Antitumor Activity of the Cardiac Glycoside αlDiginoside by Modulating Mcl-1 in Human Oral Squamous Cell Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. αlDiginoside Inhibits the Proliferation of OSCC Cells
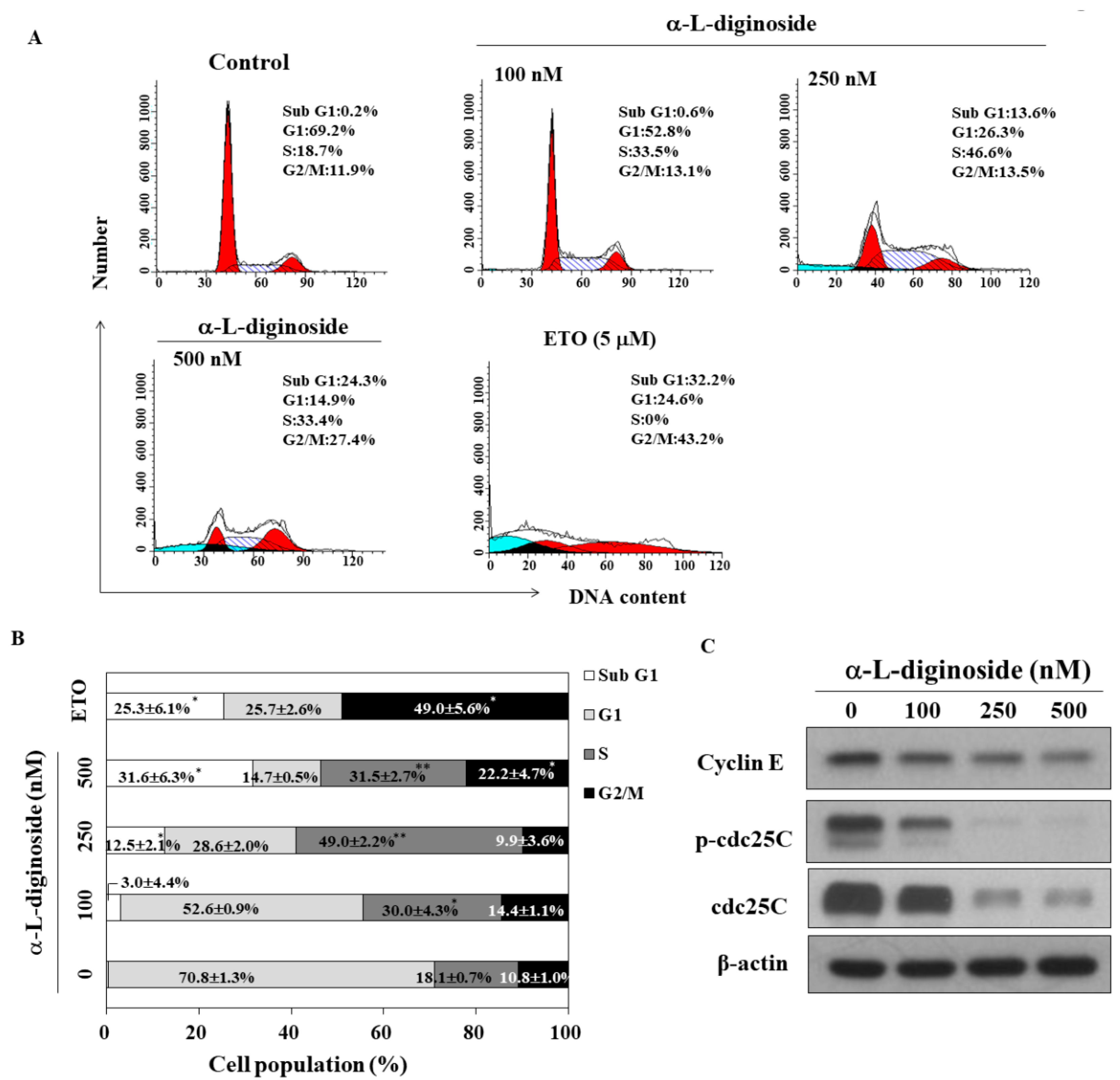
2.2. αlDiginoside Induces Cell Cycle Arrest in OSCC Cells
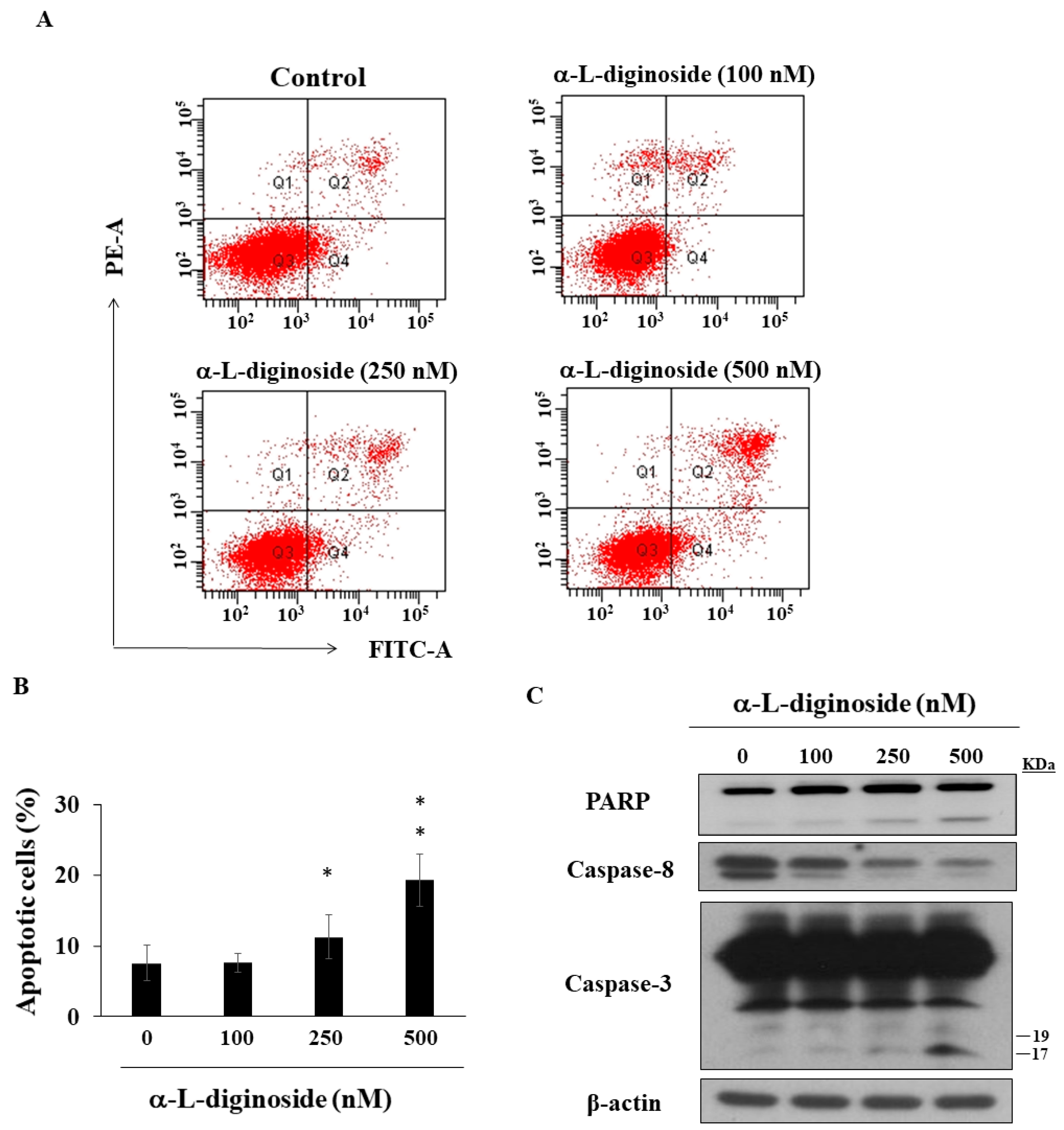
2.3. αlDiginoside Induces Apoptosis in OSCC Cells
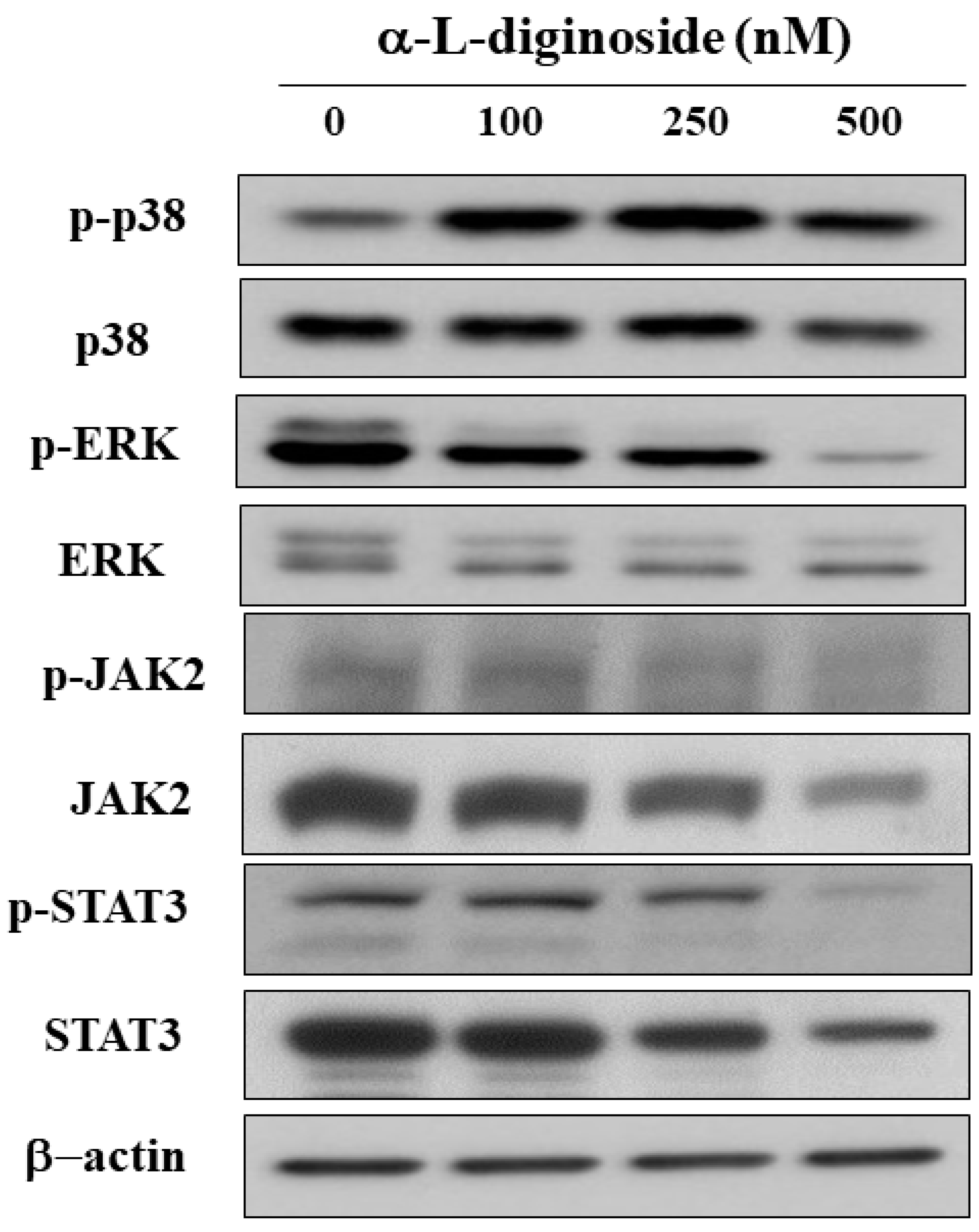
2.4. αlDiginoside Modulates JAK/STAT3 Signaling
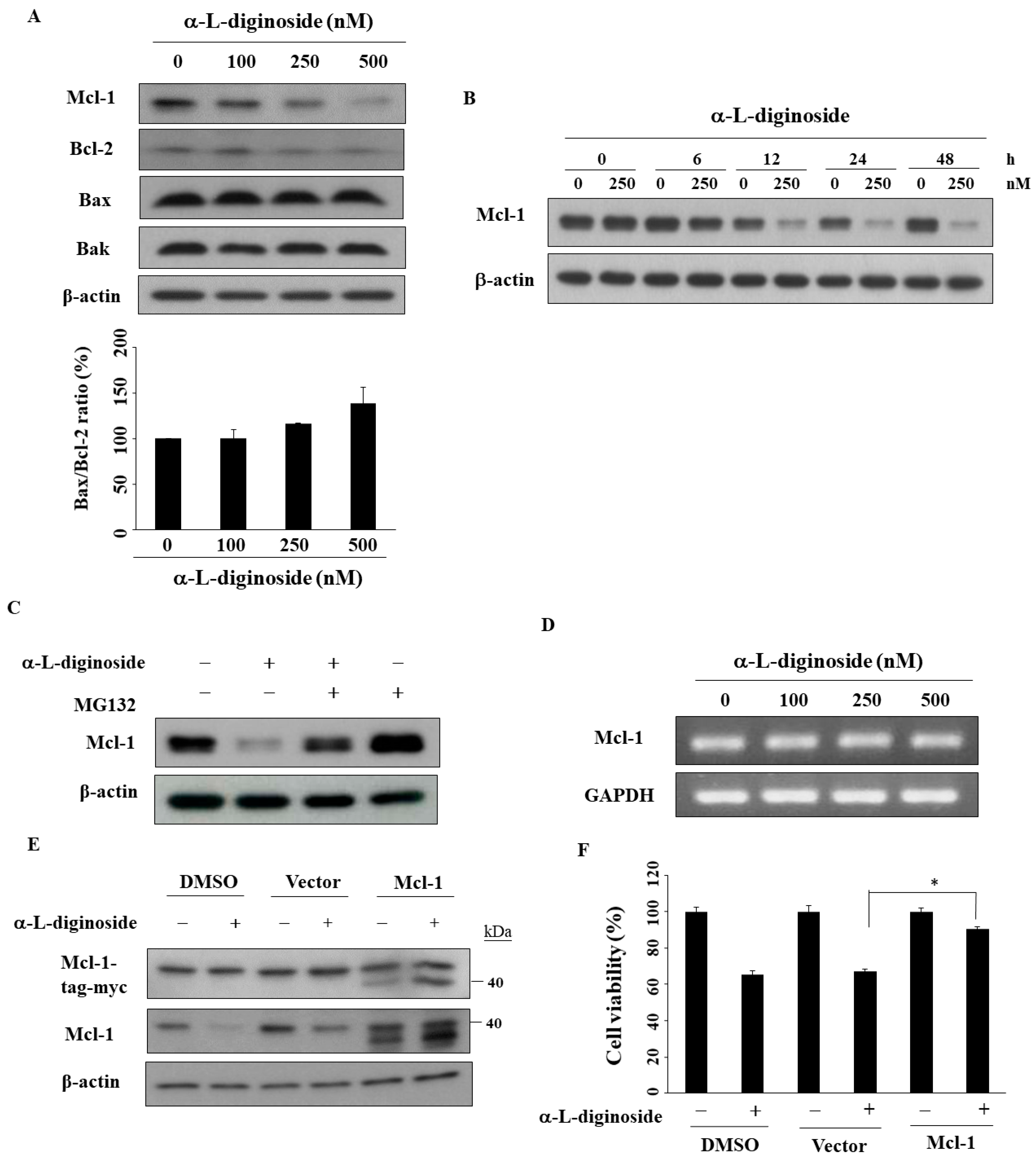
2.5. Mcl-1 Is Involved in αlDiginoside-Mediated Cell Death
3. Discussion
4. Materials and Methods
4.1. Reagents, Antibodies, and Plasmids
4.2. Cell Culture
4.3. Cell Viability Analysis
4.4. Western Blot
4.5. Flow Cytometry
4.6. Transient Transfection for Overexpression
4.7. Reverse Transcriptase-PCR (RT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| MAPKs | Mitogen-activated protein kinases |
| STAT | Signal transducer and activator of transcription |
| OSCC | Oral squamous cell carcinoma |
| CGs | Cardiac glycosides |
| JAK | Janus kinase |
| PI | Propidium iodide |
| PARP | Poly ADP-ribose polymerase |
| ERK | Extracellular signal-regulated kinases |
| MEK | MAPK kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PVDF | Polyvinylidene difluoride |
References
- Ghantous, Y.; Abu Elnaaj, I. Global incidence and risk factors of oral cancer. Harefuah 2017, 156, 645–649. [Google Scholar] [PubMed]
- Porter, S.; Gueiros, L.A.; Leao, J.C.; Fedele, S. Risk factors and etiopathogenesis of potentially premalignant oral epithelial lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rempel, V.; Safi, A.F.; Drebber, U.; Nickenig, H.J.; Neugebauer, J.; Zoller, J.E.; Kreppel, M. The