Sexual Dimorphism in the Chemical Composition of Male and Female in the Dioecious Tree, Juniperus communis L., Growing under Different Nutritional Conditions
Abstract
:1. Introduction
- (1)
- Females allocate more resources to storage and defense than males. Therefore, female leaves have a greater concentration of carbon, carbohydrates, and C:N ratio, as well as phenolic compounds, but a lower concentration of other macroelements.
- (2)
- Differences between sexes are most evident when plants are grown under nutrient limited conditions.
- (3)
- Differences between male and female plants will be most pronounced after strobili opening when females produce seeds and accompanying structures and during the most intensive period of vegetative growth. Differences would also be evident in winter (December) when additional stresses, such as low temperatures, occur.
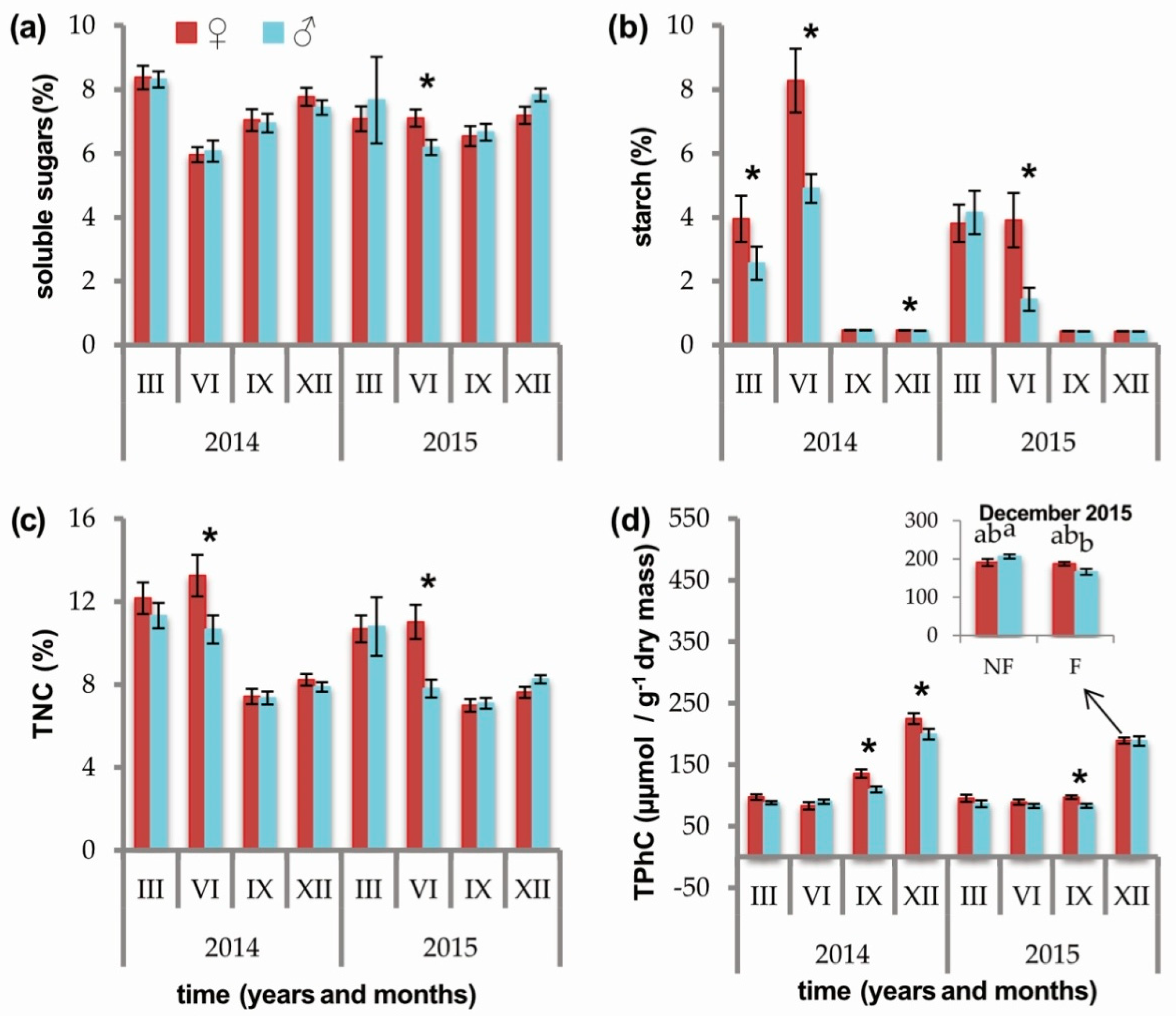
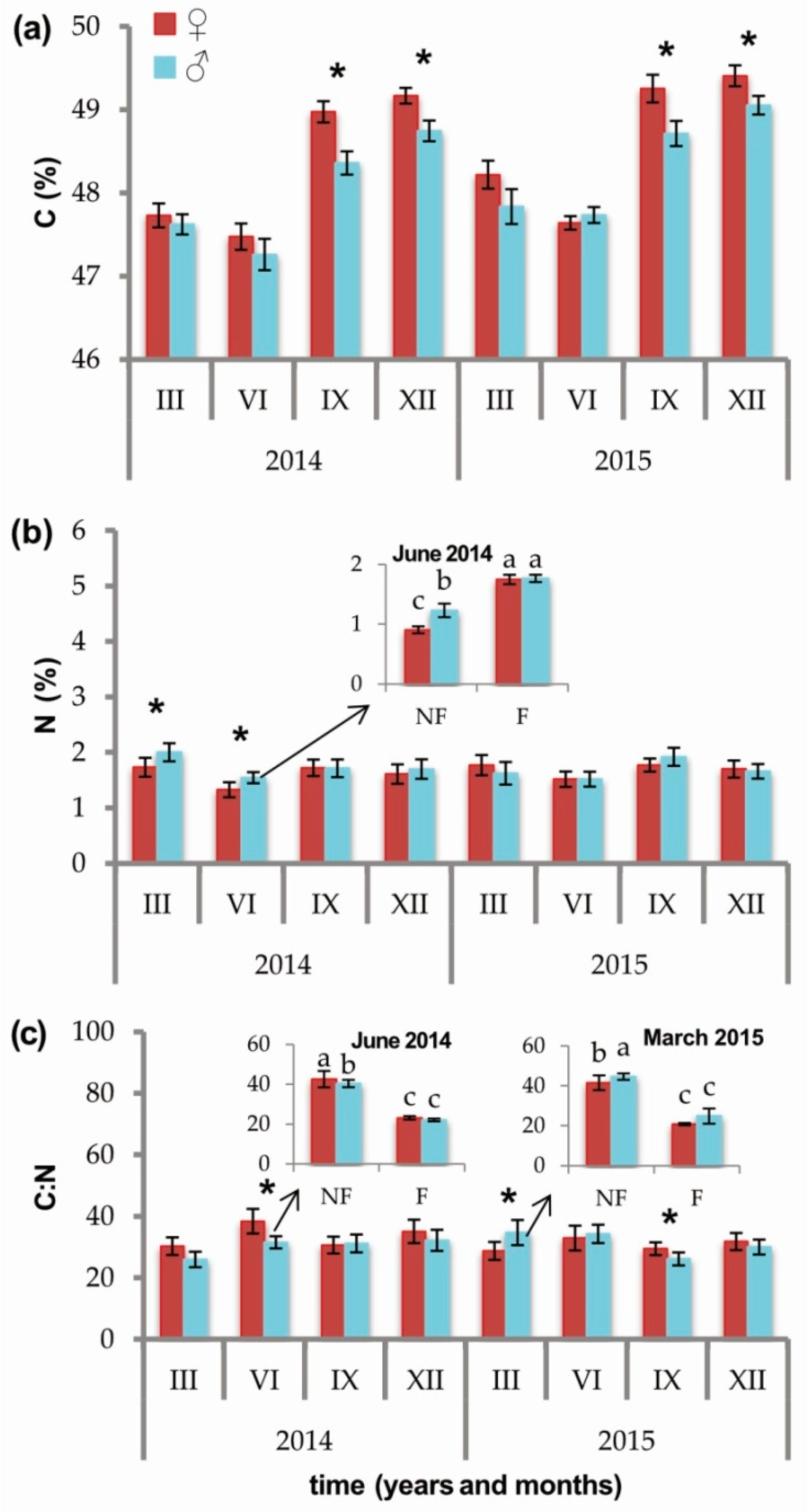
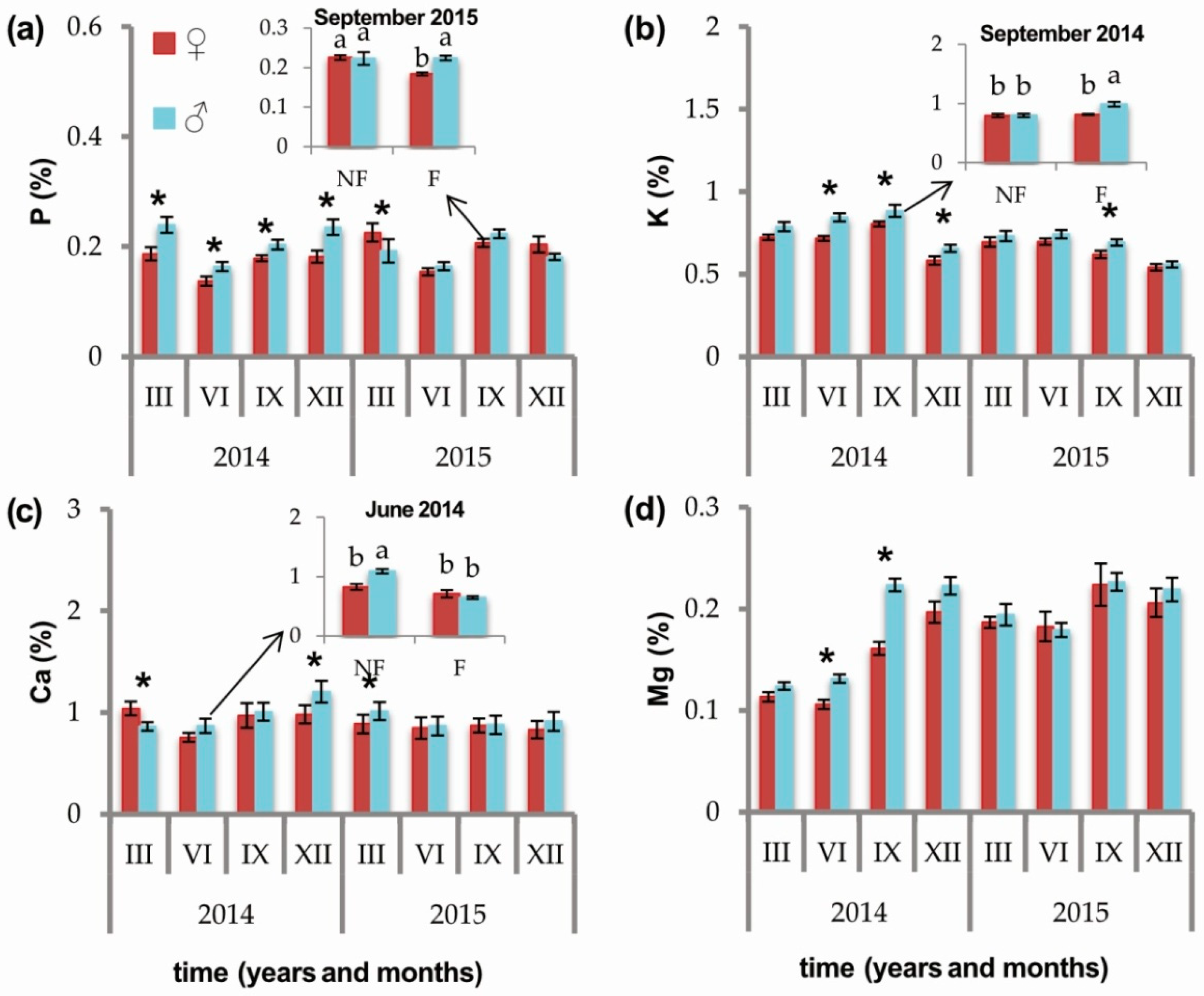
2. Results
2.1. Effect of Soil Fertilization
2.2. Effects of Sex and Sex × Soil Fertilization Interactions
3. Discussion
3.1. Carbohydrates
3.2. Carbon, Nitrogen, C:N Ratio, and Other Elements
3.3. Phenolic Compounds
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design and Sampling
4.3. Microclimate
4.4. Sugars and Starch
4.5. Elemental Analysis
4.6. Total Phenols
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TPhC | Total phenolic compounds |
| TNC | Total nonstructural carbohydrates |
References
- Gehring, J.L.; Linhart, Y.B. Sexual dimorphisms and response to low resources in the dioecious plant Silene latifolia (Caryophyllaceae). Int. J. Plant. Sci. 1993, 154, 152–162. [Google Scholar] [CrossRef]
- Sherman, D.A.; Dahlgren, J.P.; Ehrlén, J.; García, M.B. Sex and the cost of reproduction through the life course of an extremely long-lived herb. Oecologia 2019, 191, 369–375. [Google Scholar] [CrossRef]
- Elmqvist, T.; Cates, R.G.; Harper, J.K.; Gardfjell, H. Flowering in males and females of a Utah willow, Salix rigida and effects on growth, tannins, phenolic glycosides and sugars. Oikos 1991, 61, 65–72. [Google Scholar] [CrossRef]
