Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases
Abstract
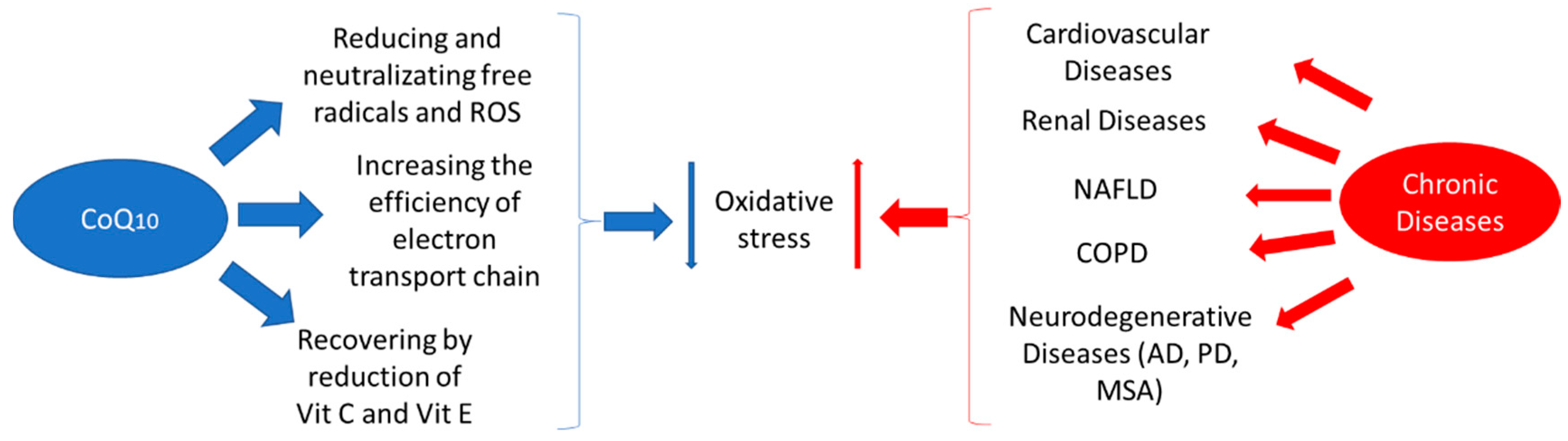
1. Introduction
2. Biology of CoQ10
3. Methodology of Review
4. CoQ10 and Cardiovascular Risk Factors
4.1. Dyslipidaemias
4.2. Hypertension
4.3. Endothelial Dysfunction
5. CoQ10 and Cardiovascular Diseases
5.1. Heart Failure and Coronary Heart Disease
5.2. Myocardial Infarction
6. CoQ10 and Chronic Kidney Disease
7. CoQ10 and Chronic Obstructive Pulmonary Diseases
8. CoQ10 and Non-alcoholic Fatty Liver Disease
9. CoQ10 and Neurodegenerative Diseases or Neural Diseases
9.1. Alzheimer’s Disease
9.2. Parkinson’s Disease
9.3. Multiple System Atrophy
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Aspartate aminotransferase |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| CoQ10 | Coenzyme Q10 |
| CRP | C-reactive protein |
| eNOS | Endothelial nitric oxide synthase |
| EPCs | Endothelial progenitor cells |
| ESRD | End-stage renal disease |
| FMD | Flow-mediated dilation |
| GGT | Gamma-glutamyl transpeptidase |
| HF | Heart failure |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| hs-CRP | High-sensitivity C-reactive protein |
| Lp(a) | Lipoprotein (a) |
| MACE | Major adverse cardiac events |
| MitoQ | Mitochondrial-targeting antioxidant |
| MSA | Multiple system atrophy |
| NAFLD | Non-alcoholic fatty liver disease |
| NFκB | Nuclear factor kappa B |
| NMD | Nitroglycerin-mediated dilation |
| PPAR-γ | Peroxisome proliferator-activated receptor-gamma |
| RCTs | Randomized clinical trials |
| ROS | Reactive oxygen species |
| SQOR | Sulfide:quinone oxidorreductase |
| TAC | Total antioxidant capacity |
| TG | Triglyceride |
References
- Festenstein, G.N.; Heaton, F.W.; Lowe, J.S.; Morton, R.A. A constituent of the unsaponifiable portion of animal tissue lipids (lambda max. 272 m mu). Biochem. J. 1955, 59, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Crane, F.; Hatefi, Y.; Lester, R.; Widmer, C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta Bioenerg. 1957, 25, 220–221. [Google Scholar] [CrossRef]
- Lenaz, G.; Fato, R.; Di Bernardo, S.; Jarreta, D.; Costa, A.; Genova, M.L.; Castelli, G.P. Localization and mobility of coenzyme Q in lipid bilayers and membranes. BioFactors 1999, 9, 87–93. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Barroso, M.P.; Martin, S.F.; Fernández-Ayala, D.J.M.; Gómez-Díaz, C.; Villalba, J.M.; Navas, P. Role of plasma membrane coenzyme Q on the regulation of apoptosis. BioFactors 1999, 9, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Yubero-Serrano, E.M.; Villalba, J.M.; Lopez-Miranda, J. Coenzyme Q10: From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2018, 59, 2240–2257. [Google Scholar] [CrossRef] [PubMed]
- Munier-Lehmann, H.; Lucas-Hourani, M.; Guillou, S.; Helynck, O.; Zanghi, G.; Noel, A.; Tangy, F.; Vidalain, P.-O.; Janin, Y.L. Original 2-(3-Alkoxy-1H-pyrazol-1-yl) pyrimidine Derivatives as Inhibitors of Human Dihydroorotate Dehydrogenase (DHODH). J. Med. Chem. 2015, 58, 860–877. [Google Scholar] [CrossRef]
- Villalba, J.M.; Navas, P. Plasma Membrane Redox System in the Control of Stress-Induced Apoptosis. Antioxid. Redox Signal. 2000, 2, 213–230. [Google Scholar] [CrossRef]
