Sarcopenia Induced by Chronic Liver Disease in Mice Requires the Expression of the Bile Acids Membrane Receptor TGR5
Abstract
:1. Introduction
2. Results
2.1. TGR5 Ablation Does Not Alter the Increased Plasma BA in Mice with CLD, but It Protects from the Decline in Muscle Mass
2.2. Muscle Function Declines in Mice with CLD via a Mechanism Dependent on TGR5 Expression
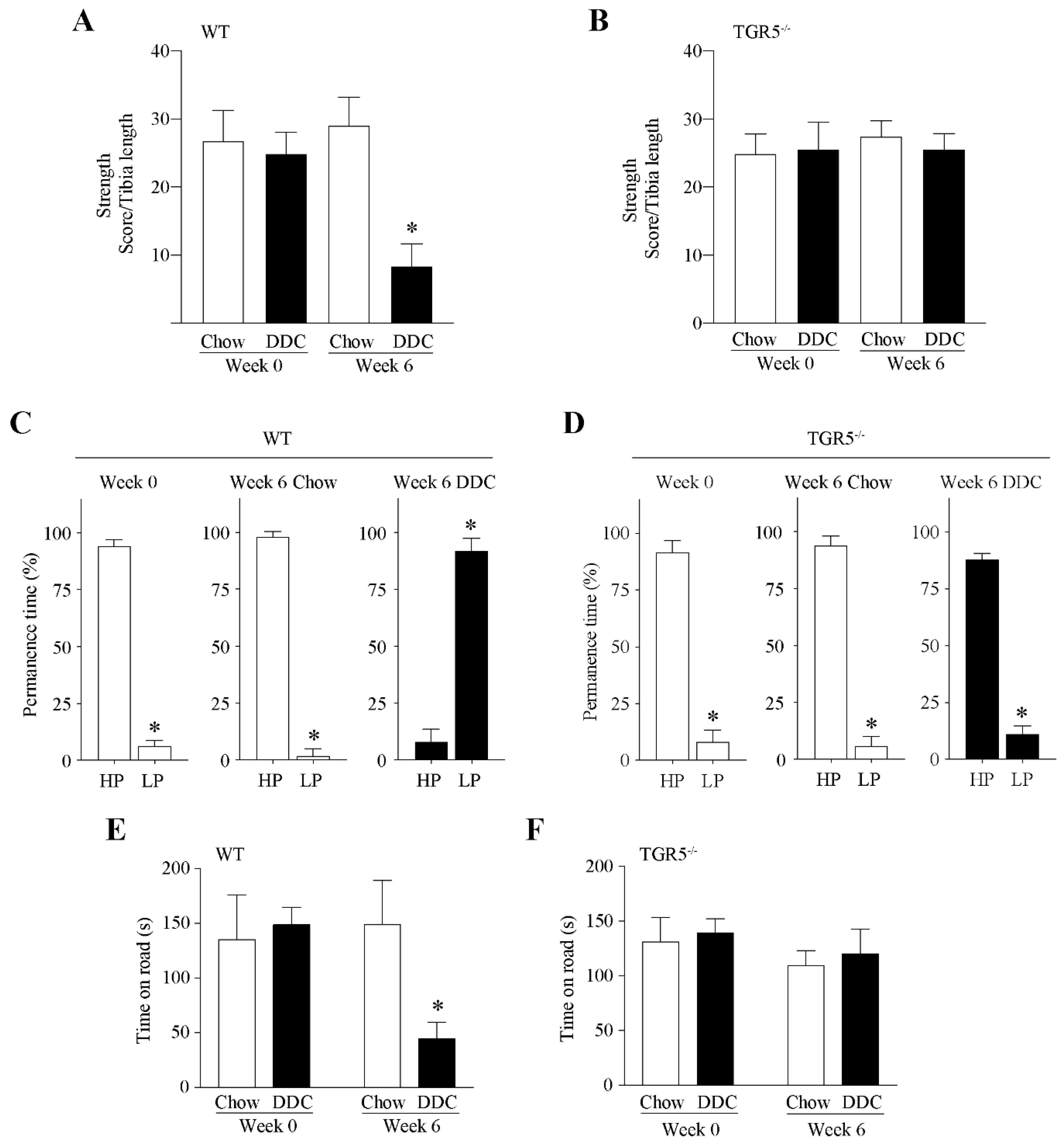
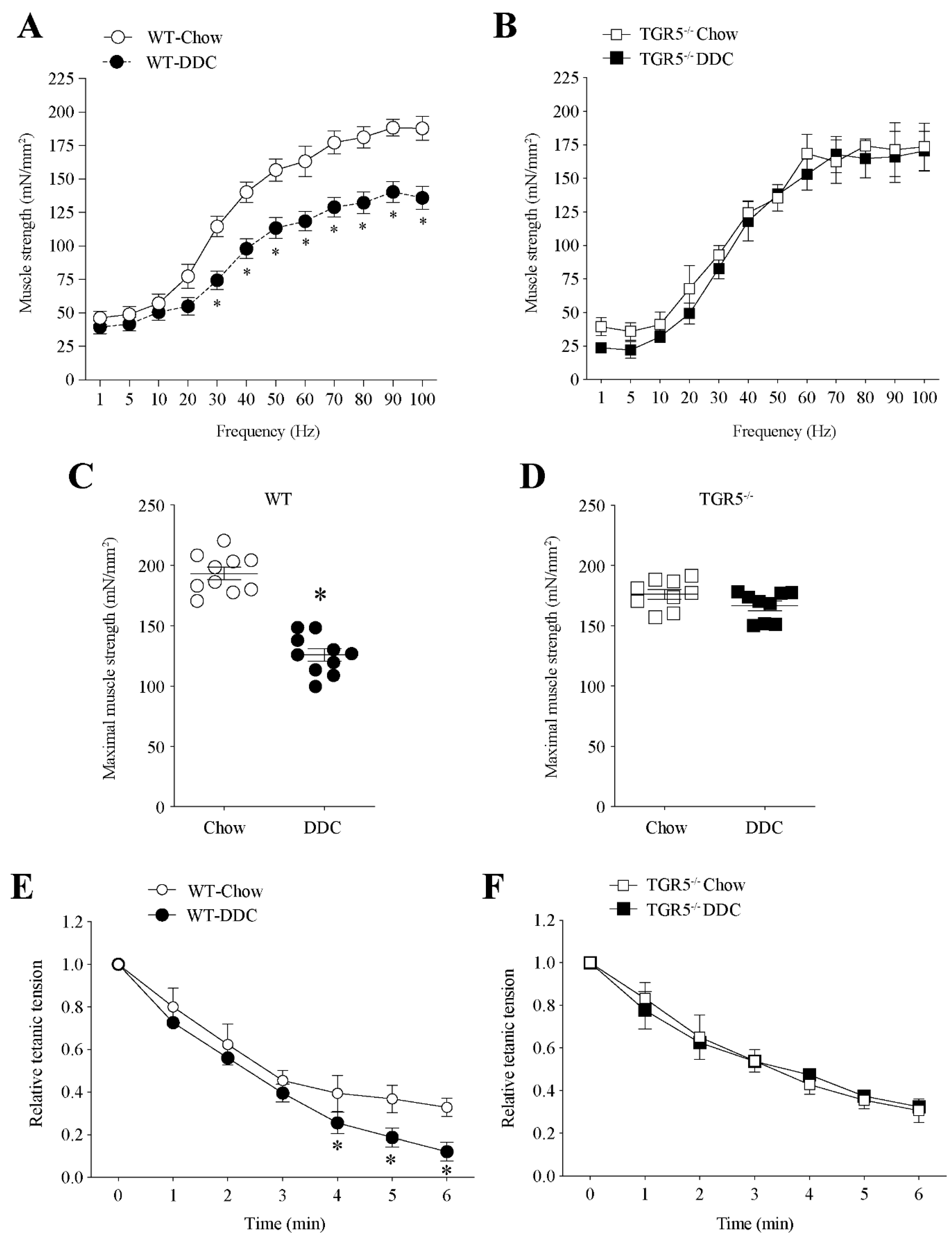
2.3. TGR5 Genetic Ablation Protects Mice with CLD from a Decrease in Muscle Strength and an Increase in Fatigue
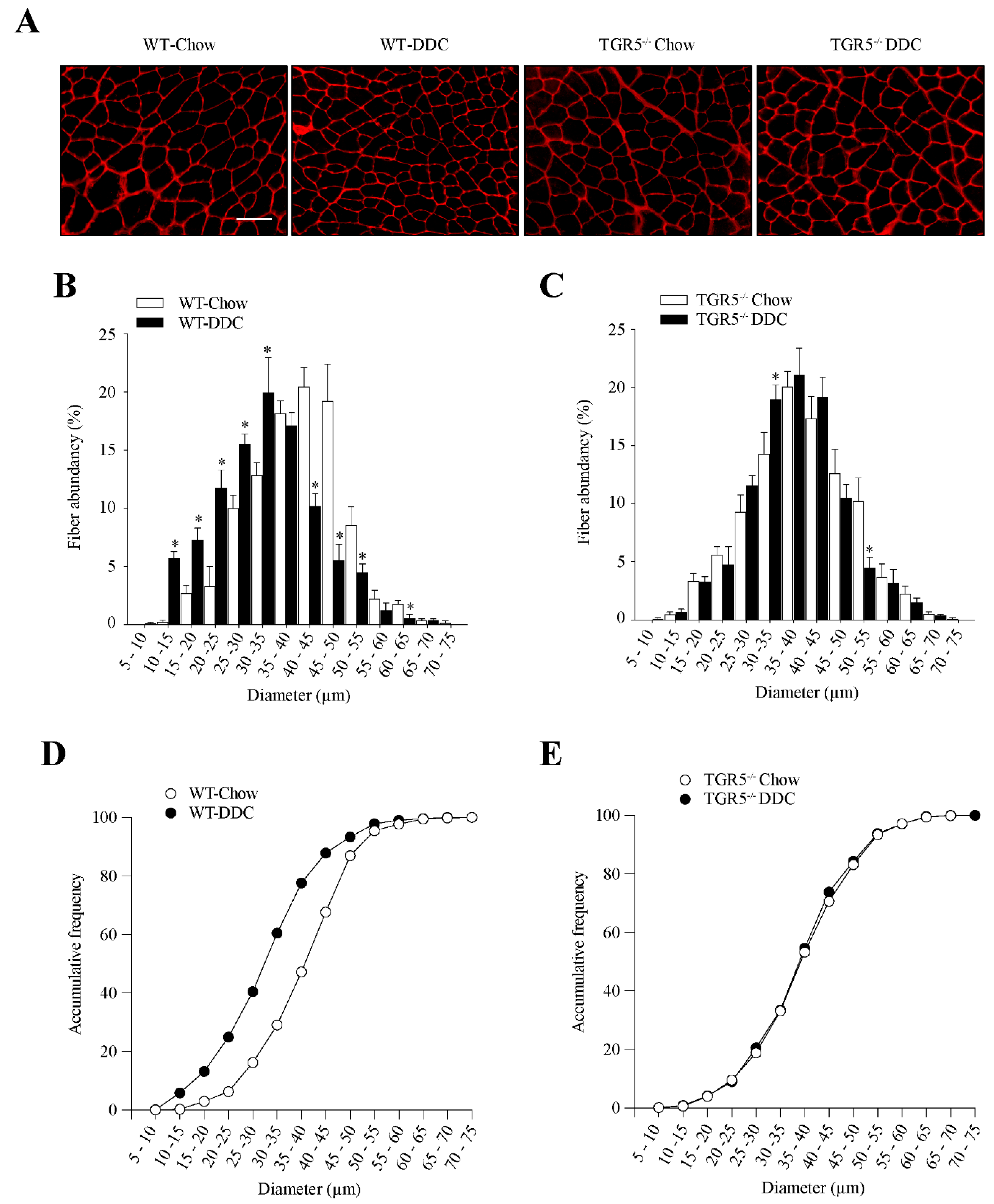
2.4. CLD-Induced Fast-to-Slow Shift in Muscle Fiber Type Composition Is Dependent on TGR5 Expression
2.5. TGR5 Ablation Protects the Muscle from a Decrease in Fiber Diameter Induced by CLD in Mice
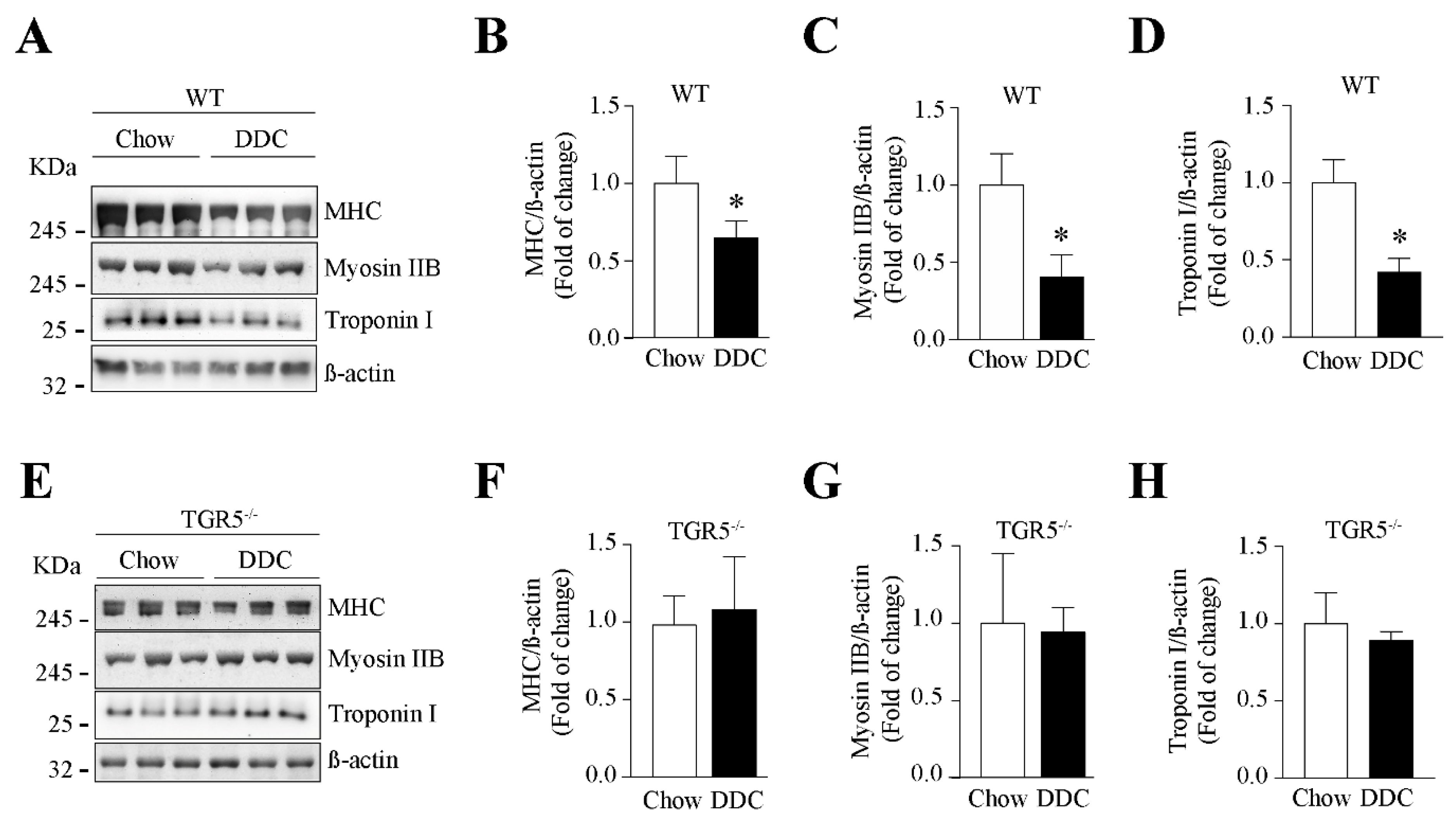
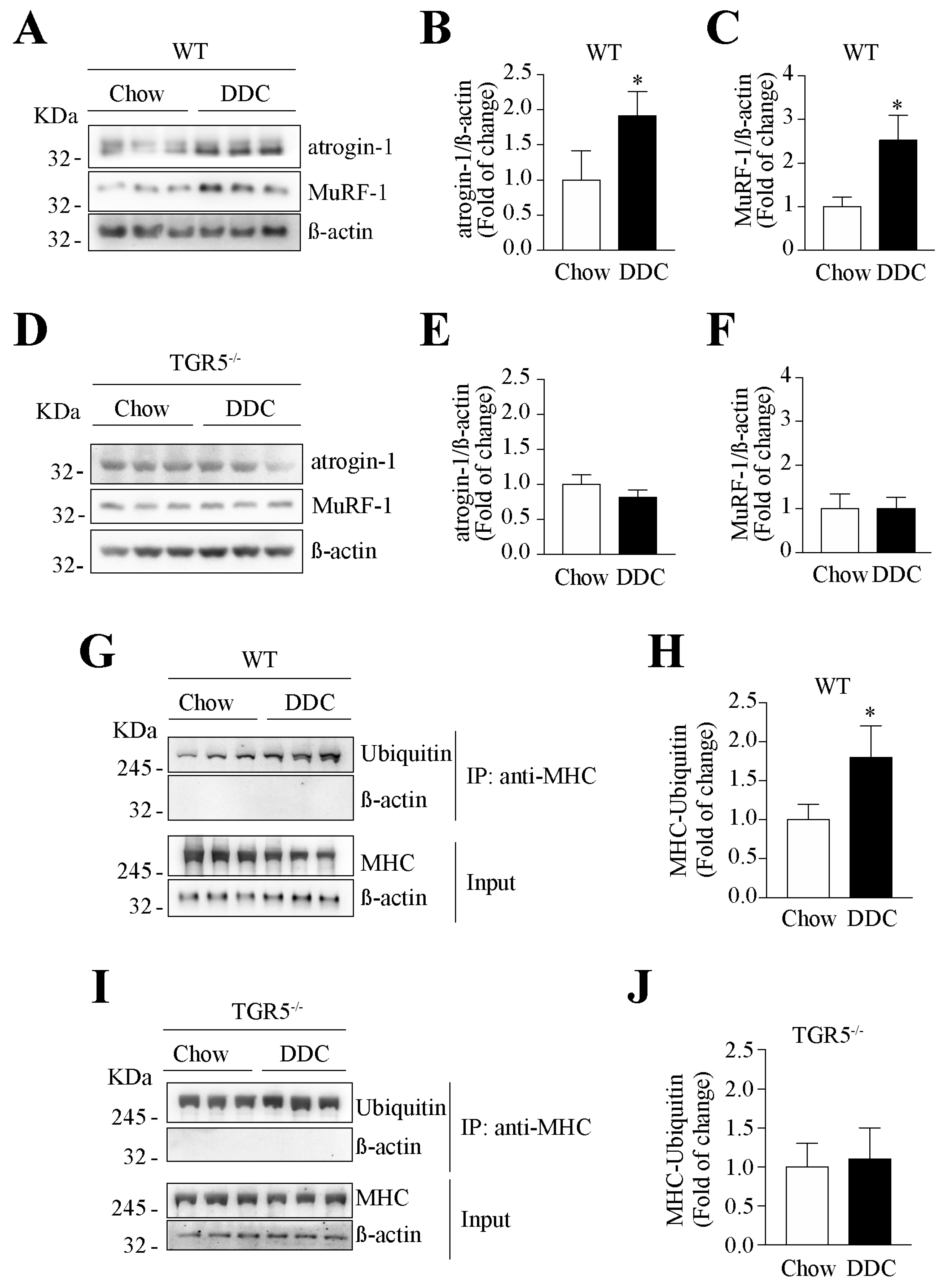
2.6. The Decreased Levels of Sarcomeric Protein and the Increased UPS Activity Induced by CLD Are Dependent on TGR5 Expression
2.7. The Muscular Oxidative Stress Induced by CLD Is Abolished in TGR5−/− Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measurement of Fat, Lean Mass, and Body Water
4.3. Plasma Bile Acids Levels
4.4. Parameters of Liver Injury
4.5. Weightlifting Test
4.6. Running Test
4.7. Rotarod
4.8. Grip Strength Test
4.9. Contractile Measurements in Isolated Skeletal Muscle
4.10. Western Blot
4.11. Co-Immunoprecipitation
4.12. RT-qPCR
4.13. Muscle Fiber Diameter Determination and Quantification
4.14. Determination of Muscle Fiber Types
4.15. Skeletal Muscle Electroporation and Luciferase Activity
