Doxorubicin Improves Cancer Cell Targeting by Filamentous Phage Gene Delivery Vectors
Abstract
:1. Introduction
2. Results
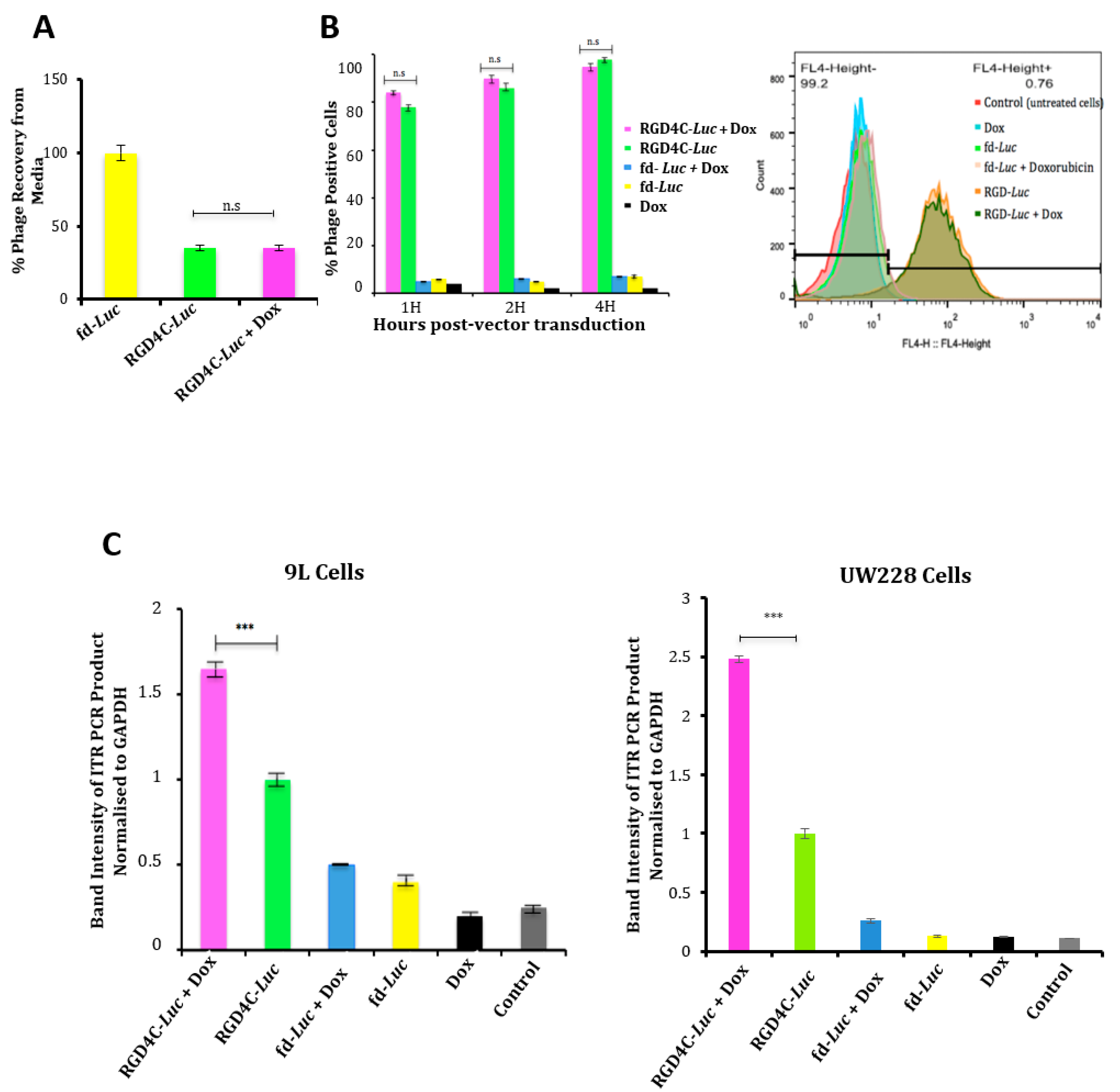
2.1. Doxorubicin Increases Targeted Gene Transfer by RGD4C/AAVP in Rat Glioma (9L) and Human Melanoma (M21) Cancer Cells In Vitro
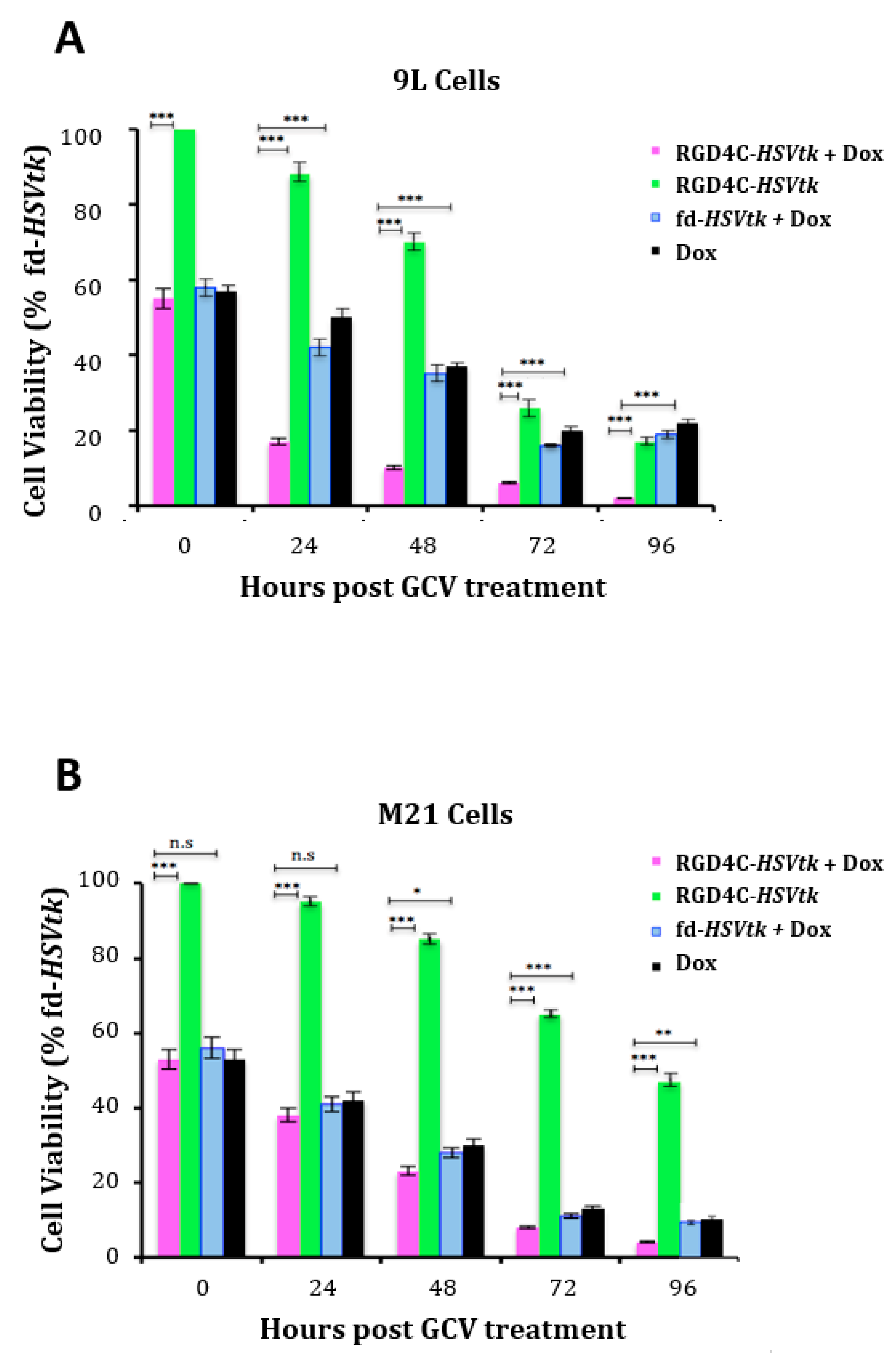
2.2. Doxorubicin Drug Treatment Boosts Cancer Cell Death by AAVP-Mediated Suicide Gene Killing
2.3. Evaluation of the Effect of Doxorubicin Treatment on Vector Cellular Trafficking
2.4. Evaluation of Efficacy of Doxorubicin and RGD4C/AAVP Combination in a Three-Dimensional (3D) Multicellular Tumor Spheroid Model
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Production, Purification and Titration of AAVP Vectors
4.3. In Vitro Cell Transduction by AAVP Vectors
4.4. Reporter Gene Assays
4.5. Determination of Tumor Cell Killing In Vitro
4.6. D Model of Multicellular Tumor Spheroid Culture and Treatment
4.7. Attachment Assay
4.8. Internalization Assay
4.9. Nuclei Extraction
4.10. Semi-Quantitative PCR Analysis
4.11. Comet Assay
4.12. Immunofluorescence Staining for Lamin B1
4.13. Western Blot for Lamin B1
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2007—An update. J. Gene Med. 2007, 9, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.D.; Nguyen, T.V.; Allen, K.L.; Ayasoufi, K.; Barry, M.A. Comparison of gene delivery to the kidney by adenovirus, Adeno-associated virus, and Lentiviral vectors after intravenous and direct kidney injections. Human Gene Ther. 2019, 30, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Monteilhet, V.; Saheb, S.; Boutin, S.; Leborgne, C.; Veron, P.; Montus, M.F.; Moullier, P.; Benveniste, O.; Masurier, C. A 10 patient case report on the impact of Plasmapheresis upon neutralizing factors against Adeno-Associated Virus (AAV) types 1, 2, 6, and 8. Mol. Ther. 2011, 19, 2084–2091. [Google Scholar] [CrossRef] [Green Version]
- Huh, H.; Wong, S.; St Jean, J.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Hajitou, A.; Trepel, M.; Lilley, C.E.; Soghomonyan, S.; Alauddin, M.M.; Marini, F.C.; Restel, B.H.; Ozawa, M.G.; Moya, C.A.; Rangel, R.; et al. A hybrid vector for ligand-directed Tumor targeting and molecular imaging. Cell 2006, 125, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Hajitou, A.; Rangel, R.; Trepel, M.; Soghomonyan, S.; Gelovani, J.G.; Alauddin, M.M.; Pasqualini, R.; Arap, W. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat. Protoc. 2007, 2, 523–531. [Google Scholar] [CrossRef]