- Bullock, S.H. Biomass and nutrient allocation in a neotropical dioecious palm. Oecologia 1984, 63, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Iszkuło, G.; Boratyński, A. Initial period of sexual maturity determines the greater growth rate of male over female in the dioecious tree Juniperus communis subsp. communis. Acta Oecol. 2011, 37, 99–102. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Liu, Q.; He, H.; Xu, X.; Dong, T. Sexual differences in growth and defence of Populus yunnanensis under drought stress. Can. J. Res. 2019, 49, 491–499. [Google Scholar] [CrossRef]
- Houssard, C.; Escarré, J.; Vartanian, N. Water stress effects on successional populations of the dioecious herb, Rumex acetosella L. New Phytol. 1992, 120, 551–559. [Google Scholar] [CrossRef]
- Bañuelos, M.-J.; Obeso, J.-R. Resource allocation in the dioecious shrub Rhamnus alpinus: The hidden costs of reproduction. Evol. Ecol. Res. 2004, 6, 397–413. [Google Scholar]
- Cipollini, M.L.; Stiles, E.W. Costs of reproduction in Nyssa sylvatica: Sexual dimorphism in reproductive frequency and nutrient flux. Oecologia 1991, 86, 585–593. [Google Scholar] [CrossRef]
- Allen, G.A.; Antos, J.A. Sex ratio variation in the dioecious shrub Oemleria cerasiformis. Am. Nat. 1993, 141, 537–553. [Google Scholar] [CrossRef]
- Espírito-Santo, M.M.; Madeira, B.G.; Neves, F.S.; Faria, M.L.; Fagundes, M.; Fernandes, G.W. Sexual differences in reproductive phenology and their consequences for the demography of Baccharis dracunculifolia (Asteraceae), a dioecious tropical shrub. Ann. Bot. 2003, 91, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Iszkuło, G.; Jasińska, A.K.; Giertych, M.J.; Boratyński, A. Do secondary sexual dimorphism and female intolerance to drought influence the sex ratio and extinction risk of Taxus baccata? Plant. Ecol. 2009, 200, 229–240. [Google Scholar] [CrossRef]
- McDowell, S.C.L.; McDowell, N.G.; Marshall, J.D.; Hultine, K. Carbon and nitrogen allocation to male and female reproduction in Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca, Pinaceae). Am. J. Bot. 2000, 87, 539–546. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, X.; Zhang, Y.; Korpelainen, H.; Li, C. Nitrogen deposition limits photosynthetic response to elevated CO2 differentially in a dioecious species. Oecologia 2011, 165, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhry, N.; Singh, S.; Siddiqui, M.B.; Khatoon, S. Impact of seasons and dioecy on therapeutic phytoconstituents of Tinospora cordifolia, a rasayana drug. Biomed. Res. Int. 2014, 2014, 902138. [Google Scholar] [CrossRef]
