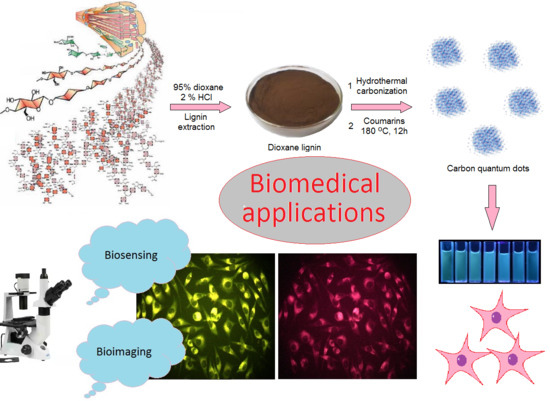
Coumarin-Modified CQDs for Biomedical Applications—Two-Step Synthesis and Characterization
Abstract
1. Introduction
2. Results and Discussion
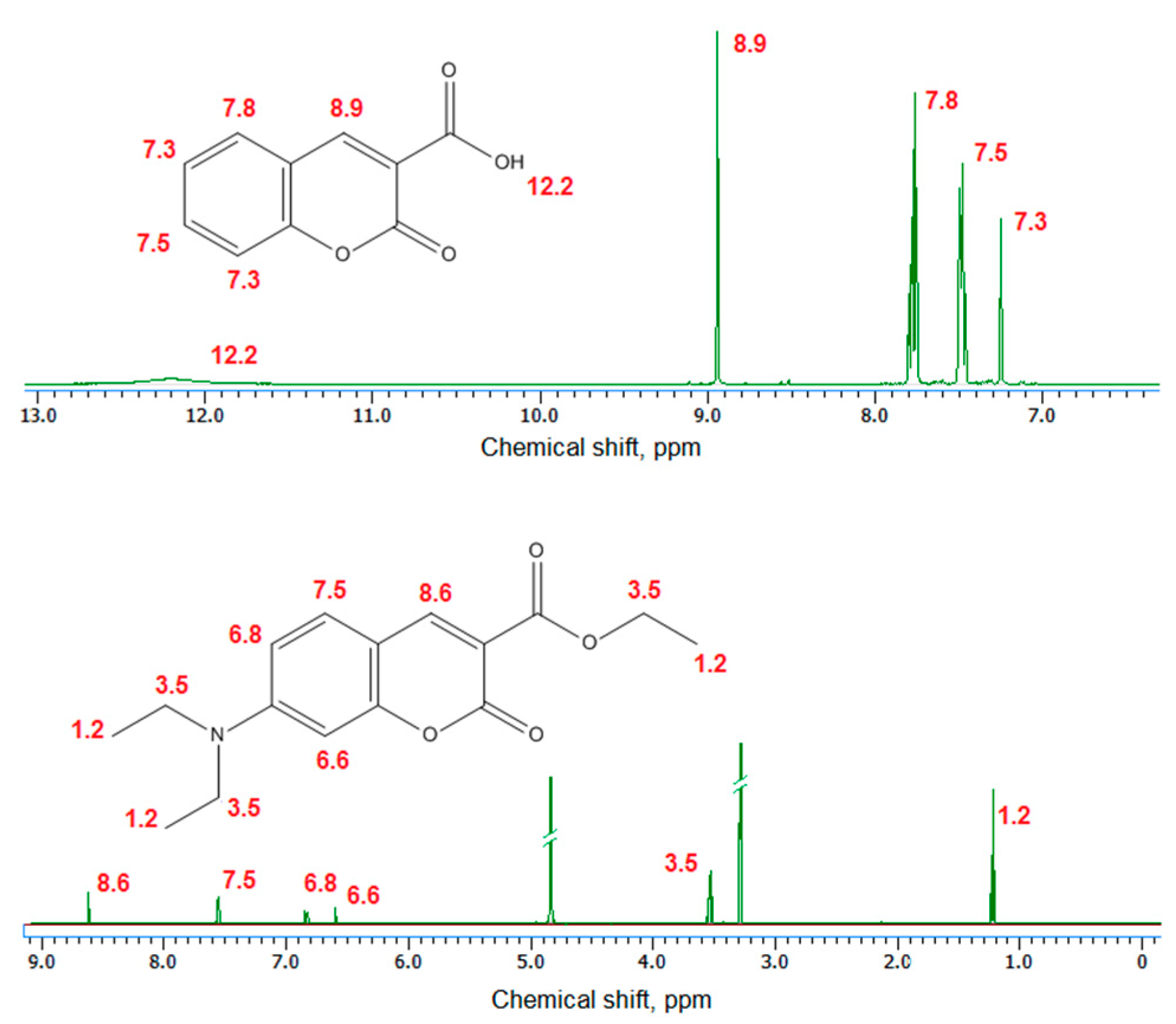
2.1. Chemical Structure Analysis
2.2. Morphology Analysis
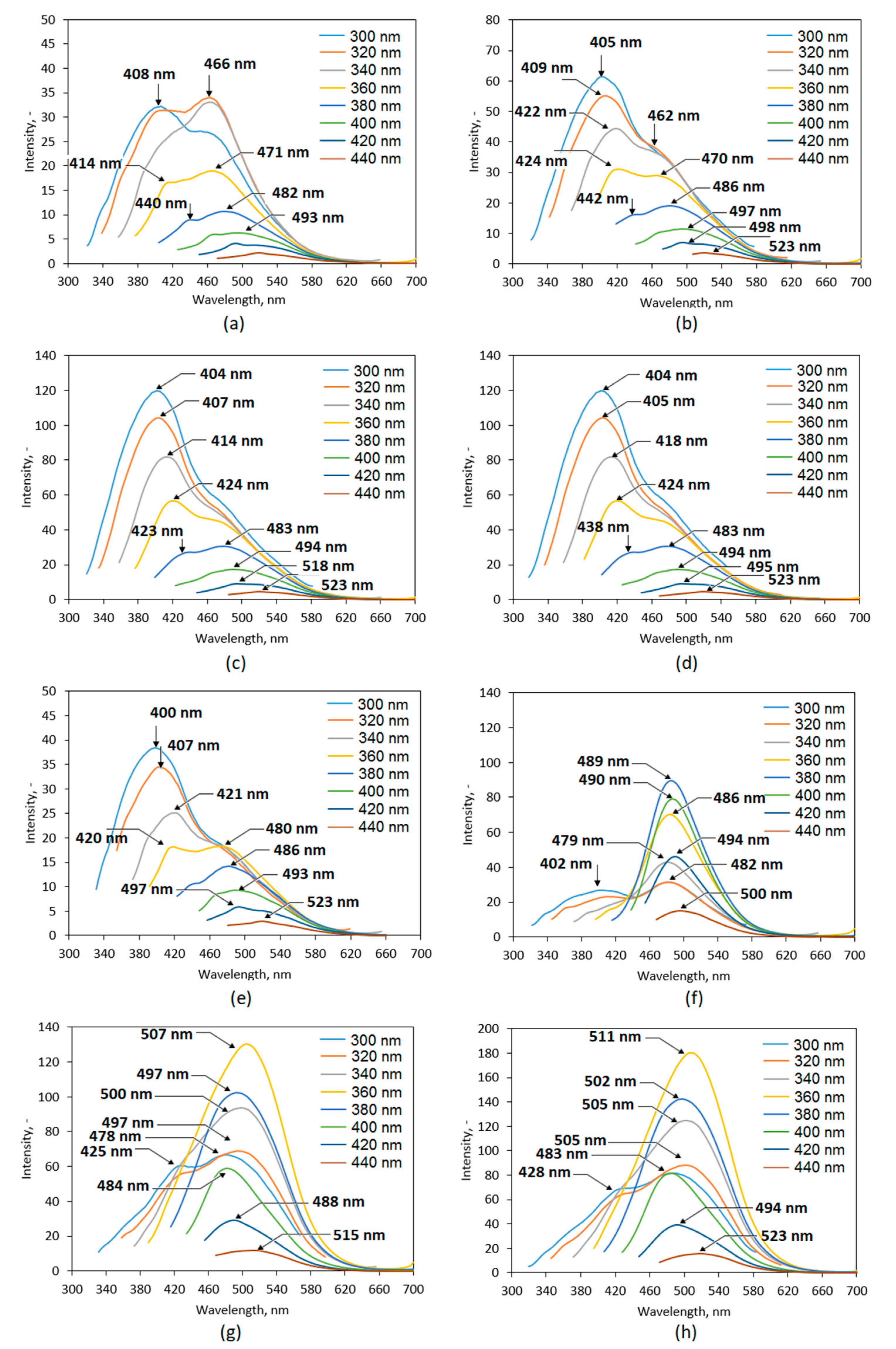
2.3. Optical Properties Analysis
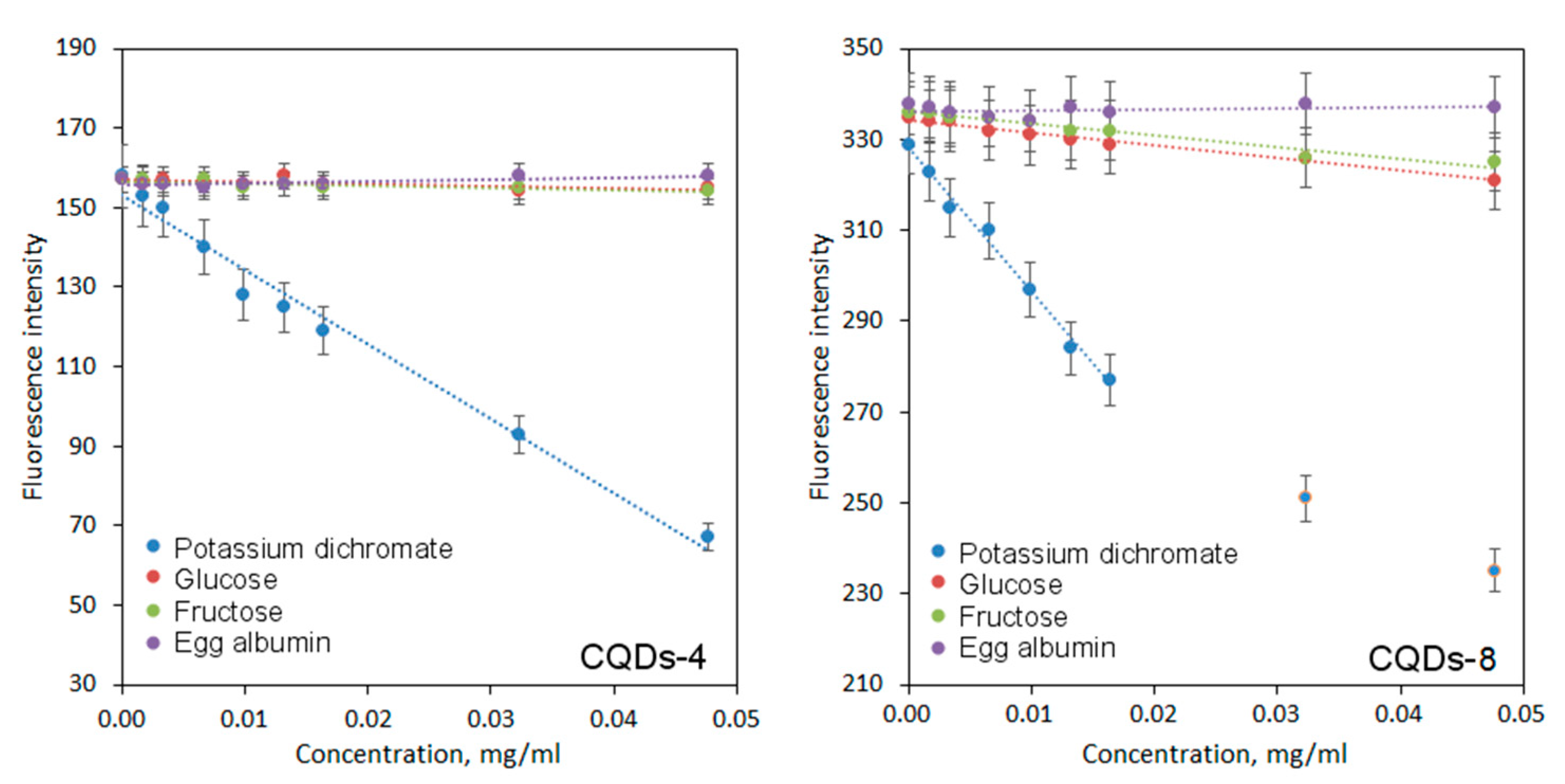
2.4. In Vitro Cytotoxicity Study
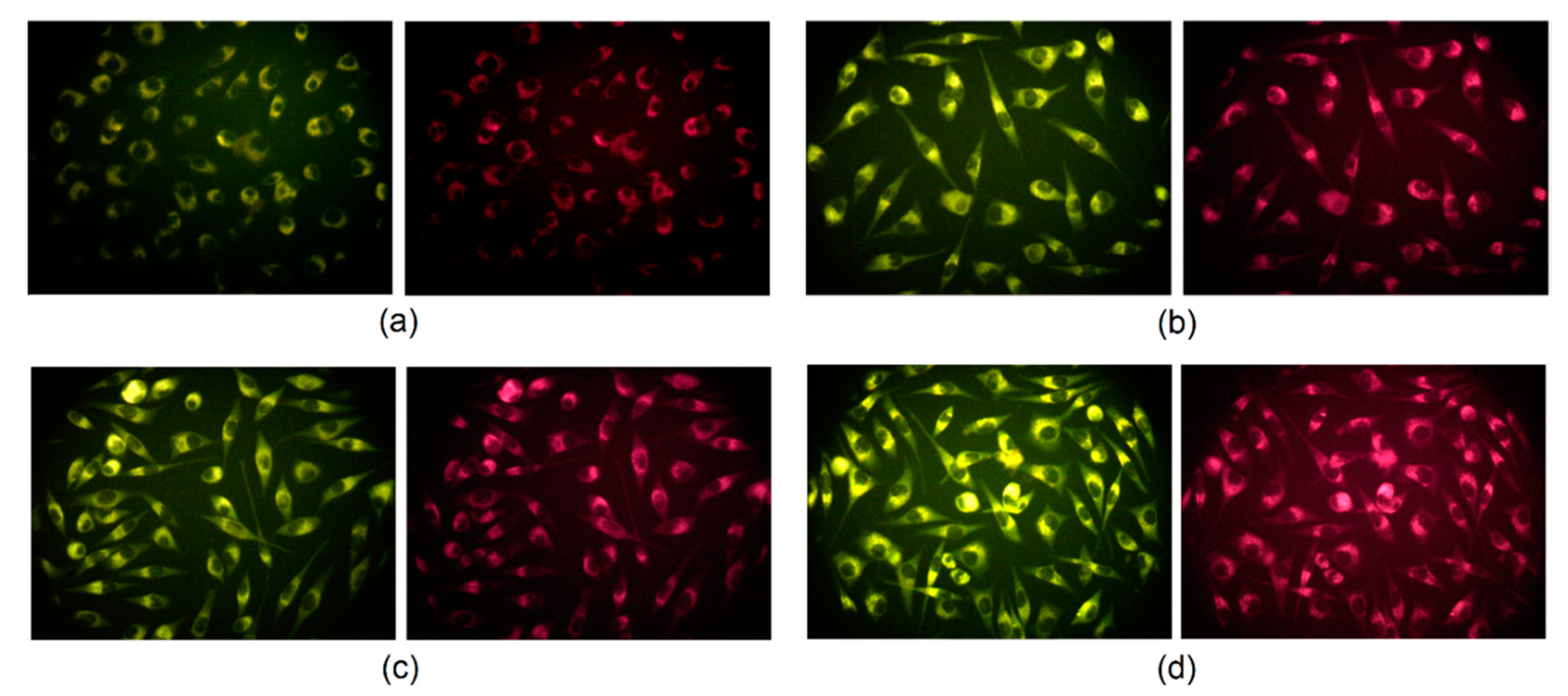
2.5. Cells Visualization Study
3. Materials and Methods
3.1. Materials
3.2. Methods
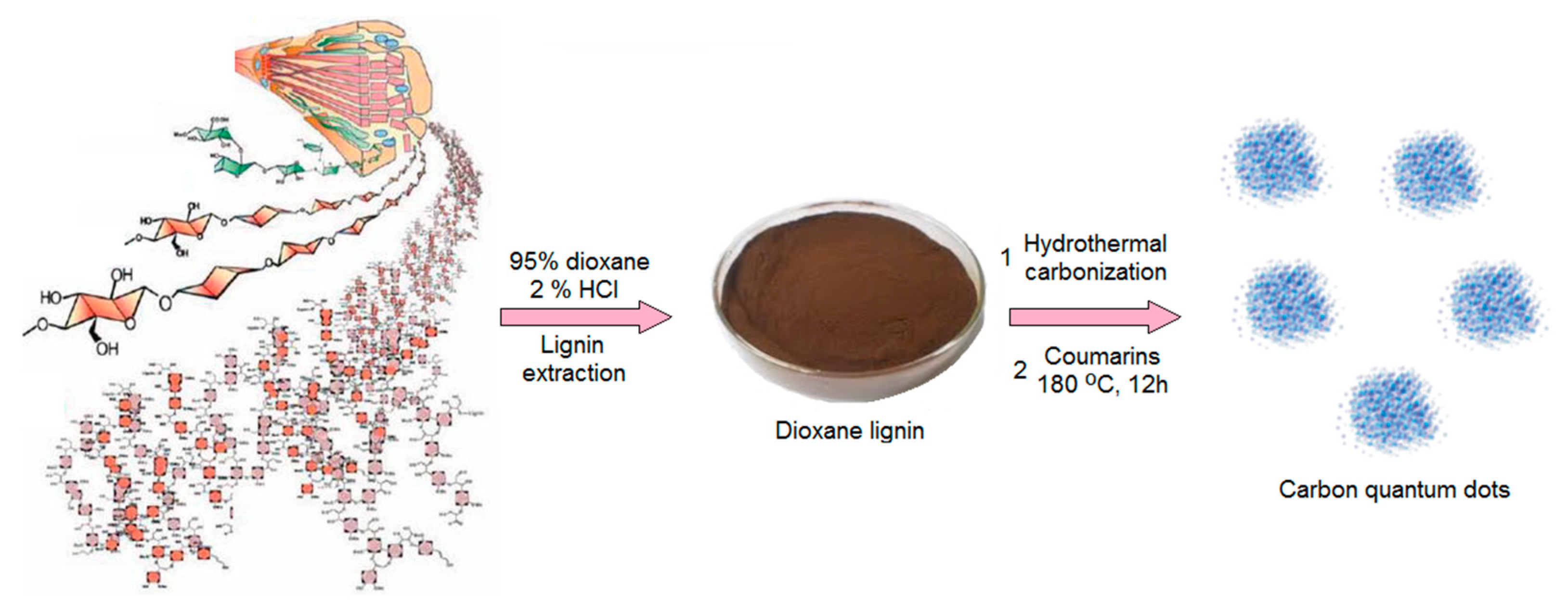
3.2.1. CQDs Preparation
3.2.2. Chemical Structure Analysis
3.2.3. Optical Properties Study
3.2.4. Morphology Study
3.2.5. In Vitro Cytotoxicity Study
3.2.6. Cells Visualization Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duong, H.D.; Sohn, O.-J.; Rhee, J.I. Development of a Ratiometric Fluorescent Glucose Sensor Using an Oxygen-Sensing Membrane Immobilized with Glucose Oxidase for the Detection of Glucose in Tears. Biosensors 2020, 10, 86. [Google Scholar] [CrossRef]