- Hajitou, A. Targeted systemic gene therapy and molecular imaging of cancer contribution of the vascular-targeted AAVP vector. Adv. Genet. 2010, 69, 65–82. [Google Scholar]
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M.; et al. Efficacy of systemic Temozolomide-activated phage-targeted gene therapy in human Glioblastoma. EMBO Mol. Med. 2019, 11. [Google Scholar] [CrossRef]
- Suwan, K.; Yata, T.; Waramit, S.; Przystal, J.M.; Stoneham, C.A.; Bentayebi, K.; Asavarut, P.; Chongchai, A.; Pothachareon, P.; Lee, K.Y.; et al. Next-generation of targeted AAVP vectors for systemic transgene delivery against cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 18571–18577. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Syrkin, G.; Adem, A.; Geha, R.; Pastoriza, J.; Vrikshajanani, C.; Smith, T.; Quinn, T.J.; Alemu, G.; Cho, H.; et al. Blockade of Inhibitors of Apoptosis (IAPs) in combination with Tumor-targeted delivery of Tumor necrosis factor-alpha leads to synergistic antitumor activity. Cancer Gene Ther. 2013, 20, 46–56. [Google Scholar] [CrossRef]
- Hajitou, A.; Lev, D.C.; Hannay, J.A.; Korchin, B.; Staquicini, F.I.; Soghomonyan, S.; Alauddin, M.M.; Benjamin, R.S.; Pollock, R.E.; Gelovani, J.G.; et al. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 4471–4476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trepel, M.; Stoneham, C.A.; Eleftherohorinou, H.; Mazarakis, N.D.; Pasqualini, R.; Arap, W.; Hajitou, A. A heterotypic bystander effect for Tumor cell killing after Adeno-associated virus/phage-mediated, vascular-targeted suicide gene transfer. Mol. Cancer. Ther. 2009, 8, 2383–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandle, A.; Hanna, E.; Lorang, D.; Hajitou, A.; Moya, C.A.; Pasqualini, R.; Arap, W.; Adem, A.; Starker, E.; Hewitt, S.; et al. Tumor vasculature-targeted delivery of Tumor necrosis factor-alpha. Cancer 2009, 115, 128–139. [Google Scholar] [CrossRef]
- Dobroff, A.S.; D’Angelo, S.; Eckhardt, B.L.; Ferrara, F.; Staquicini, D.I.; Cardo-Vila, M.; Staquicini, F.I.; Nunes, D.N.; Kim, K.; Driessen, W.H.P.; et al. Towards a Transcriptome-based Theranostic platform for Unfavorable breast cancer phenotypes. Proc. Natl. Acad. Sci. USA 2016, 113, 12780–12785. [Google Scholar] [CrossRef] [Green Version]