- Randriamanana, T.R.; Nissinen, K.; Moilanen, J.; Nybakken, L.; Julkunen-Tiitto, R. Long-term UV-B and temperature enhancements suggest that females of Salix myrsinifolia plants are more tolerant to UV-B than males. Env. Exp. Bot. 2015, 109, 296–305. [Google Scholar] [CrossRef]
- García, M.B.; Antor, R.J. Sex ratio and sexual dimorphism in the dioecious Borderea pyrenaica (Dioscoreaceae). Oecologia 1995, 101, 59–67. [Google Scholar] [CrossRef]
- DeSoto, L.; Olano, J.M.; Rozas, V. Secondary growth and carbohydrate storage patterns differ between sexes in Juniperus thurifera. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Robakowski, P.; Pers-Kamczyc, E.; Ratajczak, E.; Thomas, P.A.; Ye, Z.-P.; Rabska, M.; Iszkuło, G. Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Front. Plant. Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Laporte, M.M.; Delph, L.F. Sex-specific physiology and source-sink relations in the dioecious plant Silene latifolia. Oecologia 1996, 106, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, N.; Liu, T.; Tang, M.; Chen, H. Gender-related responses of dioecious plant Populus cathayana to AMF, drought and planting pattern. Sci. Rep. 2020, 10, 11530. [Google Scholar] [CrossRef]
- Xia, Z.; He, Y.; Yu, L.; Lv, R.; Korpelainen, H.; Li, C. Sex-specific strategies of phosphorus (P) acquisition in Populus cathayana as affected by soil P availability and distribution. New Phytol. 2020, 225, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Obeso, J.R. The costs of reproduction in plants. New Phytol. 2002, 155, 321–348. [Google Scholar] [CrossRef]
- Kachi, N.; Hirose, T. Limiting nutrients for plant growth in coastal sand dune soils. J. Ecol. 1983, 71, 937–944. [Google Scholar] [CrossRef]
- Song, H.; Cai, Z.; Liao, J.; Tang, D.; Zhang, S. Sexually differential gene expressions in poplar roots in response to nitrogen deficiency. Tree Physiol. 2019, 39, 1614–1629. [Google Scholar] [CrossRef]
- Grunes, D.L. Effect of nitrogen on the availability of soil and fertilizer phosphorus to plants. In Advances in Agronomy; Norman, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1959; Volume 11, pp. 369–396. [Google Scholar]