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Dawson Hughes, B.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Keller, K. Sarcopenia. Wien. Med. Wochenschr. 2019, 169, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of Oxidative Stress as Key Regulator of Muscle Wasting during Cachexia. Oxid. Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Abrigo, J.; Aguirre, F.; Garces, B.; Arrese, M.; Karpen, S.; Cabrera, D.; Andia, M.E.; Simon, F.; Cabello-Verrugio, C. Sarcopenia in a mice model of chronic liver disease: Role of the ubiquitin-proteasome system and oxidative stress. Pflugers Arch. 2018, 470, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology (Baltimore, Md.) 2017, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.S.; Kao, J.H. Sarcopenia and chronic liver diseases. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1229–1244. [Google Scholar] [CrossRef]
- Noh, J. Sarcopenia as a Novel Risk Factor for Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2020, 29, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990. [Google Scholar] [CrossRef] [Green Version]
- De Bandt, J.P.; Jegatheesan, P.; Tennoune-El-Hafaia, N. Muscle Loss in Chronic Liver Diseases: The Example of Nonalcoholic Liver Disease. Nutrients 2018, 10, 1195. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, D.; Cabello-Verrugio, C.; Solís, N.; Martín, D.S.; Cofré, C.; Pizarro, M.; Arab, J.P.; Abrigo, J.; Campos, F.; Irigoyen, B.; et al. Somatotropic axis dysfunction in non-alcoholic fatty liver disease: Beneficial hepatic and systemic effects of hormone supplementation. Int. J. Mol. Sci. 2018, 19, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeks, A.C.; Madill, J. Sarcopenia in liver transplantation: A review. Clin. Nutr. ESPEN 2017, 22, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.; Marin, T.; Aguirre, F.; Tacchi, F.; Vilos, C.; Simon, F.; Arrese, M.; Cabrera, D.; Cabello-Verrugio, C. N-Acetyl Cysteine attenuates the sarcopenia and muscle apoptosis induced by chronic liver disease. Curr. Mol. Med. 2019, 20, 60–71. [Google Scholar] [CrossRef]