- Kia, A.; Przystal, J.M.; Nianiaris, N.; Mazarakis, N.D.; Mintz, P.J.; Hajitou, A. Dual systemic Tumor targeting with ligand-directed phage and Grp78 promoter induces Tumor regression. Mol. Cancer Ther. 2012, 11, 2566–2577. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.L.; Yuan, Z.; Cardo-Vila, M.; Sanchez Claros, C.; Adem, A.; Cui, M.H.; Branch, C.A.; Gelovani, J.G.; Libutti, S.K.; Sidman, R.L.; et al. AAVP displaying Octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine Tumors of the pancreas. Proc. Natl. Acad. Sci. USA 2016, 113, 2466–2471. [Google Scholar] [CrossRef] [Green Version]
- Staquicini, F.I.; Ozawa, M.G.; Moya, C.A.; Driessen, W.H.; Barbu, E.M.; Nishimori, H.; Soghomonyan, S.; Flores, L.G.; Liang, X.; Paolillo, V.; et al. Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting Tumors in a mouse model of human Glioblastoma. J. Clin. Investig. 2011, 121, 161–173. [Google Scholar] [CrossRef]
- Staquicini, F.I.; Smith, T.L.; Tang, F.H.F.; Gelovani, J.G.; Giordano, R.J.; Libutti, S.K.; Sidman, R.L.; Cavenee, W.K.; Arap, W.; Pasqualini, R. Targeted AAVP-based therapy in a mouse model of human Glioblastoma: A comparison of cytotoxic versus suicide gene delivery strategies. Cancer Gene Ther. 2019, 27, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, F.; Staquicini, D.I.; Driessen, W.H.P.; D’Angelo, S.; Dobroff, A.S.; Barry, M.; Lomo, L.C.; Staquicini, F.I.; Cardo-Vila, M.; Soghomonyan, S.; et al. Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 12786–12791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoloni, M.C.; Tandle, A.; Mazcko, C.; Hanna, E.; Kachala, S.; Leblanc, A.; Newman, S.; Vail, D.; Henry, C.; Thamm, D.; et al. Launching a novel preclinical infrastructure: Comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PLoS ONE 2009, 4, e4972. [Google Scholar] [CrossRef]
- Yata, T.; Lee, K.Y.; Dharakul, T.; Songsivilai, S.; Bismarck, A.; Mintz, P.J.; Hajitou, A. Hybrid nanomaterial complexes for advanced phage-guided gene delivery. Mol. Ther. Nucleic Acids 2014, 3, e185. [Google Scholar] [CrossRef]
- Yata, T.; Lee, E.L.; Suwan, K.; Syed, N.; Asavarut, P.; Hajitou, A. Modulation of extracellular matrix in cancer is associated with enhanced Tumor cell targeting by bacteriophage vectors. Mol. Cancer 2015, 14, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Stoneham, C.A.; Hollinshead, M.; Hajitou, A. Clathrin-mediated endocytosis and subsequent Endo-Lysosomal trafficking of Adeno-associated virus/phage. J. Biol. Chem. 2012, 287, 35849–35859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przystal, J.M.; Umukoro, E.; Stoneham, C.A.; Yata, T.; O’Neill, K.; Syed, N.; Hajitou, A. Proteasome inhibition in cancer is associated with enhanced Tumor targeting by the Adeno-associated virus/phage. Mol. Oncol. 