- Pickup, J.C.; Hussain, F.; Evans, N.D.; Rolinski, O.J.; Birch, D.J. Fluorescence-based glucose sensors. Biosens. Bioelectron. 2005, 20, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Facile Synthesis of Surface-Modified Carbon Quantum Dots (CQDs) for Biosensing and Bioimaging. Materials 2020, 13, 3313. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in fluorescent carbon dots for biomedical applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Ayyala, R.S.; Bhethanabotla, V.R.; Sahiner, N. Nitrogen-Doped Arginine Carbon Dots and Its Metal Nanoparticle Composites as Antibacterial Agent. C J. Carbon Res. 2020, 6, 58. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Su, Y.; Wu, X.; Narron, R.; Yong, Q. Synthesis of Carbon Quantum Dot Nanoparticles Derived from Byproducts in Bio-Refinery Process for Cell Imaging and In Vivo Bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef]
- Nikazar, S.; Sivasankarapillai, V.S.; Rahdar, A.; Gasmi, S.; Anumol, P.S.; Shanavas, M.S. Revisiting the cytotoxicity of quantum dots: An in-depth overview. Biophys. Rev. 2020, 12, 703–718. [Google Scholar] [CrossRef]
- Semonin, O.E.; Luther, J.M.; Beard, M.C. Quantum dots for next-generation photovoltaics. Mater. Today 2012, 15, 508–515. [Google Scholar] [CrossRef]
- Huang, X.; Tong, X.; Wang, Z. Rational design of colloidal core/shell quantum dots for optoelectronic applications. J. Electron. Sci. Technol. 2020, 100018. [Google Scholar] [CrossRef]