prognostic relevance of lymph node ratio in patients with oral squamous cell carcinoma treated with neoadjuvant therapy regimen and radical surgery. J. Craniomaxillofac. Surg. 2018, 46, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotaskie, G.E.; Andreassi, B.F. Paclitaxel: A new antimitotic chemotherapeutic agent. Cancer Pract. 1994, 2, 27–33. [Google Scholar]
- Smith, G.A. Current status of vinorelbine for breast cancer. Oncology 1995, 9, 767–773. [Google Scholar]
- Cheng, C.S.; Wang, J.; Chen, J.; Kuo, K.T.; Tang, J.; Gao, H.; Chen, L.; Chen, Z.; Meng, Z. New therapeutic aspects of steroidal cardiac glycosides: The anticancer properties of Huachansu and its main active constituent Bufalin. Cancer Cell Int. 2019, 19, 92. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Yan, J.Y.; Han, X.C.; Niu, F.L.; Zhang, J.H.; Hu, W.N. Anti-proliferative effect of digoxin on breast cancer cells via inducing apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5837–5842. [Google Scholar] [PubMed]
- Chou, W.H.; Liu, K.L.; Shih, Y.L.; Chuang, Y.Y.; Chou, J.; Lu, H.F.; Jair, H.W.; Lee, M.Z.; Au, M.K.; Chung, J.G. Ouabain induces apoptotic cell death through caspase- and mitochondria-dependent pathways in human osteosarcoma U-2 OS cells. Anticancer Res. 2018, 38, 169–178. [Google Scholar]
- Kang, X.H.; Zhang, J.H.; Zhang, Q.Q.; Cui, Y.H.; Wang, Y.; Kou, W.Z.; Miao, Z.H.; Lu, P.; Wang, L.F.; Xu, Z.Y.; et al. Degradation of Mcl-1 through GSK-3beta activation regulates apoptosis induced by bufalin in non-small cell lung cancer H1975 cells. Cell. Physiol. Biochem. 