- Watmough, N.J.; Frerman, F.E. The electron transfer flavoprotein: Ubiquinone oxidoreductases. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1910–1916. [Google Scholar] [CrossRef]
- Bentinger, M.; Tekle, M.; Dallner, G. Coenzyme Q—Biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef]
- Quinzii, C.M.; DiMauro, S.; Hirano, M. Human Coenzyme Q10 Deficiency. Neurochem. Res. 2006, 32, 723–727. [Google Scholar] [CrossRef]
- Turunen, M.; Olsson, J.; Dallner, G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta Biomembr. 2004, 1660, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.W.; Belogrudov, G.I.; Lee, J.C.; Lee, P.T.; Strahan, J.; Shepherd, J.N.; Clarke, C.F. Yeast Coq5C-Methyltransferase Is Required for Stability of Other Polypeptides Involved in Coenzyme Q Biosynthesis. J. Biol. Chem. 2003, 279, 10052–10059. [Google Scholar] [CrossRef]
- Belogrudov, G.I.; Lee, P.T.; Jonassen, T.; Hsu, A.Y.; Gin, P.; Clarke, C.F. Yeast COQ4 Encodes a Mitochondrial Protein Required for Coenzyme Q Synthesis. Arch. Biochem. Biophys. 2001, 392, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta Mol. Basis Dis. 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Coenzyme Q, oxidative stress and aging. Mitochondrion 2007, 7, S103–S111. [Google Scholar] [CrossRef]
- Rodríguez-Aguilera, J.C.; Cortés, A.B.; Fernández-Ayala, D.J.M.; Navas, P. Biochemical Assessment of Coenzyme Q10 Deficiency. J. Clin. Med. 2017, 6, 27. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Zhang, J.; Sun, N.; Duan, M.; Yan, Z.; Xia, Q. Novel Lipid-Free Nanoformulation for Improving Oral Bioavailability of Coenzyme Q10. BioMed Res. Int. 2014, 2014, 793879. [Google Scholar] [CrossRef]
- Villalba, J.M.; Parrado, C.; Santos-Gonzalez, M.; Alcain, F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Investig. Drugs 2010, 19, 535–554. [Google Scholar] [CrossRef]
- Emmanuele, V.; López, L.C.; Berardo, A.; Naini, A.; Tadesse, S.; Wen, B.; D’Agostino, E.; Solomon, M.; DiMauro, S.; Quinzii, C.; et al. Heterogeneity of Coenzyme Q10 Deficiency. Arch. Neurol. 2012, 69, 978–983. [Google Scholar] [CrossRef]
- Land, J.M.; Heales, S.J.R.; Duncan, A.J.; Hargreaves, I.P. Some Observations upon Biochemical Causes of Ataxia and a New Disease Entity Ubiquinone, CoQ10 Deficiency. Neurochem. Res. 2006, 32, 837–843. [Google Scholar] [CrossRef]
- Duncan, A.J.; Heales, S.J.; Mills, K.; Eaton, S.; Land, J.M.; Hargreaves, I.P. Determination of Coenzyme Q10 Status in Blood Mononuclear Cells, Skeletal Muscle, and Plasma by HPLC with Di-Propoxy-Coenzyme Q10 as an Internal Standard. Clin. Chem. 2005, 51, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Tabrizi, R.; Moosazadeh, M.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Chamani, M.; Kolahdooz, F.; Asemi, Z. The Effects of Coenzyme Q10 Supplementation on Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2018, 24, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendía, L.E.; Stefanutti, C.; Pirro, M. Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: A systematic review and meta-analysis. Pharmacol. Res. 2016, 105, 198–209. [Google Scholar] [CrossRef]