- Meadows, V.; Kennedy, L.; Kundu, D.; Alpini, G.; Francis, H. Bile Acid Receptor Therapeutics Effects on Chronic Liver Diseases. Front. Med. (Lausanne) 2020, 7, 15. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [Green Version]
- Deutschmann, K.; Reich, M.; Klindt, C.; Droge, C.; Spomer, L.; Haussinger, D.; Keitel, V. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1319–1325. [Google Scholar] [CrossRef]
- Keitel, V.; Haussinger, D. Role of TGR5 (GPBAR1) in Liver Disease. Semin. Liver Dis. 2018, 38, 333–339. [Google Scholar] [CrossRef]
- Schaap, F.G.; Trauner, M.; Jansen, P.L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 55–67. [Google Scholar] [CrossRef]
- Horiba, T.; Katsukawa, M.; Mita, M.; Sato, R. Dietary obacunone supplementation stimulates muscle hypertrophy, and suppresses hyperglycemia and obesity through the TGR5 and PPARgamma pathway. Biochem. Biophys. Res. Commun. 2015, 463, 846–852. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hara, N.; Sugimoto, R.; Mifuji-Moroka, R.; Tanaka, H.; Eguchi, A.; Iwasa, M.; Hasegawa, H.; Iwata, K.; Takei, Y.; et al. The Associations between Circulating Bile Acids and the Muscle Volume in Patients with Non-alcoholic Fatty Liver Disease (NAFLD). Intern. Med. 2017, 56, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Kuboyama, A.; Mita, M.; Murata, S.; Shimizu, M.; Inoue, J.; Mori, K.; Sato, R. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice. J. Biol. Chem. 2018, 293, 10322–10332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrigo, J.; Gonzalez, F.; Aguirre, F.; Tacchi, F.; Gonzalez, A.; Meza, M.P.; Simon, F.; Cabrera, D.; Arrese, M.; Karpen, S.; et al. Cholic acid and deoxycholic acid induce skeletal muscle atrophy through a mechanism dependent on TGR5 receptor. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Fickert, P.; Stoger, U.; Fuchsbichler, A.; Moustafa, T.; Marschall, H.U.; Weiglein, A.H.; Tsybrovskyy, O.; Jaeschke, H.; Zatloukal, K.; Denk, H.; et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 2007, 171, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelking, L.R.; Dasher, C.A.; Hirschowitz, B.I. Within-day Fluctuations in Serum Bile-acid Concentrations among Normal Control Subjects and Patients with Hepatic Disease. Am. J. Clin. Pathol. 1980, 73, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Hatzoglou, M. Hyperammonemia and proteostasis in cirrhosis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 30–36. [Google Scholar] [CrossRef]
- Guo, C.; Chen, W.D.; Wang, Y.D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef]
- Rao, J.; Yang, C.; Yang, S.; Lu, H.; Hu, Y.; Lu, L.; Cheng, F.; Wang, X. Deficiency of TGR5 exacerbates immune-mediated cholestatic hepatic injury by stabilizing the beta-catenin destruction complex. Int. Immunol. 2020, 32, 321–334. [Google Scholar] [CrossRef] [Green Version]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buckingham, M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006, 172, 91–102. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the pathophysiology of skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, W. Bile acid receptor TGR5, NADPH Oxidase NOX5-S and CREB Mediate Bile Acid-Induced DNA Damage In Barrett’s Esophageal Adenocarcinoma Cells. Sci. Rep. 2016, 6, 31538. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Sanchez, E.; Perez, M.J.; Nytofte, N.S.; Briz, O.; Monte, M.J.; Lozano, E.; Serrano, M.A.; Marin, J.J.G. Protective role of biliverdin against bile acid-induced oxidative stress in liver cells. Free Radic. Biol. Med. 2016, 97, 466–477. [Google Scholar] [CrossRef]