2017, 41, 2067–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.H.; Zhu, X.Y.; Shi, W.D.; Liu, L.M. Huachansu injection inhibits metastasis of pancreatic cancer in mice model of human tumor xenograft. BMC Complement. Altern. Med. 2014, 14, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Shi, R.; Chang, L.; Tang, K.; Chen, K.; Yu, G.; Tian, Y.; Guo, Y.; He, W.; Song, X.; et al. Huachansu suppresses human bladder cancer cell growth through the Fas/Fasl and TNF- alpha/TNFR1 pathway in vitro and in vivo. J. Exp. Clin. Cancer Res. 2015, 34, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, S.K.; Sah, N.K.; Newman, R.A.; Cisneros, A.; Aggarwal, B.B. Oleandrin suppresses activation of nuclear transcription factor-kappaB, activator protein-1, and c-Jun NH2-terminal kinase. Cancer Res. 2000, 60, 3838–3847. [Google Scholar] [PubMed]
- Ko, Y.S.; Rugira, T.; Jin, H.; Park, S.W.; Kim, H.J. Oleandrin and its derivative odoroside A, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the STAT-3 signaling pathway. Int. J. Mol. Sci. 2018, 19, E3350. [Google Scholar] [CrossRef] [Green Version]
- Bielawski, K.; Winnicka, K.; Bielawska, A. Inhibition of DNA topoisomerases I and II, and growth inhibition of breast cancer MCF-7 cells by ouabain, digoxin and proscillaridin A. Biol. Pharm. Bull. 2006, 29, 1493–1497. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.R.; Bai, L.Y.; Chiu, S.J.; Chiu, C.F.; Lin, W.Y.; Hu, J.L.; Shieh, T.M. Divaricoside exerts antitumor effects, in part, by modulating Mcl-1 in human oral squamous cell carcinoma cells. Comput. Struct. Biotec. J. 2019, 17, 151–159. [Google Scholar] [CrossRef]
- Mekhail, T.; Kaur, H.; Ganapathi, R.; Budd, G.T.; Elson, P.; Bukowski, R.M. Phase 1 trial of Anvirzel in patients with refractory solid tumors. Investig. New Drugs 2006, 24, 423–427. [Google Scholar] [CrossRef]
