β-Adrenoceptor Activation in Breast MCF-10A Cells Induces a Pattern of Catecholamine Production Similar to that of Tumorigenic MCF-7 Cells
Abstract
1. Introduction
2. Results
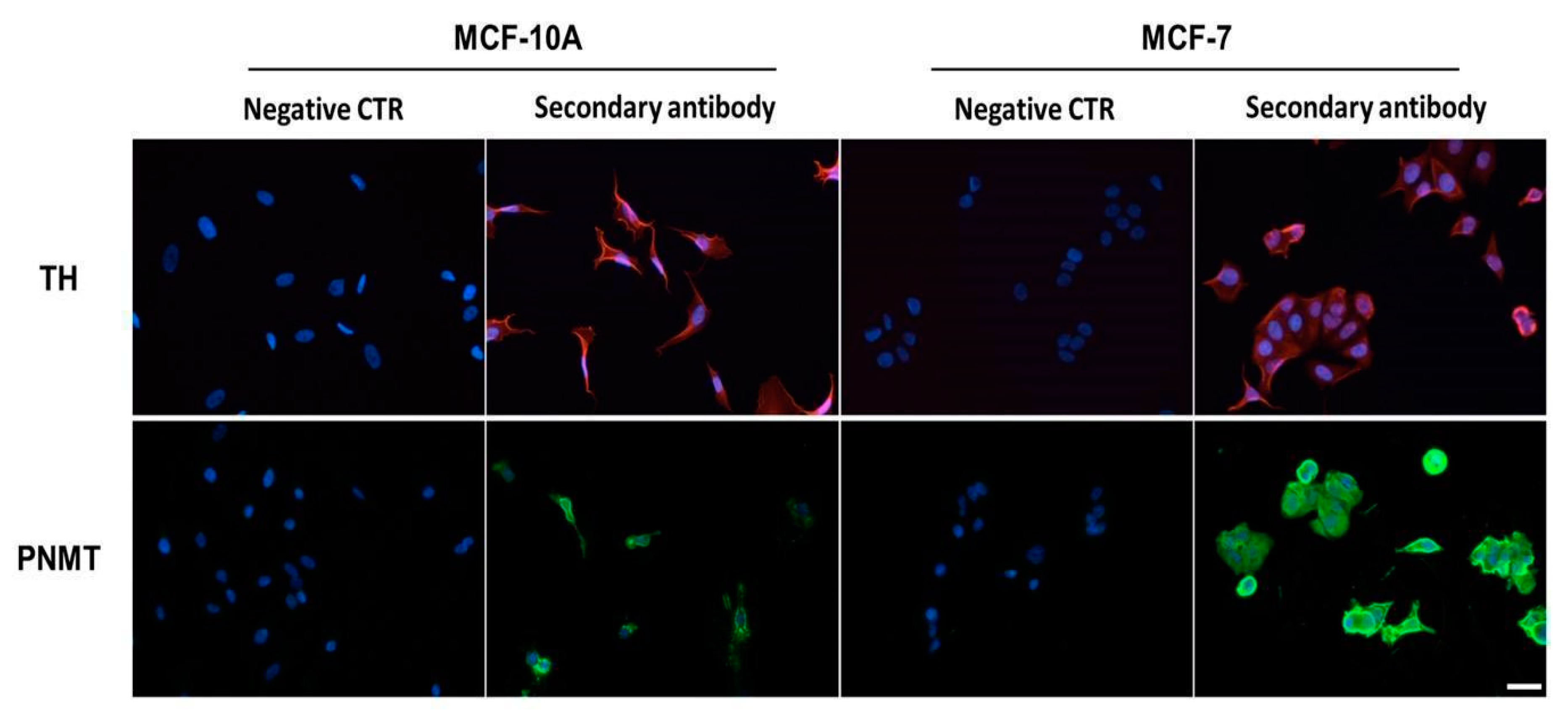
2.1. Expression of Enzymes Involved in the Biosynthesis of Catecholamines
2.2. Catecholamines Biosynthesis
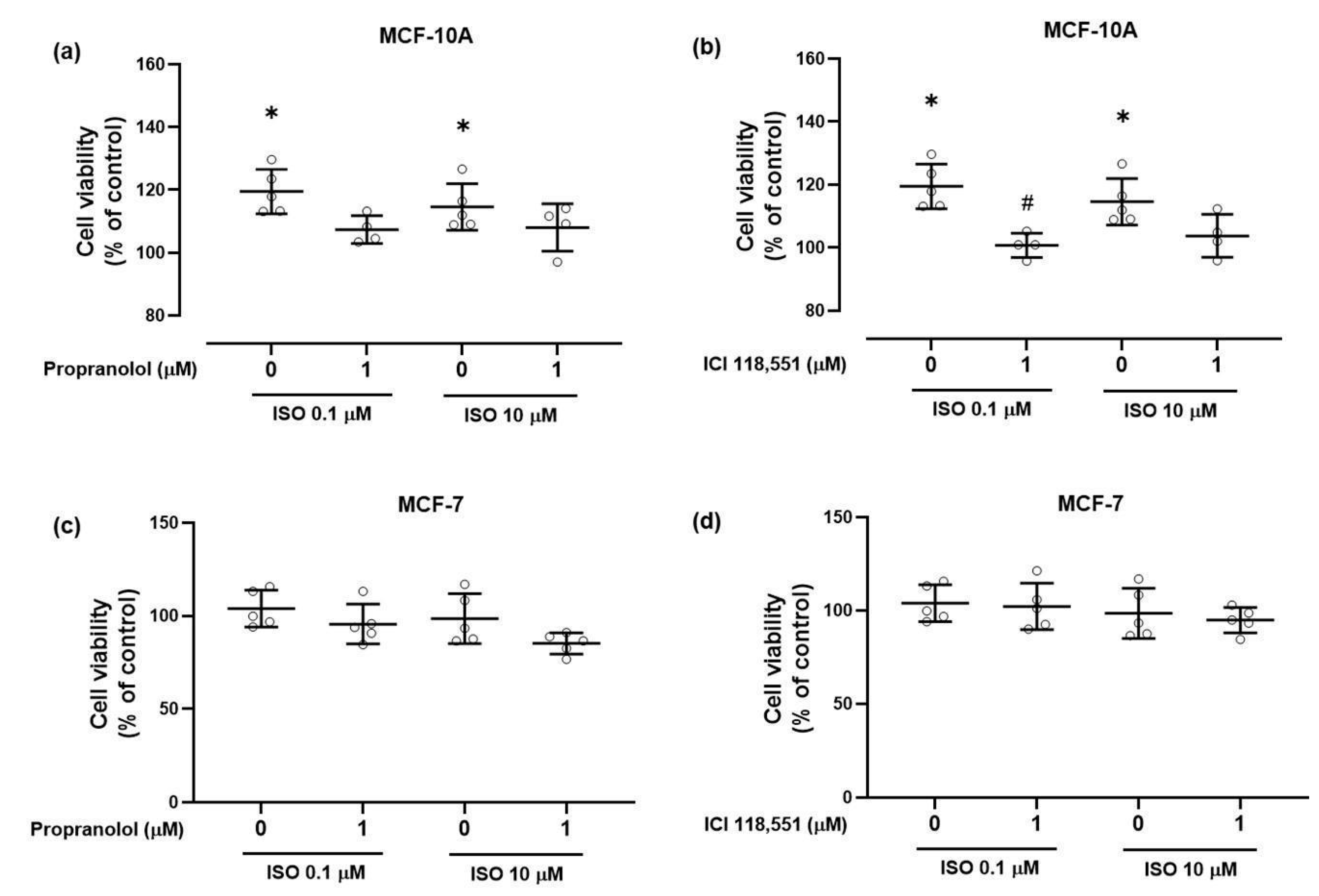
2.3. Influence of β-Adrenergic Receptor Activation
2.3.1. Cell Viability
2.3.2. Clonogenic Ability
2.3.3. Catecholamine Biosynthetic Capacity
3. Discussion
4. Materials and Methods
4.1. Drugs and Antibodies
4.2. Cells and Culture Conditions
4.3. mRNA Expression by RT-qPCR
4.4. Western Blot
4.5. Immunocytochemistry Assay
4.6. HPLC-ECD Analysis
4.7. Cell Viability Assay
4.8. Colony Formation Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Foetal bovine serum |
| ISO | Isoprenaline |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PNMT | Phenylethanolamine N-methyltransferase |
| SDS | Sodium dodecyl sulfate |
| SNS | Sympathetic nervous system |
| TH | Tyrosine hydroxylase |
| β2-AR | β2-adrenoceptors |
References
- Holmes, T.H.; Rahe, R.H. The social readjustment rating scale. J. Psychosom. Res. 1967, 11, 213–218. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Bernstein, J.; Gronostaj, M. Psychological Stress and Cellular Aging in Cancer: A Meta-Analysis. Oxidative Med. Cell. Longev. 2019, 2019, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pr. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Paran, E.; Neumann, L.; Cristal, N. Effects of mental and physical stress on plasma catecholamine levels before and afterβ-adrenoceptor blocker treatment. Eur. J. Clin. Pharmacol. 1992, 43, 11–15. [Google Scholar] [CrossRef]
- Kronenberg, F.; Côte, L.J.; Linkie, D.M.; Dyrenfurth, I.; Downey, J.A. Menopausal hot flashes: Thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas 1984, 6, 31–43. [Google Scholar] [CrossRef]
- Cignarelli, M.; Cicinelli, E.; Corso, M.; Cospite, M.; Garruti, G.; Tafaro, E.; Giorgino, R.; Schonauer, S. Biophysical and Endocrine-Metabolic Changes during Menopausal Hot Flashes: Increase in Plasma Free Fatty Acid and Norepinephrine Levels. Gynecol. Obstet. Investig. 1989, 27, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Lutgendorf, S.K.; Sood, A.K. The Neuroendocrine Impact of Chronic Stress on Cancer. Cell Cycle 2007, 6, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Chen, M.; Bucsek, M.J.; Repasky, E.A.; Hylander, B.L. Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front. Immunol. 2018, 9, 164. [Google Scholar] [CrossRef]