- Manikandan, A.; Chen, Y.-Z.; Shen, C.-C.; Sher, C.-W.; Kuo, H.-C.; Chueh, Y.-L. A critical review on two-dimensional quantum dots (2D QDs): From synthesis toward applications in energy and optoelectronics. Prog. Quantum Electron. 2019, 68, 100226. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro- and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Reshak, A. Quantum dots in photocatalytic applications: Efficiently enhancing visible light photocatalytic activity by integrating CdO quantum dots as sensitizers. Phys. Chem. Chem. Phys. 2017, 19, 24915–24927. [Google Scholar] [CrossRef]
- Huang, C.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. Photocatalysis with Quantum Dots and Visible Light for Effective Organic Synthesis. Chem. Eur. J. 2018, 24, 11530–11534. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Cui, L.; Li, C.-C.; Tang, B.; Zhang, C.-Y. Advances in the integration of quantum dots with various nanomaterials for biomedical and environmental applications. Analalyst 2018, 143, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhao, Y.; Chen, J.; Zhu, J.-J. Metal ions optical sensing by semiconductor quantum dots. J. Mater. Chem. C 2014, 2, 595–613. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, Y.; Gu, M.; Huo, X.; Ma, S.; Lu, Y.; Ning, Y.; Zhang, X.; Tian, B.; Feng, Z. Carbon Dots Derived from the Maillard Reaction for pH Sensors and Cr (VI) Detection. Nanomaterials 2020, 10, 1924. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Litvin, A.P.; Purcell-Milton, F.; Baranov, A.V.; Fedorov, A.V.; Gun’Ko, Y.K. Application of semiconductor quantum dots in bioimaging and biosensing. J. Mater. Chem. B 2017, 5, 6701–6727. [Google Scholar] [CrossRef]
- Park, Y.; Jeong, S.; Kim, S. Medically translatable quantum dots for biosensing and imaging. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 51–70. [Google Scholar] [CrossRef]
- Jin, S.; Hu, Y.; Gu, Z.; Liu, L.; Wu, H.-C. Application of Quantum Dots in Biological Imaging. J. Nanomater. 2011, 2011, 834139. [Google Scholar] [CrossRef]
- Kairdolf, B.A.; Smith, A.M.; Stokes, T.H.; Wang, M.D.; Young, A.N.; Nie, S. Semiconductor Quantum Dots for Bioimaging and Biodiagnostic Applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Reshma, V.; Mohanan, P. Quantum dots: Applications and safety consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114417. [Google Scholar] [CrossRef]