- Hong, D.S.; Henary, H.; Falchook, G.S.; Naing, A.; Fu, S.; Moulder, S.; Wheler, J.J.; Tsimberidou, A.; Durand, J.B.; Khan, R.; et al. First-in-human study of PBI-05204, an oleander-derived inhibitor of akt, fgf-2, nf-kappaBeta and p70s6k, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, X.Y.; Shou, Q.Y.; Yan, J.F.; Chen, L.; Fu, H.Y.; Wang, J.C. Bufalin inhibits pancreatic cancer by inducing cell cycle arrest via the c-Myc/NF-kappaB pathway. J. Ethnopharmacol. 2016, 193, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, P.; Wang, F.; Zhou, D.; Wang, R.; Meng, L.; Wang, X.; Zhou, M.; Chen, B.; Ouyang, J. Effects of digoxin on cell cycle, apoptosis and NF-kappaB pathway in Burkitt’s lymphoma cells and animal model. Leuk. Lymphoma 2017, 58, 1673–1685. [Google Scholar] [CrossRef]
- Molinolo, A.A.; Amornphimoltham, P.; Squarize, C.H.; Castilho, R.M.; Patel, V.; Gutkind, J.S. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009, 45, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Lakshminarayana, S.; Augustine, D.; Rao, R.S.; Patil, S.; Awan, K.H.; Venkatesiah, S.S.; Haragannavar, V.C.; Nambiar, S.; Prasad, K. Molecular pathways of oral cancer that predict prognosis and survival: A systematic review. J. Carcinog. 2018, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Oikawa, M.; Miki, Y.; Takahashi, T.; Kumamoto, H. Immunohistochemical assessment of growth factor signaling molecules: MAPK, Akt, and STAT3 pathways in oral epithelial precursor lesions and squamous cell carcinoma. Odontology 2020, 108, 91–101. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, S.; Li, J.; Jiang, C.; Ye, M.; Hu, H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis 2011, 16, 394–403. [Google Scholar] [CrossRef]