- Monte, M.D.; Calvani, M.; Cammalleri, M.; Favre, C.; Filippi, L.; Bagnoli, P. β-Adrenoceptors as drug targets in melanoma: Novel preclinical evidence for a role of β3-adrenoceptors. Br. J. Pharmacol. 2018, 176, 2496–2508. [Google Scholar] [CrossRef]
- Ritchie, A.C. The Effect of Local Injections of Adrenalin on Epidermal Carcinogenesis in the Mouse. J. Natl. Cancer Inst. 1952, 12, 839–846. [Google Scholar] [CrossRef]
- Coelho, M.; Moz, M.; Correia, G.; Teixeira, A.; Medeiros, R.; Ribeiro, L. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol. Rep. 2015, 33, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, F.; Yang, R.; Zheng, X.; Gao, H.; Zhang, P. Effect of Chronic Restraint Stress on Human Colorectal Carcinoma Growth in Mice. PLoS ONE 2013, 8, e61435. [Google Scholar] [CrossRef]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar] [PubMed]
- Yang, E.V.; Sood, A.K.; Chen, M.; Li, Y.; Eubank, T.D.; Marsh, C.B.; Jewell, S.; Flavahan, N.A.; Morrison, C.; Yeh, P.-E.; et al. Norepinephrine Up-regulates the Expression of Vascular Endothelial Growth Factor, Matrix Metalloproteinase (MMP)-2, and MMP-9 in Nasopharyngeal Carcinoma Tumor Cells. Cancer Res. 2006, 66, 10357–10364. [Google Scholar] [CrossRef]
- Barbieri, A.; Bimonte, S.; De Palma, G.; Luciano, A.; Rea, D.; Giudice, A.; Scognamiglio, G.; La Mantia, E.; Franco, R.; Perdonà, S.; et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int. J. Oncol. 2015, 47, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Guo, K.; Wang, L.; Hu, H.; Li, J.; Zhang, D.; Zhang, M. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol. Rep. 2009, 22, 825–830. [Google Scholar] [CrossRef]
- Altosaar, K.; Balaji, P.; Bond, R.A.; Bylund, D.B.; Cotecchia, S.; Devost, D.; Doze, V.A.; Eikenburg, D.C.; Gora, S.; Goupil, E.; et al. Adrenoceptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guid. Pharmacol. CITE 2019, 2019. [Google Scholar] [CrossRef]
- Lüthy, I.A.; Bruzzone, A.; Piñero, C.P.; Castillo, L.; Chiesa, I.; Vázquez, S.; Sarappa, M. Adrenoceptors: Non Conventional Target for Breast Cancer? Curr. Med. Chem. 2009, 16, 1850–1862. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Sakai, N.; Yano, H.; Uehara, H.; Nakaizumi, A.; Iseki, K. Promotion by the α-adrenoceptor agonist phenylephrine, but not by the β-adrenoceptor agonist isoproterenol, of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1998, 122, 61–65. [Google Scholar] [CrossRef]
- Kyprianou, N.; Benning, C.M. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000, 60, 4550–4555. [Google Scholar]