- Kovacs, P.; Csonka, T.; Kovacs, T.; Sari, Z.; Ujlaki, G.; Sipos, A.; Karanyi, Z.; Szeocs, D.; Hegedus, C.; Uray, K.; et al. Lithocholic Acid, a Metabolite of the Microbiome, Increases Oxidative Stress in Breast Cancer. Cancers (Basel) 2019, 11, 1255. [Google Scholar] [CrossRef] [Green Version]
- Close, R.I. Dynamic properties of mammalian skeletal muscles. Physiol. Rev. 1972, 52, 129–197. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef]
- Barclay, J.K.; Hansel, M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can. J. Physiol. Pharmacol. 1991, 69, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Bagni, M.A.; Colombini, B.; Nocella, M.; Pregno, C.; Cornachione, A.S.; Rassier, D.E. The effects of fatigue and oxidation on contractile function of intact muscle fibers and myofibrils isolated from the mouse diaphragm. Sci. Rep. 2019, 9, 4422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debold, E.P. Potential molecular mechanisms underlying muscle fatigue mediated by reactive oxygen and nitrogen species. Front. Physiol. 2015, 6, 239. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Reid, M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl. Physiol. (1985) 2008, 104, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef]
- Montgomery, J.; Englesbe, M. Sarcopenia in Liver Transplantation. Curr. Transplant. Rep. 2019, 6, 7–15. [Google Scholar] [CrossRef]
- BasuRay, S.; Smagris, E.; Cohen, J.C.; Hobbs, H.H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology 2017, 66, 1111–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavasu, P.W.; Longhurst, S.; Joel, S.P.; Slevin, M.L.; Balkwill, F.R. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J. Immunol. Methods 1992, 153, 115–124. [Google Scholar] [CrossRef]