- Jorat, M.V.; Tabrizi, R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Heydari, S.T.; Mottaghi, R.; Asemi, Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Heal. Dis. 2018, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.A.; Langsjoen, P.H.; Szabo, S.; Patil, H.; Zelinger, A. Statin cardiomyopathy? A potential role for Co-Enzyme Q10 therapy for statin-induced changes in diastolic LV performance: Description of a clinical protocol. BioFactors 2003, 18, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Tseng, Y.-F.; Yen, C.-H.; Lin, P.-T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013, 12, 142. [Google Scholar] [CrossRef]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-Associated Myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARalpha induction in 3T3-L1 preadipocytes. Cell Signal. 2012, 24, 2329–2336. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Chen, L.-H.; Chiou, S.-H.; Chiou, G.-Y.; Chen, Y.-C.; Chou, H.-Y.; Chen, L.-K.; Chen, H.-Y.; Chiu, T.-H.; Tsai, C.-S.; et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011, 55, S227–S240. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef]
- Musini, V.M.; Tejani, A.M.; Bassett, K.; Wright, J.M. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst. Rev. 2009, 2009, CD000028. [Google Scholar] [CrossRef] [PubMed]
- Digiesi, V.; Cantini, F.; Oradei, A.; Bisi, G.; Guarino, G.; Brocchi, A.; Bellandi, F.; Mancini, M.; Littarru, G. Coenzyme Q10 in essential hypertension. Mol. Asp. Med. 1994, 15, s257–s263. [Google Scholar] [CrossRef]
- Belardinelli, R.; Tiano, L.; Littarru, G.P. Oxidative stress, endothelial function and coenzyme Q10. BioFactors 2008, 32, 129–133. [Google Scholar] [CrossRef]
- González-Guardia, L.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Perez-Jimenez, F.; Garcia-Rios, A.; Marin, C.; Camargo, A.; Delgado-Casado, N.; Roche, H.M.; Perez-Jimenez, F.; et al. Effects of the Mediterranean Diet Supplemented With Coenzyme Q10 on Metabolomic Profiles in Elderly Men and Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 70, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Gonzalez-Guardia, L.; Rangel-Zuñiga, O.; Delgado-Casado, N.; Gomez-Delgado, F.; Perez-Jimenez, F.; Garcia-Rios, A.; Caballero-Villarraso, J.; Marín, C.; Gutierrez-Mariscal, F.M.; et al. Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. AGE 2011, 35, 159–170. [Google Scholar] [CrossRef]
- Young, J.M.; Florkowski, C.M.; Molyneux, S.L.; McEwan, R.G.; Frampton, C.M.; Nicholls, M.G.; Scott, R.S.; George, P.M. A Randomized, Double-Blind, Placebo-Controlled Crossover Study of Coenzyme Q10 Therapy in Hypertensive Patients With the Metabolic Syndrome. Am. J. Hypertens. 2012, 25, 261–270. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, C.; Guo, H.; Wang, J.; Lin, S.; Li, H.; Yang, Y.; Ling, W. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J. Clin. Lipidol. 2018, 12, 417–427. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Mxinwa, V.; Mokgalaboni, K.; Nkambule, B.B.; Louw, J.; Muller, C.J.F.; Tiano, L. The impact of coenzyme Q 10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2020, 3, e00118. [Google Scholar] [CrossRef]