- Vázquez, S.M.; Mladovan, A.G.; Pérez, C.; Bruzzone, A.; Baldi, A.; Lüthy, I.A. Human breast cell lines exhibit functional α2-adrenoceptors. Cancer Chemother. Pharmacol. 2005, 58, 50–61. [Google Scholar] [CrossRef]
- Lamkin, D.M.; Sung, H.Y.; Yang, G.S.; David, J.M.; Ma, J.C.; Cole, S.W.; Sloan, E.K. α2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology 2014, 51, 262–270. [Google Scholar] [CrossRef]
- Schuller, H.M.; Cole, B. Regulation of cell proliferation by β-adrenergjc receptors in a human lung adenocarcinoma cell line. Carcinogenesis 1989, 10, 1753–1755. [Google Scholar] [CrossRef]
- Yazawa, T.; Kaira, K.; Shimizu, K.; Shimizu, A.; Mori, K.; Nagashima, T.; Ohtaki, Y.; Oyama, T.; Mogi, A.; Kuwano, H. Prognostic significance of beta2-adrenergic receptor expression in non-small cell lung cancer. Am. J. Transl. Res. 2016, 8, 5059–5070. [Google Scholar] [PubMed]
- Re, G.; Badino, P.; Novelli, A.; Girardi, C.; Di Carlo, F. Evidence for functional β-adrenoceptor subtypes in cg-5 breast cancer cells. Pharmacol. Res. 1996, 33, 255–260. [Google Scholar] [CrossRef]
- Piñero, C.P.; Bruzzone, A.; Sarappa, M.G.; Castillo, L.F.; Lüthy, I.A. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Wang, H.-C.; Yuan, Z.; Huang, J.; Zheng, Q. Norepinephrine Stimulates Pancreatic Cancer Cell Proliferation, Migration and Invasion Via ß-Adrenergic Receptor-Dependent Activation of P38/MAPK Pathway. Hepatogastroenterology 2011, 59, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Maccari, S.; Buoncervello, M.; Rampin, A.; Spada, M.; Macchia, D.; Giordani, L.; Stati, T.; Bearzi, C.; Catalano, L.; Rizzi, R.; et al. Biphasic effects of propranolol on tumour growth in B16F10 melanoma-bearing mice. Br. J. Pharmacol. 2016, 174, 139–149. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Benemei, S.; Marchionni, N.; Geppetti, P.; Gandini, S. β-Blocker use and reduced disease progression in patients with thick melanoma. Melanoma Res. 2017, 27, 268–270. [Google Scholar] [CrossRef]
- Zhong, S.; Yu, D.; Zhang, X.; Chen, X.; Yang, S.; Tang, J.; Zhong, S.-L.; Wang, S. β-Blocker use and mortality in cancer patients. Eur. J. Cancer Prev. 2016, 25, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Repasky, E.A.; Eng, J.; Hylander, B.L. Stress, Metabolism and Cancer. Cancer J. 2015, 21, 97–103. [Google Scholar] [CrossRef]
- Chiba, T.; Maeda, T.; Fujita, Y.; Takeda, R.; Kikuchi, A.; Kudo, K. Stress-Induced Suppression of Milk Protein Is Involved in a Noradrenergic Mechanism in the Mammary Gland. Endocrinology 2019, 160, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Qian, L.; Wang, L.; Niu, L.; Zhang, H.; Yong, Z.; Gong, Z.; Song, L.; et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res. Treat. 2010, 125, 351–362. [Google Scholar] [CrossRef]