- Slingerland, M.; Cerella, C.; Guchelaar, H.J.; Diederich, M.; Gelderblom, H. Cardiac glycosides in cancer therapy: From preclinical investigations towards clinical trials. Investig. New Drugs 2013, 31, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Van Quaquebeke, E.; Delest, B.; Debeir, O.; Darro, F.; Kiss, R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim. Biophys. Acta 2007, 1776, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Eskiocak, U.; Gill, J.G.; Yuan, S.; Ramesh, V.; Froehlich, T.W.; Ahn, C.; Morrison, S.J. Digoxin plus trametinib therapy achieves disease control in BRAF wild-type metastatic melanoma patients. Neoplasia 2017, 19, 255–260. [Google Scholar] [CrossRef]
- Takai, N.; Ueda, T.; Nishida, M.; Nasu, K.; Narahara, H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 2008, 21, 637–643. [Google Scholar] [CrossRef]
- Xu, Z.W.; Wang, F.M.; Gao, M.J.; Chen, X.Y.; Shan, N.N.; Cheng, S.X.; Mai, X.; Zala, G.H.; Hu, W.L.; Xu, R.C. Cardiotonic steroids attenuate ERK phosphorylation and generate cell cycle arrest to block human hepatoma cell growth. J. Steroid Biochem. Mol. Biol. 2011, 125, 181–191. [Google Scholar] [CrossRef]
- Sauer, K.; Lehner, C.F. The role of cyclin E in the regulation of entry into S phase. Prog. Cell Cycle Res. 1995, 1, 125–139. [Google Scholar]
- Perdiguero, E.; Nebreda, A.R. Regulation of Cdc25C activity during the mriotic G2/M transition. Cell Cycle 2004, 3, 733–737. [Google Scholar] [CrossRef]
- Deng, L.J.; Hu, L.P.; Peng, Q.L.; Yang, X.L.; Bai, L.L.; Yiu, A.; Li, Y.; Tian, H.Y.; Ye, W.C.; Zhang, D.M. Hellebrigenin induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells through inhibition of Akt. Chem. Biol. Interact. 2014, 219, 184–194. [Google Scholar] [CrossRef]