- Gao, L.; Mao, Q.; Cao, J.; Wang, Y.; Zhou, X.-L.; Fan, L. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 221, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Watts, G.F.; Playford, D.A.; Burke, V.; Croft, K.D. Coenzyme Q10 improves blood pressure and glycaemic control: A controlled trial in subjects with type 2 diabetes. Eur. J. Clin. Nutr. 2002, 56, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Tiano, L.; Belardinelli, R.; Carnevali, P.; Principi, F.; Seddaiu, G.; Littarru, G.P. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: A double-blind, randomized controlled study. Eur. Hear. J. 2007, 28, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Gonzalez-Guardia, L.; Rangel-Zuñiga, O.; Delgado-Lista, J.; Gutierrez-Mariscal, F.M.; Perez-Martinez, P.; Delgado-Casado, N.; Cruz-Teno, C.; Tinahones, F.J.; Villalba, J.M.; et al. Mediterranean Diet Supplemented With Coenzyme Q10 Modifies the Expression of Proinflammatory and Endoplasmic Reticulum Stress–Related Genes in Elderly Men and Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011, 67, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moreno, J.; Quintana-Navarro, G.M.; Gomez-Delgado, F.; Garcia-Rios, A.; Díaz, J.F.A.; Gomez-Delgado, F.; Camargo, A.; Perez-Martinez, P.; Tinahones, F.J.; Striker, G.E.; et al. Mediterranean Diet Supplemented With Coenzyme Q 10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 73. [Google Scholar] [CrossRef]
- Tsuneki, H.; Sekizaki, N.; Suzuki, T.; Kobayashi, S.; Wada, T.; Okamoto, T.; Kimura, I.; Sasaoka, T. Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2007, 566, 1–10. [Google Scholar] [CrossRef]
- Bozkurt, B. What Is New in Heart Failure Management in 2017? Update on ACC/AHA Heart Failure Guidelines. Curr. Cardiol. Rep. 2018, 20, 39. [Google Scholar] [CrossRef]
- Kannel, W.B. Incidence and Epidemiology of Heart Failure. Hear. Fail. Rev. 2000, 5, 167–173. [Google Scholar] [CrossRef]
- Judy, W.; Stogsdill, W.; Folkers, K. Myocardial preservation by therapy with coenzyme Q10 during heart surgery. J. Mol. Med. 1993, 71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Singh, N.; Verma, S. South Asians and Cardiovascular Risk. Circulation 2006, 113. [Google Scholar] [CrossRef]
- Hughes, K. Homocysteine, folate, vitamin B12, and cardiovascular risk in Indians, Malays, and Chinese in Singapore. J. Epidemiol. Community Heal. 2000, 54, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The Effect of Coenzyme Q 10 on Morbidity and Mortality in Chronic Heart Failure. JACC: Hear. Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sobirin, M.A.; Herry, Y.; Sofia, S.N.; Uddin, I.; Rifqi, S.; Tsutsui, H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discov. Ther. 2019, 13, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 231–264. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef]
- Ulla, A.; Mohamed, M.K.; Sikder, B.; Rahman, A.T.; Sumi, F.A.; Hossain, M.; Reza, H.M.; Rahman, G.M.S.; Alam, A. Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol. Toxicol. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Mohseni, M.; Vafa, M.R.; Hajimiresmail, S.J.; Zarrati, M.; Forushani, A.R.; Bitarafan, V.; Shidfar, F. Effects of Coenzyme Q10 Supplementation on Serum Lipoproteins, Plasma Fibrinogen, and Blood Pressure in Patients With Hyperlipidemia and Myocardial Infarction. Iran. Red Crescent Med. J. 2014, 16. [Google Scholar] [CrossRef]
- Shidfar, F.; Mohseni, M.; Miresmail, S.J.H.; Forushani, A.R.; Vafa, M.; Zarrati, M. Beneficial effects of Coenzyme Q10 supplementation on lipid profile and Intereukin-6 and Intercellular adhesion Molecule-1 reduction, preliminary results of a double-blind trial in Acute Myocardial Infarction. Int. J. Prev. Med. 2015, 6, 73. [Google Scholar] [CrossRef]