2013, 7, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Suwan, K.; Waramit, S.; Aboagye, E.O.; Hajitou, A. Selective inhibition of histone Deacetylation in melanoma increases targeted gene delivery by a bacteriophage viral vector. Cancers (Basel) 2018, 10, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, F.; Cai, H.; Wu, Y.; He, G.; Tan, W.S. The efficacy of combination therapy using Adeno-associated virus-TRAIL targeting to telomerase activity and Cisplatin in a mice model of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2010, 136, 1827–1837. [Google Scholar] [CrossRef]
- Streck, C.J.; Dickson, P.V.; Ng, C.Y.; Zhou, J.; Gray, J.T.; Nathwani, A.C.; Davidoff, A.M. Adeno-associated virus vector-mediated systemic delivery of IFN-beta combined with low-dose cyclophosphamide affects Tumor regression in murine Neuroblastoma models. Clin. Cancer Res. 2005, 11, 6020–6029. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, J.; Ding, W.; Wang, X. Doxorubicin augments rAAV-2 transduction in rat neuronal cells. Neurochem. Int. 2009, 55, 521–528. [Google Scholar] [CrossRef]
- Black, M.E.; Kokoris, M.S.; Sabo, P. Herpes simplex virus-1 thymidine kinase mutants created by semi-random sequence mutagenesis improve Prodrug-mediated Tumor cell killing. Cancer Res. 2001, 61, 3022–3026. [Google Scholar]
- Hamel, W.; Magnelli, L.; Chiarugi, V.P.; Israel, M.A. Herpes simplex virus thymidine kinase/Ganciclovir-mediated apoptotic death of bystander cells. Cancer Res. 1996, 56, 2697–2702. [Google Scholar]
- Nishikawa, M.; Huang, L. Nonviral vectors in the new millennium: Delivery barriers in gene transfer. Human Gene Ther. 2001, 12, 861–870. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Middaugh, C.R. Barriers to Nonviral gene delivery. J. Pharm. Sci. 2003, 92, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Fasulo, B.; Koyama, C.; Yu, K.R.; Homola, E.M.; Hsieh, T.S.; Campbell, S.D.; Sullivan, W. Chk1 and Wee1 kinases coordinate DNA replication, chromosome condensation, and anaphase entry. Mol. Biol. Cell 2012, 23, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Tsafa, E.; Al-Bahrani, M.; Bentayebi, K.; Przystal, J.; Suwan, K.; Hajitou, A. The natural dietary Genistein boosts bacteriophage-mediated cancer cell killing by improving phage-targeted Tumor cell transduction. Oncotarget 2016, 7, 52135–52149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurnhammer, C.; Haase, M.; Muether, N.; Hausl, M.; Rauschhuber, C.; Huber, I.; Nitschko, H.; Busch, U.; Sing, A.; Ehrhardt, A.; et al. Universal real-time PCR for the detection and quantification of Adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Human Gene Ther. Methods 2012, 23, 18–28. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Singh, R.P.; Agarwal, C.; Chan, D.C.; Agarwal, R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res. 2002, 8, 3512–3519. [Google Scholar]
- Stravopodis, D.J.; Karkoulis, P.K.; Konstantakou, E.G.; Melachroinou, S.; Lampidonis, A.D.; Anastasiou, D.; Kachrilas, S.; Messini-Nikolaki, N.; Papassideri, I.S.; Aravantinos, G.; et al. Grade-dependent effects on cell cycle progression and apoptosis in response to doxorubicin in human bladder cancer cell lines. Int. J. Oncol. 2009, 34, 137–160. [Google Scholar]
- Duarte, S.; Carle, G.; Faneca, H.; de Lima, M.C.; Pierrefite-Carle, V. Suicide gene therapy in cancer: Where do we stand now? Cancer Lett. 2012, 324, 160–170. [Google Scholar] [CrossRef]
- Yan, Z.; Zak, R.; Zhang, Y.; Ding, W.; Godwin, S.; Munson, K.; Peluso, R.; Engelhardt, J.F. Distinct classes of proteasome-modulating agents cooperatively augment recombinant Adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J. Virol. 2004, 78, 2863–2874. [Google Scholar] [CrossRef] [Green Version]
- Beaudouin, J.; Gerlich, D.; Daigle, N.; Eils, R.; Ellenberg, J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 2002, 108, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Machowska, M.; Piekarowicz, K.; Rzepecki, R. Regulation of Lamin properties and functions: Does phosphorylation do it all? Open Biol. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechat, T.; Adam, S.A.; Taimen, P.; Shimi, T.; Goldman, R.D. Nuclear lamins. Cold Spring Harb. Perspect. Biol. 2010, 2, a000547. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, F.K.; Samulski, T.; Shenk, T.; Samulski, R.J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant Adeno-associated virus vectors. J. Virol. 1996, 70, 3227–3234. [Google Scholar] [CrossRef] [Green Version]