- Fickert, P.; Zollner, G.; Fuchsbichler, A.; Stumptner, C.; Pojer, C.; Zenz, R.; Lammert, F.; Stieger, B.; Meier, P.J.; Zatloukal, K.; et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology 2001, 121, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Miranda, V.; Abrigo, J.; Rivera, J.C.; Araya-Duran, I.; Aravena, J.; Simon, F.; Pacheco, N.; Gonzalez-Nilo, F.D.; Cabello-Verrugio, C. The complex of PAMAM-OH dendrimer with Angiotensin (1-7) prevented the disuse-induced skeletal muscle atrophy in mice. Int. J. Nanomed. 2017, 12, 1985–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonetto, A.; Andersson, D.C.; Waning, D.L. Assessment of muscle mass and strength in mice. BoneKEY Rep. 2015, 4, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aartsma-Rus, A.; van Putten, M. Assessing functional performance in the mdx mouse model. J. Vis. Exp. 2014, e51303. [Google Scholar] [CrossRef] [Green Version]
- Cabello-Verrugio, C.; Morales, M.G.; Cabrera, D.; Vio, C.P.; Brandan, E. Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J. Cell. Mol. Med. 2012, 16, 752–764. [Google Scholar] [CrossRef]
- Aravena, J.; Abrigo, J.; Gonzalez, F.; Aguirre, F.; Gonzalez, A.; Simon, F.; Cabello-Verrugio, C. Angiotensin (1-7) Decreases Myostatin-Induced NF-kappaB Signaling and Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2020, 21, 1167. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.G.; Abrigo, J.; Acuna, M.J.; Santos, R.A.; Bader, M.; Brandan, E.; Simon, F.; Olguin, H.; Cabrera, D.; Cabello-Verrugio, C. Angiotensin-(1-7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis. Model. Mech. 2016, 9, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Meneses, C.; Morales, M.G.; Abrigo, J.; Simon, F.; Brandan, E.; Cabello-Verrugio, C. The angiotensin-(1-7)/Mas axis reduces myonuclear apoptosis during recovery from angiotensin II-induced skeletal muscle atrophy in mice. Pflugers Arch. 2015, 467, 1975–1984. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Liu, F.; Fry, C.S.; Mula, J.; Jackson, J.R.; Lee, J.D.; Peterson, C.A.; Yang, L. Automated fiber-type-specific cross-sectional area assessment and myonuclei counting in skeletal muscle. J. Appl. Physiol. (1985) 2013, 115, 1714–1724. [Google Scholar] [CrossRef] [Green Version]
- McMahon, J.M.; Signori, E.; Wells, K.E.; Fazio, V.M.; Wells, D.J. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase–increased expression with reduced muscle damage. Gene Ther. 2001, 8, 1264–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbanda, P.S.; Prabhakar, S.; Chawla, Y.K.; Das, C.P.; Syal, P. Peripheral neuropathy in liver cirrhosis. J. Gastroenterol. Hepatol. 2003, 18, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Auwerx, J.; Schoonjans, K. Bile acids and the membrane bile acid receptor TGR5--connecting nutrition and metabolism. Thyroid 2008, 18, 167–174. [Google Scholar] [CrossRef] [PubMed]



| Physiological Parameters | Wild-Type (WT) | TGR5−/− | |||
|---|---|---|---|---|---|
| Chow | DDC | Chow | DDC | ||
| Muscle mass (mg) | TA | 30.4 ± 2.3 | 23.2 ± 2.9 * | 28.4 ± 3.9 | 29.4 ± 2.0 |
| GAST | 86.0 ± 9.2 | 62.7 ± 16.2 * | 93.0 ± 4.2 | 89.0 ± 2.2 | |
| EDL | 9.9 ± 1.6 | 7.8 ± 1.1 * | 8.9 ± 1.9 | 8.3 ± 1.0 | |
| SOL | 8.5 ± 1.7 | 6.2 ± 1.0 * | 7.5 ± 1.0 | 8.5 ± 1.1 | |
| Liver mass (g) | 1.3 ± 0.1 | 1.9 ± 0.2 * | 1.1 ± 0.2 | 2.2 ± 0.3 #,* | |
| ALT (IU/L) | 167 ± 16 | 545 ± 32 * | 145 ± 18 | 497 ± 29 #,* | |
| ALP (IU/L) | 90 ± 10 | 982 ± 74 * | 119 ± 22 | 1091 ± 98 #,* | |
| Bilirubin total (mg/dL) | 0.3 ± 0.1 | 1.8 ± 0.3 * | 0.5 ± 0.2 | 2.3 ± 0.4 #,* | |
| Fat (g) | 1.31 ± 0.09 | 0.85 ± 0.03 * | 1.86 ± 0.18 * | 1.79 ± 0.13 * | |
| Lean (g) | 12.9 ± 0.3 | 9.3 ± 0.3 * | 11.3 ± 0.7 | 10.7 ± 0.3 | |