- Mirhashemi, S.M.; Najafi, V.; Raygan, F.; Asemi, Z. The effects of coenzyme Q10 supplementation on cardiometabolic markers in overweight type 2 diabetic patients with stable myocardial infarction: A randomized, double-blind, placebo-controlled trial. ARYA Atheroscler. 2016, 12, 158–165. [Google Scholar] [PubMed]
- Senior, R.; Basu, S.; Kinsey, C.; Schaeffer, S.; Lahiri, A. Carvedilol prevents remodeling in patients with left ventricular dysfunction after acute myocardial infarction. Am. Hear. J. 1999, 137, 646–652. [Google Scholar] [CrossRef]
- Anavekar, N.S.; McMurray, J.J.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Bakhshayeshkaram, M.; Lankarani, K.B.; Mirhosseini, N.; Tabrizi, R.; Akbari, M.; Dabbaghmanesh, M.H.; Asemi, Z. The Effects of Coenzyme Q10 Supplementation on Metabolic Profiles of Patients with Chronic Kidney Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2018, 24, 3710–3723. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Çwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of Oxidative Stress Markers in Chronic Kidney Disease. Kidney Blood Press. Res. 2011, 34, 12–19. [Google Scholar] [CrossRef]
- Lippa, S.; Colacicco, L.; Bondanini, F.; Callà, C.; Gozzo, M.L.; Ciccariello, M.; Angelitti, A.G. Plasma levels of coenzyme Q10, vitamin E and lipids in uremic patients on conservative therapy and hemodialysis treatment: Some possible biochemical and clinical implications. Clin. Chim. Acta 2000, 292, 81–91. [Google Scholar] [CrossRef]
- Singh, R.B.; Khanna, H.K.; Niaz, M.A. Randomized, Double-blind Placebo-controlled Trial of Coenzyme Q10 in Chronic Renal Failure: Discovery of a New Role. J. Nutr. Environ. Med. 2000, 10, 281–288. [Google Scholar] [CrossRef]
- Yeung, C.K.; Iv, F.T.B.; Claessens, A.J.; Roshanravan, B.; Linke, L.; Sundell, M.B.; Ahmad, S.; Shao, B.; Shen, D.D.; Ikizler, T.A.; et al. Coenzyme Q10 dose-escalation study in hemodialysis patients: Safety, tolerability, and effect on oxidative stress. BMC Nephrol. 2015, 16, 1–8. [Google Scholar] [CrossRef]
- Rivara, M.B.; Yeung, C.K.; Robinson-Cohen, C.; Phillips, B.R.; Ruzinski, J.; Rock, D.; Linke, L.; Shen, D.D.; Ikizler, T.A.; Himmelfarb, J. Effect of Coenzyme Q10 on Biomarkers of Oxidative Stress and Cardiac Function in Hemodialysis Patients: The CoQ10 Biomarker Trial. Am. J. Kidney Dis. 2017, 69, 389–399. [Google Scholar] [CrossRef]
- Kleiner, G.; Barca, E.; Ziosi, M.; Emmanuele, V.; Xu, Y.; Hidalgo-Gutierrez, A.; Qiao, C.; Tadesse, S.; Area-Gomez, E.; Lopez, L.C.; et al. CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3708–3722. [Google Scholar] [CrossRef] [PubMed]
- Sunnetcioglu, A.; Alp, H.H.; Ndan, B.S.; Balaharoglu, R.; Gunbatar, H. Evaluation of Oxidative Damage and Antioxidant Mechanisms in COPD, Lung Cancer, and Obstructive Sleep Apnea Syndrome. Respir. Care 2015, 61, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Hagiwara, S.-I.; Saitoh, E.; Ieki, R.; Okamura, T.; Ota, T.; Iguchi, M.; Yuasa, K.; Kodaka, T.; Koishi, T.; et al. Increased oxidative stress in patients with chronic obstructive pulmonary disease (COPD) as measured by redox status of plasma coenzyme Q10. Pathophysiology 2006, 13, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S. Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Di, A.; Mehta, D.; Malik, A.B. ROS-activated calcium signaling mechanisms regulating endothelial barrier function. Cell Calcium 2016, 60, 163–171. [Google Scholar] [CrossRef]