- Yu, J.; Shendre, S.; Koh, W.-K.; Liu, B.; Li, M.; Hou, S.; Hettiarachchi, C.; Delikanli, S.; Hernández-Martínez, P.; Birowosuto, M.D.; et al. Electrically control amplified spontaneous emission in colloidal quantum dots. Sci. Adv. 2019, 5, eaav3140. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, G.; Ding, C.; Liu, F.; Liu, D.; Masuda, T.; Yoshino, K.; Hayase, S.; Wang, R.; Shen, Q. Surface-Modified Graphene Oxide/Lead Sulfide Hybrid Film-Forming Ink for High-Efficiency Bulk Nano-Heterojunction Colloidal Quantum Dot Solar Cells. Nano-Micro Lett. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Deng, X.; Feng, Y.; Li, H.; Du, Z.; Teng, Q.; Wang, H. N-doped carbon quantum dots as fluorescent probes for highly selective and sensitive detection of Fe3+ ions. Particuology 2018, 41, 94–100. [Google Scholar] [CrossRef]
- Lin, F.; Bao, Y.-W.; Wu, F.-G. Carbon Dots for Sensing and Killing Microorganisms. C J. Carbon Res. 2019, 5, 33. [Google Scholar] [CrossRef]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.; Mahmoud, A.H.; Ibrahim, B.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, J.; Huh, Y.; Kim, D. Benzo[g]coumarin-Based Fluorescent Probes for Bioimaging Applications. J. Anal. Methods Chem. 2018, 2018, 5249765. [Google Scholar] [CrossRef]