- Krstić, Đ.; Vukojević, V.; Mutić, J.; Akšić, M.F.; Ličina, V.; Milojković-Opsenica, D.; Trifković, J. Distribution of elements in seeds of some wild and cultivated fruits. Nutrition and authenticity aspects. J. Sci. Food Agric. 2019, 99, 546–554. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant. Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Spencer, D.F.; Ryan, F.J.; Aung, L.; Ksander, G.G. Soluble sugar concentrations associated with tuber and winter bud sprouting. J. Aquat. Plant. Manag. 2001, 39, 45–47. [Google Scholar]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Dyjeta, K.; Giertych, M.J.; Thomas, P.; Iszkuło, G. Males and females of Juniperus communis L. and Taxus baccata L. show different seasonal patterns of nitrogen and carbon content in needles. Acta Physiol. Plant. 2017, 39, 191. [Google Scholar] [CrossRef] [Green Version]
- Rabska, M.; Robakowski, P.; Ratajczak, E.; Żytkowiak, R.; Iszkuło, G.; Pers-Kamczyc, E. Photochemistry differs between male and female Juniperus communis L. independently of nutritional availability. Trees 2020. [Google Scholar] [CrossRef]
- Vamosi, J.C.; Vamosi, S.M. Present day risk of extinction may exacerbate the lower species richness of dioecious clades. Divers. Distrib. 2005, 11, 25–32. [Google Scholar] [CrossRef]
- Verheyen, K.; Adriaenssens, S.; Gruwez, R.; Michalczyk, I.; Ward, L.; Rosseel, Y.; Broeck, A.; García, D. Juniperus communis: Victim of the combined action of climate warming and nitrogen deposition? Plant. Biol. 2009, 11, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, P.L.; Arista, M.; Talavera, S. Sex ratio and reproductive effort in the dioecious Juniperus communis subsp. alpina (Suter) Čelak. (Cupressaceae) along an altitudinal gradient. Ann. Bot. 2002, 89, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; EL Barghathi, M.; Polwart, A. Biological Flora of the British Isles: Juniperus communis L. J. Ecol. 2007, 95, 1404–1440. [Google Scholar] [CrossRef]
- Tonnabel, J.; David, P.; Pannell, J.R. Sex-specific strategies of resource allocation in response to competition for light in a dioecious plant. Oecologia 2017, 185, 675–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zhang, S.; Lei, Y.; Xu, G.; Zhang, D. Alternative growth and defensive strategies reveal potential and gender specific trade-offs in dioecious plants Salix paraplesia to nutrient availability. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, S.A.; Volkmar, K.M. Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant. Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Blake-Mahmud, J.; Struwe, L. Death, sex, and sugars: Variations in nonstructural carbohydrate concentrations in a sexually plastic tree. Am. J. Bot. 2020, 107, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Dong, T.; Duan, B. Sex-specific carbon and nitrogen partitioning under N deposition in Populus cathayana. Trees 2014, 28, 793–806. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, D.; Korpelainen, H.; Li, C. Metabolic and physiological analyses reveal that Populus cathayana males adopt an energy-saving strategy to cope with phosphorus deficiency. Tree Physiol. 2019, 39, 1630–1645. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Madsen, J.D.; Owens, C.S. Seasonal biomass and carbohydrate allocation in dioecious Hydrilla. J. Aquat. Plant. Manag. 1998, 36, 138–145. [Google Scholar]
- Hansen, J.; Beck, E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1994, 8, 172–182. [Google Scholar] [CrossRef]
- Fischer, C.; Hiill, W. Food reserves of Scots pine (Pinus sylvestris L.) I. Seasonal changes in the carbohydrate and fat reserves of pine needles. Trees 1991, 5, 187–195. [Google Scholar] [CrossRef]
- Keeling, P.L.; Bacon, P.J.; Holt, D.C. Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 1993, 191, 342–348. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O.; Tenhunen, J.D. An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiol. 1998, 18, 681–696. [Google Scholar] [CrossRef] [Green Version]
- Nowak, K.; Giertych, M.J.; Pers-Kamczyc, E.; Thomas, P.A.; Iszkuło, G. Rich but not poor conditions determine sex-specific differences in growth rate of dioecious plants. J. Plant Res. in preparation.
- Rabie, A.L.; Wells, J.D.; Dent, L.K. The nitrogen content of pollen protein. J. Apic. Res. 1983, 22, 119–123. [Google Scholar] [CrossRef]
- Selaković, S.; Vujić, V.; Stanisavljević, N.; Jovanović, Ž.; Radović, S.; Cvetković, D. Ontogenetic stage, plant vigor and sex mediate herbivory loads in a dioecious understory herb. Acta Oecologica 2017, 85, 184–190. [Google Scholar] [CrossRef]
- Montesinos, D.; Villar-Salvador, P.; García-Fayos, P.; Verdú, M. Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytol. 2012, 193, 705–712. [Google Scholar] [CrossRef]
