochrome P450 enzymes |
| DS | Dietary supplements |
| GSH | Glutathione |
| MED | Mouse equivalent dose |
| MSM | Methylsulfonylmethane |
| NEM | N-ethylmaleimide |
| PCR | Polymerase chain reaction |
References
- Alves, P.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020, 157, 104822. [Google Scholar] [CrossRef] [PubMed]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Pina, S.D.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Überall, M.A. A Review of Scientific Evidence for THC:CBD Oromucosal Spray (Nabiximols) in the Management of Chronic Pain. J. Pain Res. 2020, 13, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, K.; Pack, A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Research 2019, 8, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Shahinas, J. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J. Clin. Med. Res. 2020, 12, 129–141. [Google Scholar] [CrossRef]
- Carvalho, R.K.; Santos, M.L.; Souza, M.R.; Rocha, T.L.; Guimarães, F.S.; Anselmo-Franci, J.A.; Mazaro-Costa, R. Chronic exposure to cannabidiol induces reproductive toxicity in male Swiss mice. J. Appl. Toxicol. 2018, 38, 1545. [Google Scholar] [CrossRef] [Green Version]
- Carty, D.R.; Thornton, C.; Gledhill, J.H.; Willett, K.L. Developmental Effects of Cannabidiol and Δ9-Tetrahydrocannabinol in Zebrafish. Toxicol. Sci. 2018, 162, 137–145. [Google Scholar] [CrossRef] [Green Version]
- ElBatsh, M.M.; Assareh, N.; Marsden, C.A.; Kendall, D.A. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology 2012, 221, 239–247. [Google Scholar] [CrossRef]
- Mato, S.; Sánchez-Gómez, M.V.; Matute, C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia 2010, 58, 1739–1747. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Rosenkrantz, H.; Fleischman, R.W.; Grant, R.J. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol. Appl. Pharmacol. 1981, 58, 118–131. [Google Scholar] [CrossRef]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef] [Green Version]
- Skinner, C.M.; Nookaew, I.; Ewing, L.E.; Wongsurawat, T.; Jenjaroenpun, P.; Quick, C.M.; Yee, E.U.; Piccolo, B.D.; ElSohly, M.; Walker, L.A.; et al. Potential Probiotic or Trigger of Gut Inflammation—The Janus-Faced Nature of Cannabidiol-Rich Cannabis Extract. J. Diet. Suppl. 2020, 17, 543–560. [Google Scholar] [CrossRef]
- Ewing, L.E.; McGill, M.R.; Yee, E.U.; Quick, C.M.; Skinner, C.M.; Kennon-McGill, S.; Clemens, M.; Vazquez, J.H.; McCullough, S.S.; Williams, D.K.; et al. Paradoxical Patterns of Sinusoidal Obstruction Syndrome-Like Liver Injury in Aged Female CD-1 Mice Triggered by Cannabidiol-Rich Cannabis Extract and Acetaminophen Co-Administration. Molecules 2019, 24, 2256. [Google Scholar] [CrossRef] [Green Version]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Qian, Y.; Gurley, B.J.; Markowitz, J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019, 39, 462–471. [Google Scholar] [CrossRef]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. Dell’Istituto Super. Sanita 2020, 56, 285–291. [Google Scholar] [CrossRef]
- Walker, L.A.; Koturbash, I.; Kingston, R.; ElSohly, M.A.; Yates, C.R.; Gurley, B.J.; Khan, I. Cannabidiol (CBD) in Dietary Supplements: Perspectives on Science, Safety, and Potential Regulatory Approaches. J. Diet. Suppl. 2020, 17, 493–502. [Google Scholar] [CrossRef]
- Laux, L.C.; Bebin, E.M.; Checketts, D.; Chez, M.; Flamini, R.; Marsh, E.D.; Miller, I.; Nichol, K.; Park, Y.; Segal, E.; et al. Long-term safety and efficacy of cannabidiol in children and adults with treatmentresistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res. 2019, 154, 13–20. [Google Scholar] [CrossRef]
- Gurley, B.J.; Yates, C.R.; Markowitz, J.S. “…Not Intended to Diagnose, Treat, Cure or Prevent Any Disease.” 25 Years of Botanical Dietary Supplement Research and the Lessons Learned. Clin. Pharmacol. Ther. 2018, 104, 470–483. [Google Scholar] [CrossRef]
