Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model
Abstract
:1. Introduction
2. Result
2.1. hASCs Alleviate Motor Deficits in MPTP-Induced PD Mice
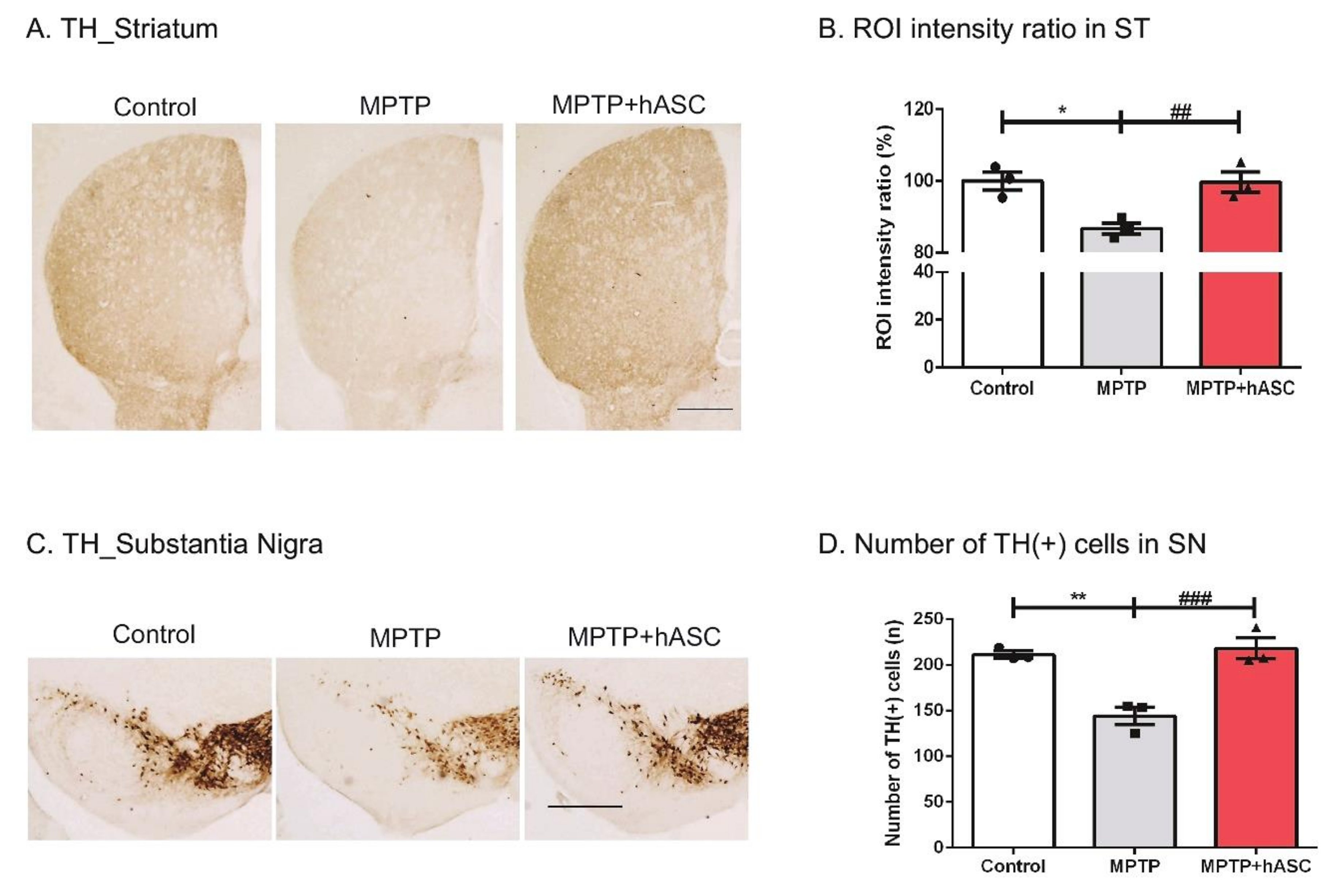
2.2. hASCs Alleviate TH-Positive Cells in MPTP-Induced PD Mice
2.3. hASCs Increase the Levels of BDNF and GDNF Expression in MPTP-Induced PD Mice
3. Discussion
4. Materials and Methods
4.1. Production of MPTP-Induced PD Mouse Model and hASC Administration
4.2. Behavior Test
4.3. Tissue Sampling
4.4. Immunohistochemistry
4.5. Western Blotting
4.6. Statistics
Supplementary Materials
Author contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| DAB | diaminobenzidine |
| DAT | dopamine Transporter |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GDNF | glial cell-derived neurotrophic factor |
| hASCs | human adipose-derived stem cells |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MSC | mesenchymal stem cells |
| NTF | neurotrophic factor |
| PD | Parkinson’s disease |
| SN | substantia nigra |
| ST | striatum |
| TH | tyrosine hydroxylase |
References
- Blaszczyk, J.W. Motor deficiency in Parkinson’s disease. Acta Neurobiol. Exp. 1998, 58, 79–93. [Google Scholar]
- Brooks, D.J. The early diagnosis of Parkinson’s disease. Ann. Neurol. 1998, 44, S10–S18. [Google Scholar] [CrossRef]
- Siderowf, A.; Lang, A.E. Premotor Parkinson’s disease: Concepts and definitions. Mov. Disord. 2012, 27, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Cenci, M.A. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007, 30, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivest, J.; Barclay, C.L.; Suchowersky, O. COMT inhibitors in Parkinson’s disease. Can. J. Neurol. Sci. 1999, 26, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, L.; Vecsei, L. Monoamine oxidase B inhibitors in Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2017, 16, 425–439. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, J.; De Backer, J.P.; Wilczak, N.; Herroelen, L. Dopamine agonists used in the treatment of Parkinson’s disease and their selectivity for the D1, D2, and D3 dopamine receptors in human striatum. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 1147–1154. [Google Scholar] [CrossRef]
- Ptaszek, L.M.; Mansour, M.; Ruskin, J.N.; Chien, K.R. Towards regenerative therapy for cardiac disease. Lancet 2012, 379, 933–942. [Google Scholar] [CrossRef]
- Saki, N.; Jalalifar, M.A.; Soleimani, M.; Hajizamani, S.; Rahim, F. Adverse effect of high glucose concentration on stem cell therapy. Int. J. Hematol. Oncol. Stem Cell Res. 2013, 7, 34–40. [Google Scholar]