- De Benedetto, F.; Pastorelli, R.; Ferrario, M.; de Blasio, F.; Marinari, S.; Brunelli, L. Supplementation with Qter ((R)) and Creatine improves functional performance in COPD patients on long term oxygen therapy. Respir. Med. 2018, 142, 86–93. [Google Scholar] [CrossRef]
- Tauskela, J.S. MitoQ—A mitochondria-targeted antioxidant. IDrugs Investig. Drugs J. 2007, 10, 399. [Google Scholar]
- Chen, S.; Wang, Y.; Zhang, H.; Chen, R.; Lv, F.; Li, Z.; Jiang, T.; Lin, D.; Zhang, H.; Yang, L.; et al. The Antioxidant MitoQ Protects Against CSE-Induced Endothelial Barrier Injury and Inflammation by Inhibiting ROS and Autophagy in Human Umbilical Vein Endothelial Cells. Int. J. Biol. Sci. 2019, 15, 1440–1451. [Google Scholar] [CrossRef]
- Erickson, S.K. Nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, S412–S416. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Cho, Y.-K.; Kang, M.-S.; Yoo, T.-W.; Park, J.-H.; Kim, H.J.; Park, D.I.; Sohn, C.-I.; Jeon, W.-K.; Kim, B.-I.; et al. The association between increased alanine aminotransferase activity and metabolic factors in nonalcoholic fatty liver disease. Metabolism 2006, 55, 1604–1609. [Google Scholar] [CrossRef]
- Pessayre, D. Role of mitochondria in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2007, 22, S20–S27. [Google Scholar] [CrossRef]
- Novo, E.; Busletta, C.; Di Bonzo, L.V.; Povero, D.; Paternostro, C.; Mareschi, K.; Ferrero, I.; David, E.; Bertolani, C.; Caligiuri, A.; et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J. Hepatol. 2011, 54, 964–974. [Google Scholar] [CrossRef]
- Singal, A.K.; Jampana, S.C.; Weinman, S. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011, 31, 1432–1448. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Laredj, L.N.; Licitra, F.; Puccio, H. The molecular genetics of coenzyme Q biosynthesis in health and disease. Biochimie 2014, 100, 78–87. [Google Scholar] [CrossRef]
- Ling, W.; Chen, X.; Xue, H.; Zhang, P.; Fang, W.; Chen, X.; Ling, W. Coenzyme Q10 attenuates high-fat diet-induced non-alcoholic fatty liver disease through activation of the AMPK pathway. Food Funct. 2019, 10, 814–823. [Google Scholar] [CrossRef]
- Zahedi, H.; Eghtesadi, S.; Seifirad, S.; Rezaee, N.; Shidfar, F.; Heydari, I.; Golestan, B.; Jazayeri, S. Effects of CoQ10 Supplementation on Lipid Profiles and Glycemic Control in Patients with Type 2 Diabetes: A randomized, double blind, placebo-controlled trial. J. Diabetes Metab. Disord. 2014, 13, 81. [Google Scholar] [CrossRef]
- Pala, R.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ali, S.; Cinar, V.; Atalay, M.; Sahin, K. Coenzyme Q10 Supplementation Modulates NFkappaB and Nrf2 Pathways in Exercise Training. J. Sports Sci. Med. 2016, 15, 196–203. [Google Scholar] [PubMed]