- Su, E.Y.; Chu, Y.L.; Chueh, F.S.; Ma, Y.S.; Peng, S.F.; Huang, W.W.; Liao, C.L.; Huang, A.C.; Chung, J.G. Bufalin induces apoptotic cell death in human nasopharyngeal carcinoma cells through mitochondrial ROS and TRAIL Pathways. Am. J. Chin. Med. 2019, 47, 237–257. [Google Scholar] [CrossRef]
- King, K.L.; Cidlowski, J.A. Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 1998, 60, 601–617. [Google Scholar] [CrossRef]
- Yong, L.; Ma, Y.; Zhu, B.; Liu, X.; Wang, P.; Liang, C.; He, G.; Zhao, Z.; Liu, Z.; Liu, X. Oleandrin synergizes with cisplatin in human osteosarcoma cells by enhancing cell apoptosis through activation of the p38 MAPK signaling pathway. Cancer Chemother. Pharmacol. 2018, 82, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.; Kumavath, R.; Tan, T.Z.; Ampasala, D.R.; Kumar, A.P. Peruvoside targets apoptosis and autophagy through MAPK Wnt/beta-catenin and PI3K/AKT/mTOR signaling pathways in human cancers. Life Sci. 2020, 241, 117147. [Google Scholar] [CrossRef]
- Reddy, D.; Ghosh, P.; Kumavath, R. Strophanthidin attenuates MAPK, PI3K/AKT/mTOR, and Wnt/beta-Catenin signaling pathways in human cancers. Front. Oncol. 2019, 9, 1469. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.C.; Bonzon, C.; Green, D.R. The machinery of programmed cell death. Pharmacol. Ther. 2001, 92, 57–70. [Google Scholar] [CrossRef]
- Affar, E.B.; Shah, R.G.; Dallaire, A.K.; Castonguay, V.; Shah, G.M. Role of poly(ADP-ribose) polymerase in rapid intracellular acidification induced by alkylating DNA damage. Proc. Natl. Acad. Sci. USA 2002, 99, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Laudisi, F.; Cherubini, F.; Monteleone, G.; Stolfi, C. STAT3 interactors as potential therapeutic targets for cancer treatment. Int. J. Mol. Sci. 2018, 19, E1787. [Google Scholar] [CrossRef] [Green Version]