- Turton-Weeks, S.M.; Barone, G.W.; Gurley, B.J.; Ketel, B.L.; Lightfoot, M.L.; Abul-Ezz, S.R. St John’s wort: A hidden risk for transplant patients. Prog. Transplant. 2001, 11, 116–120. [Google Scholar] [CrossRef]
- Barone, G.W.; Gurley, B.J.; Ketel, B.L.; Lightfoot, M.L.; Abul-Ezz, S.R. Drug interaction between St. John’s wort and cyclosporine. Ann. Pharmacother. 2000, 34, 1013–1016. [Google Scholar] [CrossRef]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and safety of a novel dietary supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef]
- Herschler, R.J. Dietary and Pharmaceutical Uses of Methyl-Sulfonylmethane and Compositions Comprising it. U.S. Patent 4514421, 1982. [Google Scholar]
- Herschler, R.J. Methylsulfonylmethane in Dietary Products. U.S. Patent 4616039, 1985. [Google Scholar]
- Herschler, R.J. Dietary Products and Uses Comprising Methylsulfonylmethane. U.S. Patent 4863748, 1986. [Google Scholar]
- Herschler, R.J. Solid Pharmaceutical Compositions Comprising MSM and their Production. U.S. Patent 4568547, 1984. [Google Scholar]
- Debbi, E.M.; Agar, G.; Fichman, G.; Ziv, Y.B.; Kardosh, R.; Halperin, N.; Elbaz, A.; Beer, Y.; Debi, R. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: A randomized controlled study. BMC Complement. Altern. Med. 2011, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.S.; Axelrod, L.J.; Howard, P.; Buratovich, N.; Waters, R.F. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: A pilot clinical trial. Osteoarthr. Cartil. 2006, 14, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.; Bloomer, R.J.; Benjamin, R.L.; Buddington, R.K. Small Intestinal Absorption of Methylsulfonylmethane (MSM) and Accumulation of the Sulfur Moiety in Selected Tissues of Mice. Nutrients 2017, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Gurley, B.J.; Murphy, T.P.; Gul, W.; Walker, L.A.; ElSohly, M. Content versus Label Claims in Cannabidiol (CBD)-Containing Products Obtained from Commercial Outlets in the State of Mississippi. J. Diet. Suppl. 2020, 17, 599–607. [Google Scholar] [CrossRef]
- Kim, S.H.; Smith, A.J.; Sanberg, P.R.; Tan, J.; Shytle, R.D.; Giunta, B. MSM ameliorates HIV-1 tat induced neuronal oxidative stress via rebalance of the glutathione cycle. Am. J. Transl. Res. 2015, 7, 328–338. [Google Scholar]
- Borzellca, J.F.; Sipes, I.G.; Wallace, K.B. Dossier in Support of the Generally Recognized as Safe (GRAS) Status of Optimsm (Methylsulfonylmethane; MSM) as a Food Ingredient; Food and Drug Administration: Vero Beach, FL, USA, 2007. [Google Scholar]
- Maronpot, R.R.; Yoshizawa, K.; Nyska, A.; Harada, T.; Flake, G.; Mueller, G.; Singh, B.; Ward, J.M. Hepatic enzyme induction: Histopathology. Toxicol. Pathol. 2010, 38, 776–795. [Google Scholar] [CrossRef] [PubMed]
- Giraud, C.; Tran, A.; Rey, E.; Vincent, J.; Tréluyer, J.M.; Pons, G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: Importance of CYP2C19. Drug Metab. Dispos. 2004, 32, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huddart, R.; Leeder, J.S.; Altman, R.B.; Klein, T.E. PharmGKB summary: Clobazam pathway, pharmacokinetics. Pharmacogenet. Genomics 2018, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.A.; Appleton, J.; Ames, G.B. Pharmacokinetics and distribution of [35S]methylsulfonylmethane following oral administration to rats. J. Agric. Food Chem. 2007, 55, 1033–1038. [Google Scholar] [CrossRef]
- Gerhards, E.; Gibian, H. The metabolism of dimethyl sulfoxide and its metabolic effects in man and animals. Ann. N. Y. Acad. Sci. 1967, 141, 65–76. [Google Scholar] [CrossRef]
- Otsuki, S.; Qian, W.; Ishihara, A.; Kabe, T. Elucidation of dimethylsulfone metabolism in rat using a 35S radioisotope tracer method. Nutr. Res. 2002, 22, 313–322. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Q.; Zhao, L.; Zhang, Q.; Wu, Y.; Hu, H.; Liu, L.; Liu, X.; Zhu, Y.; Guo, A.; et al. Time serial transcriptome reveals Cyp2c29 as a key gene in hepatocellular carcinoma development. Cancer Biol. Med. 2020, 17, 401–417. [Google Scholar] [CrossRef]