- Farsi, F.; Mohammadshahi, M.; Alavinejad, P.; Rezazadeh, A.; Zarei, M.; Engali, K.A. Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of Systemic Inflammation, and Adipokines in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2015, 35, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Alipour, B.; Jafarvand, E.; Khoshbaten, M. Oral Coenzyme Q10 Supplementation in Patients with Nonalcoholic Fatty Liver Disease: Effects on Serum Vaspin, Chemerin, Pentraxin 3, Insulin Resistance and Oxidative Stress. Arch. Med. Res. 2014, 45, 589–595. [Google Scholar] [CrossRef]
- Young, A.J.; Johnson, S.; Steffens, D.C.; Doraiswamy, P.M. Coenzyme Q10: A Review of Its Promise as a Neuroprotectant. CNS Spectrums 2007, 12, 62–68. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Firoz, C.K.; Baeesa, S.S.; Ashraf, G.M.; Akhtar, S.; Kamal, W.; Kamal, M.A.; Tabrez, S. Synopsis on the Linkage of Alzheimer’s and Parkinson’s Disease with Chronic Diseases. CNS Neurosci. Ther. 2014, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ul Islam, B.; Khan, M.S.; Jabir, N.R.; Kamal, M.A.; Tabrez, S. Elucidating Treatment of Alzheimer’s Disease via Different Receptors. Curr. Top. Med. Chem. 2017, 17, 1400–1407. [Google Scholar] [CrossRef]
- Duberley, K.E.C.; Abramov, A.Y.; Chalasani, A.; Heales, S.J.R.; Rahman, S.; Hargreaves, I. Human neuronal coenzyme Q10 deficiency results in global loss of mitochondrial respiratory chain activity, increased mitochondrial oxidative stress and reversal of ATP synthase activity: Implications for pathogenesis and treatment. J. Inherit. Metab. Dis. 2012, 36, 63–73. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Schulz, K.L.; Rhein, V.; Gotz, J. Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol. Neurobiol. 2010, 41, 107–114. [Google Scholar] [CrossRef]
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Drose, S.; Brandt, U.; et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20057–20062. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Kanwar, A.; Vegh, C.; Marginean, A.; Elliott, A.; Guilbeault, N.; Badour, A.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10 (a Nanomicellar Water-Soluble Formulation of CoQ10) Treatment Inhibits Alzheimer-Type Behavioral and Pathological Symptoms in a Double Transgenic Mouse (TgAPEswe, PSEN1dE9) Model of Alzheimer’s Disease. J. Alzheimer Dis. 2017, 61, 221–236. [Google Scholar] [CrossRef]
- Gutierrez-Mariscal, F.M.; Yubero-Serrano, E.M.; Rangel-Zuñiga, O.A.; Marín, C.; García-Rios, A.; Perez-Jimenez, F.; Gomez-Delgado, F.; Malagón, M.M.; Tinahones, F.J.; Perez-Jimenez, F.; et al. Postprandial Activation of P53-Dependent DNA Repair Is Modified by Mediterranean Diet Supplemented With Coenzyme Q10 in Elderly Subjects. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 69, 886–893. [Google Scholar] [CrossRef]
- Vegh, C.; Pupulin, S.; Wear, D.; Culmone, L.; Huggard, R.; Ma, D.; Pandey, S. Resumption of Autophagy by Ubisol-Q10 in Presenilin-1 Mutated Fibroblasts and Transgenic AD Mice: Implications for Inhibition of Senescence and Neuroprotection. Oxidative Med. Cell. Longev. 2019, 2019, 7404815. [Google Scholar] [CrossRef]
- Komaki, A.; Faraji, N.; Komaki, A.; Shahidi, S.; Etaee, F.; Raoufi, S.; Mirzaei, F. Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer’s disease. Brain Res. Bull. 2019, 147, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [PubMed]