- Rebe, C.; Ghiringhelli, F. STAT3, a Master Regulator of Anti-Tumor Immune Response. Cancers 2019, 11, E1280. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Li, E.; Liu, Y.; Gao, Y.; Sun, H.; Ma, G.; Wang, Z.; Liu, X.; Wang, Q.; Qu, X.; et al. Inhibition of Jak-STAT3 pathway enhances bufalin-induced apoptosis in colon cancer SW620 cells. World J. Surg. Oncol. 2012, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.H.; Li, M.Y.; Wang, Z.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Jin, C.; Xu, G.; Piao, L.; Paio, H.; et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine 2020, 68, 153172. [Google Scholar] [CrossRef]
- Palve, V.; Mallick, S.; Ghaisas, G.; Kannan, S.; Teni, T. Overexpression of Mcl-1L splice variant is associated with poor prognosis and chemoresistance in oral cnacers. PLoS ONE 2014, 9, e111927. [Google Scholar] [CrossRef]
- Cerella, C.; Muller, F.; Gaigneaux, A.; Radogna, F.; Viry, E.; Chateauvieux, S.; Dicato, M.; Diederich, M. Early downregulation of Mcl-1 regulates apoptosis triggered by cardiac glycoside UNBS1450. Cell Death Dis. 2015, 6, e1782. [Google Scholar] [CrossRef]
- Pongrakhananon, V.; Stueckle, T.A.; Wang, H.L.; O’Doherty, G.A.; Dinu, C.Z.; Chanvorachote, P.; Rojanasakul, Y. Monosaccharide digitoxin derivative sensitize human non-small cell lung cancer cells to anoikis through Mcl-1 proteasomal degradation. Biochem. Pharmacol. 2014, 88, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.F.; Abe, F.; Yamauchi, T.; Taki, M. Cardenolide glycosides of Strophanthus divaricatus. Phytochemistry 1987, 26, 2351–2355. [Google Scholar] [CrossRef]