- Joung, Y.H.; Darvin, P.; Kang, D.Y.; Sp, N.; Byun, H.J.; Lee, C.-H.; Lee, H.K.; Yang, Y.M. Methylsulfonylmethane Inhibits RANKL-Induced Osteoclastogenesis in BMMs by Suppressing NF-κB and STAT3 Activities. PLoS ONE 2016, 11, e0159891. [Google Scholar] [CrossRef] [Green Version]
- Amirshahrokhi, K.; Khalili, A.-R. Methylsulfonylmethane is effective against gastric mucosal injury. Eur. J. Pharmacol. 2017, 811, 240–248. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Bohlooli, S. Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice. Inflammation 2013, 36, 1111–1121. [Google Scholar] [CrossRef]
- Bohlooli, S.; Mohammadi, S.; Amirshahrokhi, K.; Mirzanejad-Asl, H.; Yosefi, M.; Mohammadi-Nei, A.; Chinifroush, M.M. Effect of Methylsulfonylmethane Pretreatment on Aceta-minophen Induced Hepatotoxicity in Rats. Iran. J. Basic Med. Sci. 2013, 16, 896–900. [Google Scholar] [PubMed]
- Pecora, F.; Gualeni, B.; Forlino, A.; Superti-Furga, A.; Tenni, R.; Cetta, G.; Rossi, A. In vivo contribution of amino acid sulfur to cartilage proteoglycan sulfation. Biochem. J. 2006, 398, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, R.; El Morsy, E.M. Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats. Arch. Pharm. Res. 2013, 36, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Wojcikowski, K.; Gobe, G. Animal studies on medicinal herbs: Predictability, dose conversion and potential value. Phytother. Res. 2014, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 23 September 2020).
- Sousa-Lima, I.; Park, S.-Y.; Chung, M.; Jung, H.J.; Kang, M.-C.; Gaspar, J.M.; Seo, J.A.; Macedo, M.P.; Park, K.S.; Mantzoros, C.; et al. Methylsulfonylmethane (MSM), an organosulfur compound, is effective against obesity-induced metabolic disorders in mice. Metabolism 2016, 65, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Skinner, C.M.; Lin, H.; Ewing, L.E.; Kosanke, S.D.; Williams, D.K.; Avula, B.; Khan, I.A.; ElSohly, M.A.; Gurley, B.J.; et al. Safety assessment of the dietary supplement OxyELITETM Pro (New Formula) in inbred and outbred mouse strains. Food Chem. Toxicol. 2017, 109, 194–209. [Google Scholar] [CrossRef]
- Mcgill, M.R.; Jaeschke, H. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol. Mech. Methods 2015, 25, 589–595. [Google Scholar] [CrossRef] [Green Version]




| Phytoconstituent | Levels, % |
|---|---|
| Cannabidiol | 57.9 |
| Cannabichromene | 2.03 |
| Δ9-tetrahydrocannabinol | 1.69 |
| Cannabigerol | 1.07 |
| Δ8-tetrahydrocannabinol | <0.01 |
| Tetrahydrocannabivarin | <0.01 |
| Residual Solvent | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutanzi, K.R.; Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration. Int. J. Mol. Sci. 2020, 21, 7808. https://doi.org/10.3390/ijms21207808
Kutanzi KR, Ewing LE, Skinner CM, Quick CM, Kennon-McGill S, McGill MR, Walker LA, ElSohly MA, Gurley BJ, Koturbash I. Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration. International Journal of Molecular Sciences. 2020; 21(20):7808. https://doi.org/10.3390/ijms21207808
Chicago/Turabian StyleKutanzi, Kristy R., Laura E. Ewing, Charles M. Skinner, Charles M. Quick, Stefanie Kennon-McGill, Mitchell R. McGill, Larry A. Walker, Mahmoud A. ElSohly, Bill J. Gurley, and Igor Koturbash. 2020. "Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration" International Journal of Molecular Sciences 21, no. 20: 7808. https://doi.org/10.3390/ijms21207808
APA StyleKutanzi, K. R., Ewing, L. E., Skinner, C. M., Quick, C. M., Kennon-McGill, S., McGill, M. R., Walker, L. A., ElSohly, M. A., Gurley, B. J., & Koturbash, I. (2020). Safety and Molecular-Toxicological Implications of Cannabidiol-Rich Cannabis Extract and Methylsulfonylmethane Co-Administration. International Journal of Molecular Sciences, 21(20), 7808. https://doi.org/10.3390/ijms21207808