- Li, X.; Huo, F.; Yue, Y.; Zhang, Y.; Yin, C. A coumarin-based “off-on” sensor for fluorescence selectivily discriminating GSH from Cys/Hcy and its bioimaging in living cells. Sens. Actuators B Chem. 2017, 253, 42–49. [Google Scholar] [CrossRef]
- Tsao, K.K.; Lee, A.C.; Racine, K.É.; Keillor, J.W. Site-Specific Fluorogenic Protein Labelling Agent for Bioconjugation. Biomolecules 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.M.; Seoane-Rivero, R.; Navarro, R.; Marcos-Fernández, A. Coumarins into Polyurethanes for Smart and Functional Materials. Polymers 2020, 12, 630. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, Y.; Sun, Y.; Fan, J.; Li, X.; Zhang, Z. Photoluminescent lignin hybridized carbon quantum dots composites for bioimaging applications. Int. J. Biol. Macromol. 2019, 122, 954–961. [Google Scholar] [CrossRef] [PubMed]
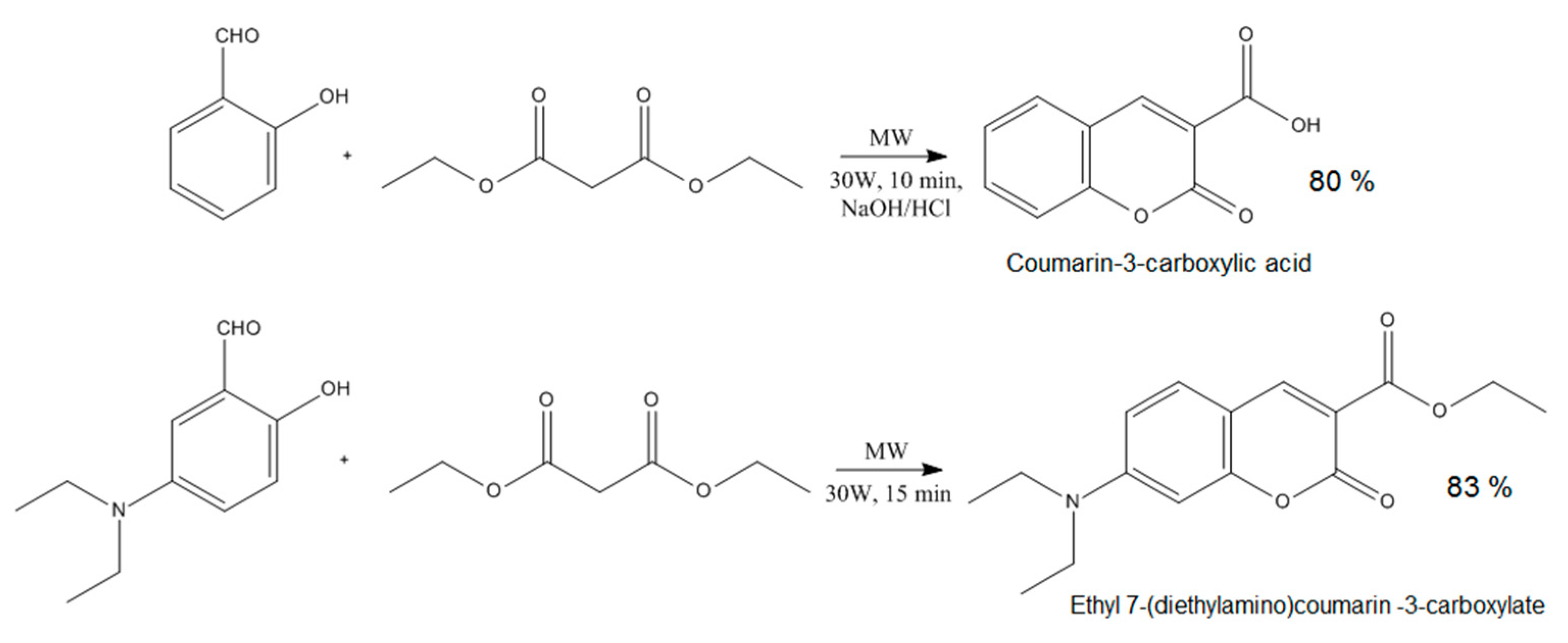
- Martínez, J.; Sánchez, L.; Pérez, F.J.; Carranza, V.; Delgado, F.; Reyes, L.; Miranda, R. Uncatalysed Production of Coumarin-3-carboxylic Acids: A Green Approach. J. Chem. 2016, 2016, 4678107. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Ren, M.; Li, W.; Zhang, X.; Vajtai, R.; Ajayan, P.M.; Tour, J.M.; Wang, L. Sustainable Synthesis of Bright Green Fluorescent Nitrogen-Doped Carbon Quantum Dots from Alkali Lignin. ChemSusChem 2019, 12, 4202–4210. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.; Van Dam, J.E. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