| Water (g) | Total | 11.2 ± 0.3 | 8.0 ± 0.3 * | 9.3 ± 0.3 | 8.9 ± 0.3 * |
| Free | 0.107 ± 0.061 | 0.075 ± 0.053 | 0.073 ± 0.025 | 0.095 ± 0.051 | |
| Hydration ratio (%) | 85.9 | 85.2 | 81.7 | 82.3 | |
| Glycemic index (g/dL) | 143.0 ± 5.6 | 78.8 ± 4.4 * | 162.4 ± 8.4 * | 81.0 ± 4.6 #,* | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrigo, J.; Campos, F.; Gonzalez, F.; Aguirre, F.; Gonzalez, A.; Huerta-Salgado, C.; Conejeros, S.; Simon, F.; Arrese, M.; Cabrera, D.; et al. Sarcopenia Induced by Chronic Liver Disease in Mice Requires the Expression of the Bile Acids Membrane Receptor TGR5. Int. J. Mol. Sci. 2020, 21, 7922. https://doi.org/10.3390/ijms21217922
Abrigo J, Campos F, Gonzalez F, Aguirre F, Gonzalez A, Huerta-Salgado C, Conejeros S, Simon F, Arrese M, Cabrera D, et al. Sarcopenia Induced by Chronic Liver Disease in Mice Requires the Expression of the Bile Acids Membrane Receptor TGR5. International Journal of Molecular Sciences. 2020; 21(21):7922. https://doi.org/10.3390/ijms21217922
Chicago/Turabian StyleAbrigo, Johanna, Fabián Campos, Francisco Gonzalez, Francisco Aguirre, Andrea Gonzalez, Camila Huerta-Salgado, Sabrina Conejeros, Felipe Simon, Marco Arrese, Daniel Cabrera, and et al. 2020. "Sarcopenia Induced by Chronic Liver Disease in Mice Requires the Expression of the Bile Acids Membrane Receptor TGR5" International Journal of Molecular Sciences 21, no. 21: 7922. https://doi.org/10.3390/ijms21217922
APA StyleAbrigo, J., Campos, F., Gonzalez, F., Aguirre, F., Gonzalez, A., Huerta-Salgado, C., Conejeros, S., Simon, F., Arrese, M., Cabrera, D., Elorza, A. A., & Cabello-Verrugio, C. (2020). Sarcopenia Induced by Chronic Liver Disease in Mice Requires the Expression of the Bile Acids Membrane Receptor TGR5. International Journal of Molecular Sciences, 21(21), 7922. https://doi.org/10.3390/ijms21217922