- Randriamanana, T.R.; Lavola, A.; Julkunen-Tiitto, R. Interactive effects of supplemental UV-B and temperature in European aspen seedlings: Implications for growth, leaf traits, phenolic defense and associated organisms. Plant. Physiol. Biochem. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Miljković, D.; Selaković, S.; Vujić, V.; Stanisavljević, N.; Radović, S.; Cvetković, D. Patterns of herbivore damage, developmental stability, morphological and biochemical traits in female and male Mercurialis perennis in contrasting light habitats. Alp. Bot. 2018, 128, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Sobuj, N.; Virjamo, V.; Zhang, Y.; Nybakken, L.; Julkunen-Tiitto, R. Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L.). Env. Exp. Bot. 2018, 146, 34–44. [Google Scholar] [CrossRef]
- Sampedro, L.; Moreira, X.; Zas, R. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. J. Ecol. 2011, 99, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Randriamanana, T.R.; Nybakken, L.; Lavola, A.; Aphalo, P.J.; Nissinen, K.; Julkunen-Tiitto, R. Sex-related differences in growth and carbon allocation to defence in Populus tremula as explained by current plant defence theories. Tree Physiol. 2014, 34, 471–487. [Google Scholar] [CrossRef]
- Zahra, P.; Majid, R.; Amin, B. Seasonal changes of peroxidase, polyphenol oxidase enzyme activity and phenol content during and after rest in pistachio (Pistacia vera L.) flower buds. World Appl. Sci. J. 2009, 6, 1193–1199. [Google Scholar]
- Messier, C.; Puttonen, P. Spatial and temporal variation in the Bight environment of developing scots pine stands: The basis for a quick and efficient method of characterizing Bight. Can. J. Res. 1995, 25, 343–354. [Google Scholar] [CrossRef]
- Haissig, B.E.; Dickson, R.E. Starch measurement in plant tissue using enzymatic hydrolysis. Physiol. Plant. 1979, 47, 151–157. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Oleksyn, J.; Karolewski, P.; Giertych, M.J.; Zytkowiak, R.; Reich, P.B.; Tjoelker, M.G. Primary and secondary host plants differ in leaf-level photosynthetic response to herbivory: Evidence from Alnus and Betula grazed by the alder beetle, Agelastica alni. New Phytol. 1998, 140, 239–249. [Google Scholar] [CrossRef]
- PN-EN ISO 11885:2009. Water Quality. Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (ISO 11885:2007), Polish version PN-EN ISO 11885:2009; Polski Komitet Normalizacyjny: Warszawa, Poland, 2009; pp. 1–42. ISBN 978-832-518-149-9. [Google Scholar]
- Johnson, G.; Schaal, L.A. Accumulation of phenolic substances and ascorbic acid in potato tuber tissue upon injury and their possible role in disease resistance. Am. Potato J. 1957, 34, 200–209. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bliss, C.I. The transformation of percentages for use in the analysis of variance. Ohio J. Sci. 1938, 38, 9–12. [Google Scholar]