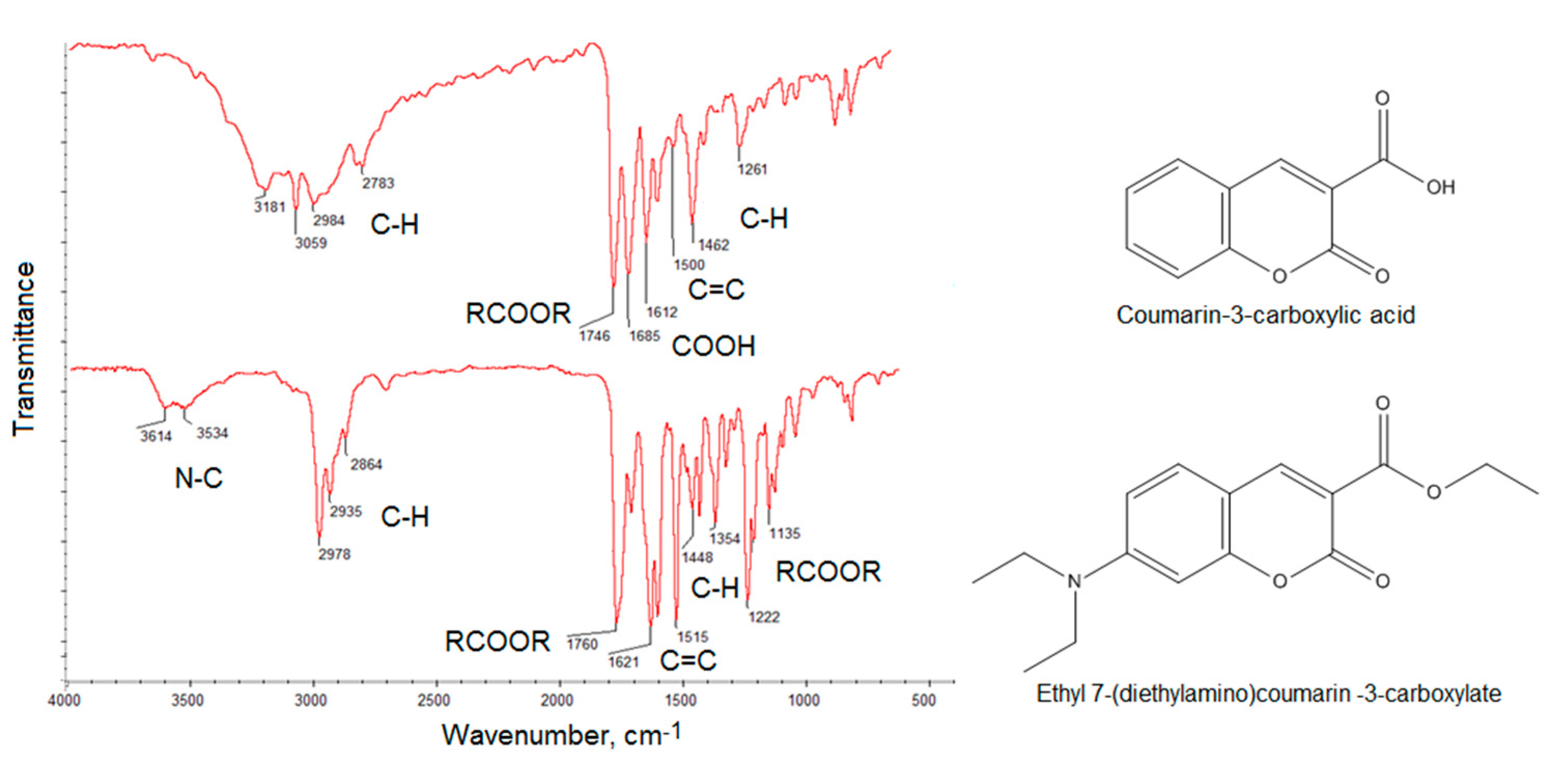
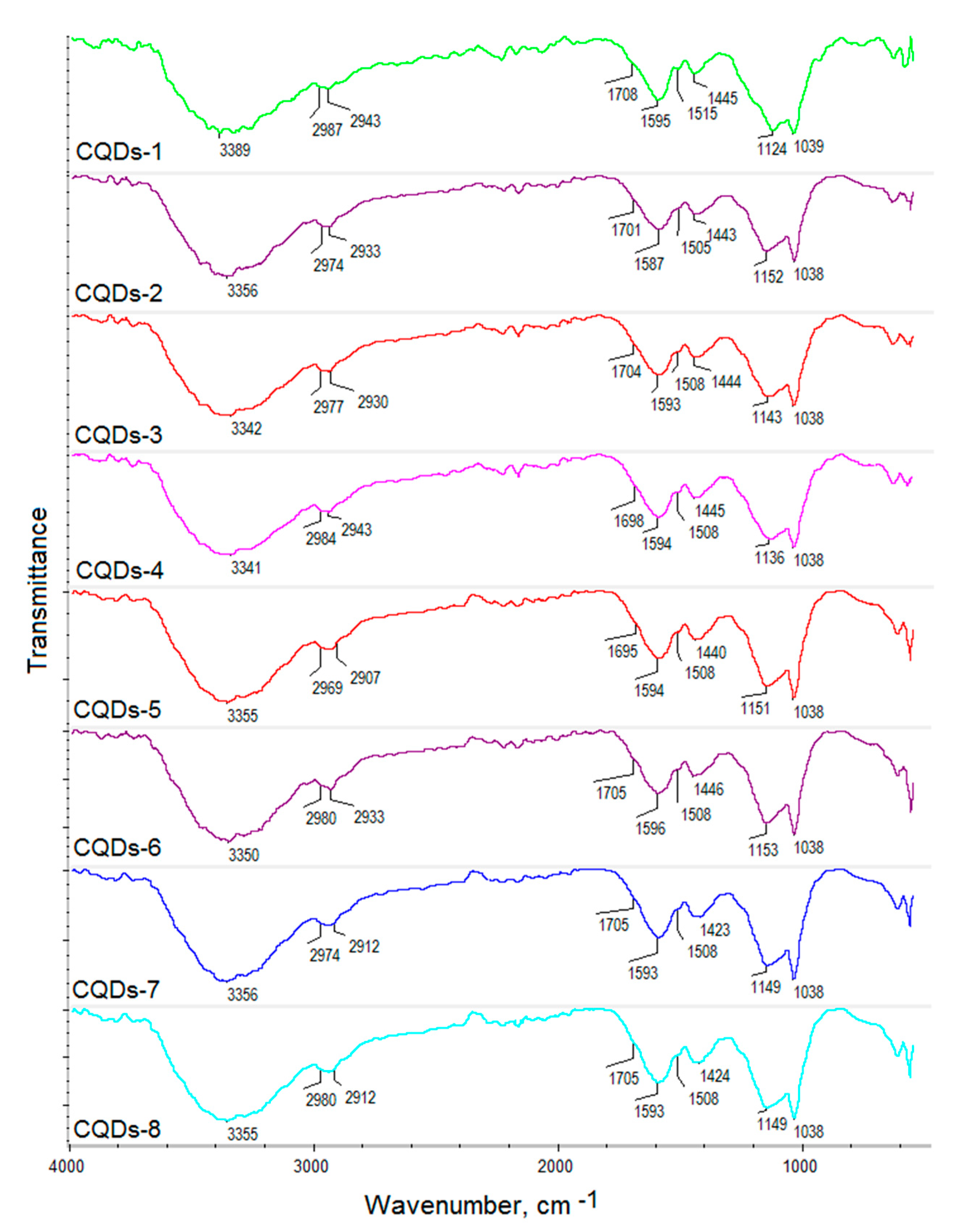
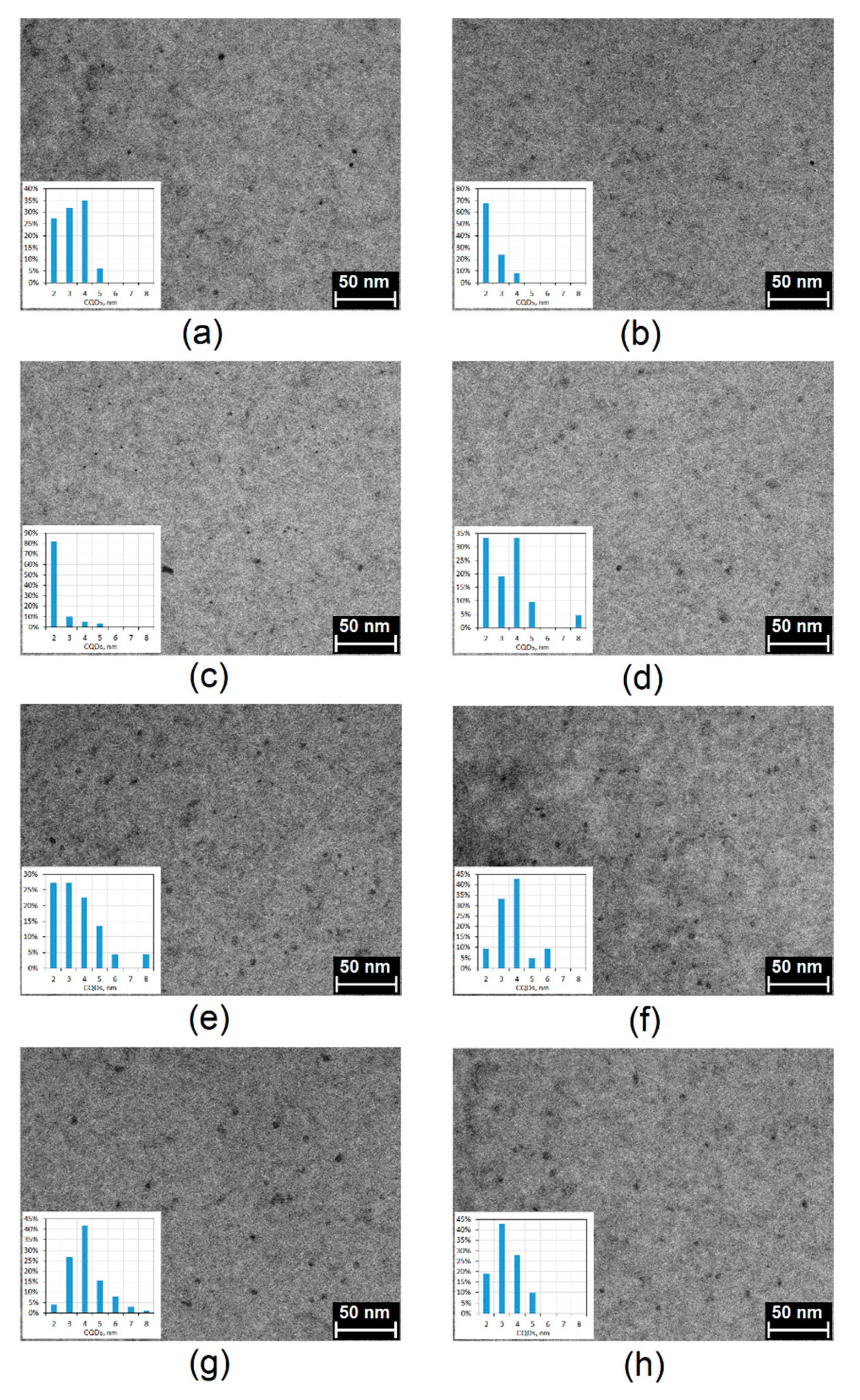
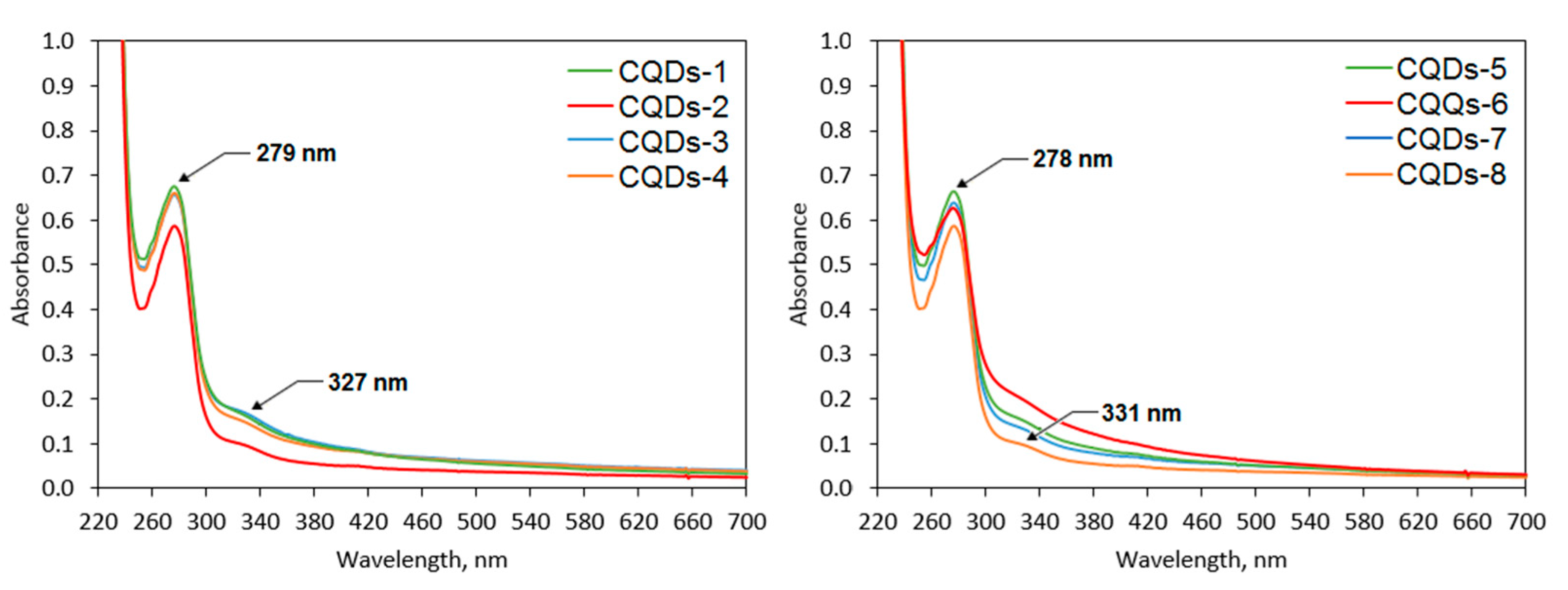


| Sample | Fluorescence Quantum Yield, % | Photostability after 7 Days, % | Photostability after 30 Days, % |
|---|---|---|---|
| CQDs-1 | 3.4 | 3.4 | 3.3 |
| CQDs-2 | 6.7 | 6.6 | 6.4 |
| CQDs-3 | 11.2 | 11.1 | 11.0 |
| CQDs-4 | 11.1 | 11.0 | 10.6 |
| CQDs-5 | 3.5 | 3.3 | 3.3 |
| CQDs-6 | 9.8 | 9.6 | 9.5 |
| CQDs-7 | 14.7 | 14.5 | 14.2 |
| CQDs-8 | 18.4 | 18.1 | 17.8 |
| Sample | Analite | Regression Equation |
|---|---|---|
| CQDs-4 | Potassium dichromate | y = −1874.4x + 153.16 |
| Glucose | y = −48.505x + 156.82 | |
| Fructose | y = −58.180x + 156.62 | |
| Egg albumin | y = 47.451x + 155.75 | |
| CQDs-8 | Potassium dichromate | y = −3190.7x + 328.27 |
| Glucose | y = −279.9x + 334.29 | |
| Fructose | y = −257.02x + 336.07 | |
| Egg albumin | y = 21.161x + 336.14 |
| Sample | Lignin, g; Propylene Carbonate, mL; H2SO4, g | Hydrothermal Carbonization Time, h | Coumarin, g | Reaction Time, min (MW 30 W) |
|---|---|---|---|---|