- Weng, J.R.; Bai, L.Y.; Ko, H.H.; Tsai, Y.T. Cyclocommunol induces apoptosis in human oral squamous cell carcinoma partially through a Mcl-1-dependent mechanism. Phytomedicine 2018, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.F.; Chin, H.K.; Huang, W.J.; Bai, L.Y.; Huang, H.Y.; Weng, J.R. Induction of apoptosis and autophagy in breast cancer cells by a novel HDAC8 inhibitor. Biomolecules 2019, 9, E824. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.Y.; Chiu, C.F.; Chiu, S.J.; Chu, P.C.; Weng, J.R. FTY720 induces autophagy-associated apoptosis in human oral squamous carcinoma cells, in part, through a reactive oxygen species/Mcl-1-dependent mechanism. Sci. Rep. 2017, 7, 5600. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, J.-R.; Lin, W.-Y.; Bai, L.-Y.; Hu, J.-L.; Feng, C.-H. Antitumor Activity of the Cardiac Glycoside αlDiginoside by Modulating Mcl-1 in Human Oral Squamous Cell Carcinoma Cells. Int. J. Mol. Sci. 2020, 21, 7947. https://doi.org/10.3390/ijms21217947
Weng J-R, Lin W-Y, Bai L-Y, Hu J-L, Feng C-H. Antitumor Activity of the Cardiac Glycoside αlDiginoside by Modulating Mcl-1 in Human Oral Squamous Cell Carcinoma Cells. International Journal of Molecular Sciences. 2020; 21(21):7947. https://doi.org/10.3390/ijms21217947
Chicago/Turabian StyleWeng, Jing-Ru, Wei-Yu Lin, Li-Yuan Bai, Jing-Lan Hu, and Chia-Hsien Feng. 2020. "Antitumor Activity of the Cardiac Glycoside αlDiginoside by Modulating Mcl-1 in Human Oral Squamous Cell Carcinoma Cells" International Journal of Molecular Sciences 21, no. 21: 7947. https://doi.org/10.3390/ijms21217947