- Menyhárt, O.; Harami-Papp, H.; Sukumar, S.; Schäfer, R.; Magnani, L.; De Barrios, O.; Győrffy, B. Guidelines for the selection of functional assays to evaluate the hallmarks of cancer. Biochim. Biophys. Acta Bioenerg. 2016, 1866, 300–319. [Google Scholar] [CrossRef]
- Obeid, E.I.; Conzen, S.D. The role of adrenergic signaling in breast cancer biology. Cancer Biomark. 2013, 13, 161–169. [Google Scholar] [CrossRef]
- Flatmark, T.; Stevens, R.C. Structural Insight into the Aromatic Amino Acid Hydroxylases and Their Disease-Related Mutant Forms. Chem. Rev. 1999, 99, 2137–2160. [Google Scholar] [CrossRef]
- Ekobayashi, K.; Nagatsu, T. Molecular genetics of tyrosine 3-monooxygenase and inherited diseases. Biochem. Biophys. Res. Commun. 2005, 338, 267–270. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nat. Cell Biol. 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Schnell, P.O.; Ignacak, M.L.; Bauer, A.L.; Striet, J.B.; Paulding, W.R.; Czyzyk-Krzeska, M.F. Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J. Neurochem. 2003, 85, 483–491. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yokota, A.; Harada, H.; Huang, G. Hypoxia/pseudohypoxia-mediated activation of hypoxia-inducible factor-1α in cancer. Cancer Sci. 2019, 110, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Mohlin, S.; Wigerup, C.; Jögi, A.; Påhlman, S. Hypoxia, pseudohypoxia and cellular differentiation. Exp. Cell Res. 2017, 356, 192–196. [Google Scholar] [CrossRef]
- Brown, S.T.; Kelly, K.F.; Daniel, J.M.; Nurse, C.A. Hypoxia inducible factor (HIF)-2α is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J. Neurochem. 2009, 110, 622–630. [Google Scholar] [CrossRef]
- Hui, A.S.; Striet, J.B.; Gudelsky, G.A.; Soukhova, G.K.; Gozal, E.; Beitner-Johnson, D.; Guo, S.-Z.; Sachleben, L.R.; Haycock, J.W.; Gozal, D.; et al. Regulation of Catecholamines by Sustained and Intermittent Hypoxia in Neuroendocrine Cells and Sympathetic Neurons. Hypertension 2003, 42, 1130–1136. [Google Scholar] [CrossRef]
- Wong, D.L.; Tai, T.C.; Wong-Faull, D.C.; Claycomb, R.; Siddall, B.J.; Bell, R.A.; Kvetnansky, R. Stress and Adrenergic Function: HIF1α, a Potential Regulatory Switch. Cell. Mol. Neurobiol. 2010, 30, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Waldmeier, P.; Hedwall, P.R.; Maître, L. On the role of α-methyldopamine in the antihypertensive effect of α-methyldopa. Naunyn-Schmiedebergs Arch. Pharmacol. 1975, 289, 303–314. [Google Scholar] [CrossRef]