| CQDs-1 | 0.05; 3; 0.05 | 12 | Coumarin-3-carboxylic acid, 0.05 | 2 |
| CQDs-2 | 5 | |||
| CQDs-3 | 10 | |||
| CQDs-4 | 20 | |||
| CQDs-5 | 7-(diethylaminocoumarin)-3-carboxylate, 0.05 | 2 | ||
| CQDs-6 | 5 | |||
| CQDs-7 | 10 | |||
| CQDs-8 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Coumarin-Modified CQDs for Biomedical Applications—Two-Step Synthesis and Characterization. Int. J. Mol. Sci. 2020, 21, 8073. https://doi.org/10.3390/ijms21218073
Janus Ł, Radwan-Pragłowska J, Piątkowski M, Bogdał D. Coumarin-Modified CQDs for Biomedical Applications—Two-Step Synthesis and Characterization. International Journal of Molecular Sciences. 2020; 21(21):8073. https://doi.org/10.3390/ijms21218073
Chicago/Turabian StyleJanus, Łukasz, Julia Radwan-Pragłowska, Marek Piątkowski, and Dariusz Bogdał. 2020. "Coumarin-Modified CQDs for Biomedical Applications—Two-Step Synthesis and Characterization" International Journal of Molecular Sciences 21, no. 21: 8073. https://doi.org/10.3390/ijms21218073
APA StyleJanus, Ł., Radwan-Pragłowska, J., Piątkowski, M., & Bogdał, D. (2020). Coumarin-Modified CQDs for Biomedical Applications—Two-Step Synthesis and Characterization. International Journal of Molecular Sciences, 21(21), 8073. https://doi.org/10.3390/ijms21218073