- Muthukumaran, K.; Leahy, S.; Harrison, K.; Sikorska, M.; Sandhu, J.K.; Cohen, J.; Keshan, C.; Lopatin, D.; Miller, H.; Borowy-Borowski, H.; et al. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: Potential for therapeutic application in Parkinson’s disease. BMC Neurosci. 2014, 15, 21. [Google Scholar] [CrossRef]
- Sikorska, M.; Lanthier, P.; Miller, H.; Beyers, M.; Sodja, C.; Zurakowski, B.; Gangaraju, S.; Pandey, S.; Sandhu, J.K. Nanomicellar formulation of coenzyme Q10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: Potential use as an adjuvant treatment in Parkinson’s disease. Neurobiol. Aging 2014, 35, 2329–2346. [Google Scholar] [CrossRef]
- Onaolapo, O.J.; Odeniyi, A.O.; Jonathan, S.O.; Samuel, M.O.; Amadiegwu, D.; Olawale, A.; Tiamiyu, A.O.; Ojo, F.O.; Yahaya, H.A.; Ayeni, O.J.; et al. An investigation of the anti-Parkinsonism potential of co-enzyme Q10 and co-enzyme Q10 /levodopa-carbidopa combination in mice. Curr. Aging Sci. 2019. [Google Scholar] [CrossRef]
- Mitsui, J.; Matsukawa, T.; Yasuda, T.; Ishiura, H.; Tsuji, S. Plasma Coenzyme Q10 Levels in Patients With Multiple System Atrophy. JAMA Neurol. 2016, 73, 977. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Yoshimoto, M.; Tsuji, S.; Takahashi, H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci. Lett. 1998, 249, 180–182. [Google Scholar] [CrossRef]
- Burn, D.J.; Jaros, E. Multiple system atrophy: Cellular and molecular pathology. Mol. Pathol. 2001, 54, 419–426. [Google Scholar] [PubMed]
- The Multiple-System Atrophy Research Collaboration Mutations in COQ2 in Familial and Sporadic Multiple-System Atrophy. N. Engl. J. Med. 2013, 369, 233–244. [CrossRef]
- Mitsui, J.; Koguchi, K.; Momose, T.; Takahashi, M.; Matsukawa, T.; Yasuda, T.; Tokushige, S.-I.; Ishiura, H.; Goto, J.; Nakazaki, S.; et al. Three-Year Follow-Up of High-Dose Ubiquinol Supplementation in a Case of Familial Multiple System Atrophy with Compound Heterozygous COQ2 Mutations. Cerebellum 2017, 16, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, F.K.; Okamoto, S.; Mitsui, J.; Sone, T.; Ishikawa, M.; Yamamoto, Y.; Kanegae, Y.; Nakatake, Y.; Imaizumi, K.; Ishiura, H.; et al. The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci. Rep. 2018, 8, 14215. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Mariscal, F.M.; Arenas-de Larriva, A.P.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020, 21, 7870. https://doi.org/10.3390/ijms21217870
Gutierrez-Mariscal FM, Arenas-de Larriva AP, Limia-Perez L, Romero-Cabrera JL, Yubero-Serrano EM, López-Miranda J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. International Journal of Molecular Sciences. 2020; 21(21):7870. https://doi.org/10.3390/ijms21217870
Chicago/Turabian StyleGutierrez-Mariscal, Francisco Miguel, Antonio Pablo Arenas-de Larriva, Laura Limia-Perez, Juan Luis Romero-Cabrera, Elena Maria Yubero-Serrano, and Jose López-Miranda. 2020. "Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases" International Journal of Molecular Sciences 21, no. 21: 7870. https://doi.org/10.3390/ijms21217870
APA StyleGutierrez-Mariscal, F. M., Arenas-de Larriva, A. P., Limia-Perez, L., Romero-Cabrera, J. L., Yubero-Serrano, E. M., & López-Miranda, J. (2020). Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. International Journal of Molecular Sciences, 21(21), 7870. https://doi.org/10.3390/ijms21217870