- Yalkinoglu, A.O.; Heilbronn, R.; Burkle, A.; Schlehofer, J.R.; zur Hausen, H. DNA amplification of Adeno-associated virus as a response to cellular Genotoxic stress. Cancer Res. 1988, 48, 3123–3129. [Google Scholar]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Peterse, J.L.; van‘t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Barber, B.; Farr, A.M.; Ivanov, B.; Novich, M. Overall survival in patients with metastatic melanoma. Curr. Med. Res. Opin. 2015, 31, 987–991. [Google Scholar] [CrossRef]
- Citron, M.L. Dose-dense chemotherapy: Principles, clinical results and future perspectives. Breast Care (Basel) 2008, 3, 251–255. [Google Scholar] [CrossRef]
- Ferguson, J.E.; Dodwell, D.J.; Seymour, A.M.; Richards, M.A.; Howell, A. High dose, dose-intensive chemotherapy with doxorubicin and cyclophosphamide for the treatment of advanced breast cancer. Br. J. Cancer 1993, 67, 825–829. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C. Phage therapy’s latest makeover. Nat. Biotechnol. 2019, 37, 581–586. [Google Scholar] [CrossRef]
- Lang, L.H. FDA approves use of bacteriophages to be added to meat and poultry products. Gastroenterology 2006, 131, 1370. [Google Scholar] [CrossRef] [PubMed]
- Krag, D.N.; Shukla, G.S.; Shen, G.P.; Pero, S.; Ashikaga, T.; Fuller, S.; Weaver, D.L.; Burdette-Radoux, S.; Thomas, C. Selection of Tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 2006, 66, 7724–7733. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafa, E.; Bentayebi, K.; Topanurak, S.; Yata, T.; Przystal, J.; Fongmoon, D.; Hajji, N.; Waramit, S.; Suwan, K.; Hajitou, A. Doxorubicin Improves Cancer Cell Targeting by Filamentous Phage Gene Delivery Vectors. Int. J. Mol. Sci. 2020, 21, 7867. https://doi.org/10.3390/ijms21217867
Tsafa E, Bentayebi K, Topanurak S, Yata T, Przystal J, Fongmoon D, Hajji N, Waramit S, Suwan K, Hajitou A. Doxorubicin Improves Cancer Cell Targeting by Filamentous Phage Gene Delivery Vectors. International Journal of Molecular Sciences. 2020; 21(21):7867. https://doi.org/10.3390/ijms21217867
Chicago/Turabian StyleTsafa, Effrosyni, Kaoutar Bentayebi, Supachai Topanurak, Teerapong Yata, Justyna Przystal, Duriya Fongmoon, Nabil Hajji, Sajee Waramit, Keittisak Suwan, and Amin Hajitou. 2020. "Doxorubicin Improves Cancer Cell Targeting by Filamentous Phage Gene Delivery Vectors" International Journal of Molecular Sciences 21, no. 21: 7867. https://doi.org/10.3390/ijms21217867