- Cui, B.; Luo, Y.; Tian, P.; Peng, F.; Lu, J.; Yang, Y.; Su, Q.; Liu, B.; Yu, J.; Luo, X.; et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Investig. 2019, 129, 1030–1046. [Google Scholar] [CrossRef]
- Ouyang, X.; Zhu, Z.; Yang, C.; Wang, L.; Ding, G.; Jiang, F. Epinephrine increases malignancy of breast cancer through p38 MAPK signaling pathway in depressive disorders. Int. J. Clin. Exp. Pathol. 2019, 12, 1932–1946. [Google Scholar]
- Zhang, Z.; Wang, Y.; Li, Q. Mechanisms underlying the effects of stress on tumorigenesis and metastasis (Review). Int. J. Oncol. 2018, 53, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, S.M.; Pignataro, O.; Luthy, I.A. α2-Adrenergic effect on human breast cancer MCF-7 cells. Breast Cancer Res. Treat. 1999, 55, 41–49. [Google Scholar] [CrossRef]
- Starke, K.; Borowski, E.; Endo, T. Preferential blockade of presynaptic α-adrenoceptors by yohimbine. Eur. J. Pharmacol. 1975, 34, 385–388. [Google Scholar] [CrossRef]
- Bylund, D.B.; Blaxall, H.S.; Iversen, L.J.; Caron, M.G.; Lefkowitz, R.J.; Lomasney, J.W. Pharmacological characteristics of alpha 2-adrenergic receptors: Comparison of pharmacologically defined subtypes with subtypes identified by molecular cloning. Mol. Pharmacol. 1992, 42, 1–5. [Google Scholar] [PubMed]
- Kenakin, T. Drug efficacy at G protein–coupledreceptors. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, A.; Saulière, A.; Finana, F.; Sénard, J.; Lüthy, I.; Galés, C. Dosage-dependent regulation of cell proliferation and adhesion through dual β 2 -adrenergic receptor/cAMP signals. FASEB J. 2013, 28, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta Blockers and Breast Cancer Mortality: A Population-Based Study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zänker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-Blocker Drug Therapy Reduces Secondary Cancer Formation in Breast Cancer and Improves Cancer Specific Survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef]
- Chang, P.-Y.; Huang, W.-Y.; Lin, C.-L.; Huang, T.-C.; Wu, Y.-Y.; Chen, J.-H.; Kao, C.-H. Propranolol reduces cancer risk: A population-based cohort study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2016, 8, 6446–6460. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Frank, S.; Cryer, P.E. Adrenomedullary response to maximal stress in humans. Am. J. Med. 1984, 77, 779–784. [Google Scholar] [CrossRef]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef]