- Dean, A.; Voss, D.; Draguljić, D. Design and Analysis of Experiments, 2nd ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 103–138. ISBN 978-3-319-52250-0. [Google Scholar] [CrossRef]
| Dates | Monthly Temperature (°C) | Relative Air Humidity (%) | |||
|---|---|---|---|---|---|
| Mean | Minimum | Maximum | |||
| I year | March 2014 | 7.47 ± 7.97 | −6.0 | 29.5 | 76.51 ± 21.43 |
| June 2014 | 18.14 ± 7.95 | 4.0 | 40.5 | 69.70 ± 22.49 | |
| September 2014 | 16.49 ± 7.32 | −1.0 | 36.0 | 78.82 ± 20.82 | |
| December 2014 | 1.58 ± 3.33 | −8.0 | 14.5 | 92.21 ± 8.37 | |
| II year | March 2015 | 5.68 ± 6.53 | −8.5 | 26.0 | 76.99 ± 20.54 |
| June 2015 | 17.89 ± 7.95 | 4.0 | 43.0 | 72.34 ± 23.32 | |
| September 2015 | 15.10 ± 7.31 | 0.5 | 44.5 | 76.31 ± 19.13 | |
| December 2015 | −2.00 ± 5.97 | −15.5 | 13.5 | 91.37 ± 8.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabska, M.; Pers-Kamczyc, E.; Żytkowiak, R.; Adamczyk, D.; Iszkuło, G. Sexual Dimorphism in the Chemical Composition of Male and Female in the Dioecious Tree, Juniperus communis L., Growing under Different Nutritional Conditions. Int. J. Mol. Sci. 2020, 21, 8094. https://doi.org/10.3390/ijms21218094
Rabska M, Pers-Kamczyc E, Żytkowiak R, Adamczyk D, Iszkuło G. Sexual Dimorphism in the Chemical Composition of Male and Female in the Dioecious Tree, Juniperus communis L., Growing under Different Nutritional Conditions. International Journal of Molecular Sciences. 2020; 21(21):8094. https://doi.org/10.3390/ijms21218094
Chicago/Turabian StyleRabska, Mariola, Emilia Pers-Kamczyc, Roma Żytkowiak, Dawid Adamczyk, and Grzegorz Iszkuło. 2020. "Sexual Dimorphism in the Chemical Composition of Male and Female in the Dioecious Tree, Juniperus communis L., Growing under Different Nutritional Conditions" International Journal of Molecular Sciences 21, no. 21: 8094. https://doi.org/10.3390/ijms21218094
APA StyleRabska, M., Pers-Kamczyc, E., Żytkowiak, R., Adamczyk, D., & Iszkuło, G. (2020). Sexual Dimorphism in the Chemical Composition of Male and Female in the Dioecious Tree, Juniperus communis L., Growing under Different Nutritional Conditions. International Journal of Molecular Sciences, 21(21), 8094. https://doi.org/10.3390/ijms21218094