- Rebar, R.W.; Spitzer, I.B. The physiology and measurement of hot flushes. Am. J. Obstet. Gynecol. 1987, 156, 1284–1288. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Walker, A.K.; Martelli, D.; Ziegler, A.I.; Lambert, G.W.; Phillips, S.E.; Hill, S.J.; Martelli, D.; Sloan, E.K. Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology 2019, 99, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Dibner, M.D.; Insel, P.A. Serum catecholamines desensitize beta-adrenergic receptors of cultured C6 glioma cells. J. Biol. Chem. 1981, 256, 7343–7346. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Soares, A.S.; Costa, V.M.; Diniz, C.; Fresco, P. The combination of Cl-IB-MECA with paclitaxel: A new anti-metastatic therapeutic strategy for melanoma. Cancer Chemother. Pharmacol. 2014, 74, 847–860. [Google Scholar] [CrossRef] [PubMed]


| Noradrenaline (nM) | Adrenaline (nM) | |
|---|---|---|
| MCF-10A cells | 1.78 ± 0.36 | 1.65 ± 0.29 |
| MCF-7 cells | 1.89 ± 0.51 | 11.02 ± 1.05 * |
| MCF-10A Cells [α-methylNA (nM)] | MCF-7 Cells [α-methylNA (nM)] | |
|---|---|---|
| 10 µM α-methylDOPA | 165.4 ± 8.9 | 11.0 ± 1.1 * |
| 100 µM α-methylDOPA | 316.3 ± 6.7 | 46.1 ± 2.8 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaro, F.; Silva, D.; Reguengo, H.; Oliveira, J.C.; Quintas, C.; Vale, N.; Gonçalves, J.; Fresco, P. β-Adrenoceptor Activation in Breast MCF-10A Cells Induces a Pattern of Catecholamine Production Similar to that of Tumorigenic MCF-7 Cells. Int. J. Mol. Sci. 2020, 21, 7968. https://doi.org/10.3390/ijms21217968
Amaro F, Silva D, Reguengo H, Oliveira JC, Quintas C, Vale N, Gonçalves J, Fresco P. β-Adrenoceptor Activation in Breast MCF-10A Cells Induces a Pattern of Catecholamine Production Similar to that of Tumorigenic MCF-7 Cells. International Journal of Molecular Sciences. 2020; 21(21):7968. https://doi.org/10.3390/ijms21217968
Chicago/Turabian StyleAmaro, Filipa, Dany Silva, Henrique Reguengo, José C. Oliveira, Clara Quintas, Nuno Vale, Jorge Gonçalves, and Paula Fresco. 2020. "β-Adrenoceptor Activation in Breast MCF-10A Cells Induces a Pattern of Catecholamine Production Similar to that of Tumorigenic MCF-7 Cells" International Journal of Molecular Sciences 21, no. 21: 7968. https://doi.org/10.3390/ijms21217968
APA StyleAmaro, F., Silva, D., Reguengo, H., Oliveira, J. C., Quintas, C., Vale, N., Gonçalves, J., & Fresco, P. (2020). β-Adrenoceptor Activation in Breast MCF-10A Cells Induces a Pattern of Catecholamine Production Similar to that of Tumorigenic MCF-7 Cells. International Journal of Molecular Sciences, 21(21), 7968. https://doi.org/10.3390/ijms21217968