- Lyon, L. Stem cell therapies in neurology: The good, the bad and the unknown. Brain 2018, 141, e77. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The international society for cellular therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; et al.; American Association of Neurological Surgeons, (A.S.N.C.); Interventional Radiology Society of, Europe; World Stroke, Organization Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Am. J. Neuroradiol. 2018, 39, E61–E76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Wrage, P.C.; Keefer, E.W.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Autologous transplants of adipose-derived adult stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp. Neurol. 2008, 210, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Schwerk, A.; Altschuler, J.; Roch, M.; Gossen, M.; Winter, C.; Berg, J.; Kurtz, A.; Akyuz, L.; Steiner, B. Adipose-derived human mesenchymal stem cells induce long-term neurogenic and anti-inflammatory effects and improve cognitive but not motor performance in a rat model of Parkinson’s disease. Regen. Med. 2015, 10, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Kim, H.J.; Oh, J.H.; Park, H.G.; Ra, J.C.; Chang, K.A.; Suh, Y.H. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson’s disease. Neurobiol. Aging 2015, 36, 2885–2892. [Google Scholar] [CrossRef] [Green Version]
- Bove, J.; Perier, C. Neurotoxin-based models of Parkinson’s disease. Neuroscience 2012, 211, 51–76. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Heikkila, R.E. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol. 1986, 129, 339–345. [Google Scholar] [CrossRef]
- Meredith, G.E.; Rademacher, D.J. MPTP mouse models of Parkinson’s disease: An update. J. Parkinsons Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petroske, E.; Meredith, G.E.; Callen, S.; Totterdell, S.; Lau, Y.S. Mouse model of Parkinsonism: A comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 2001, 106, 589–601. [Google Scholar] [CrossRef]
- Fornai, F.; Schluter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G.; et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricaurte, G.A.; Langston, J.W.; Delanney, L.E.; Irwin, I.; Peroutka, S.J.; Forno, L.S. Fate of nigrostriatal neurons in young mature mice given 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: A neurochemical and morphological reassessment. Brain Res. 1986, 376, 117–124. [Google Scholar] [CrossRef]
- Schwerk, A.; Altschuler, J.; Roch, M.; Gossen, M.; Winter, C.; Berg, J.; Kurtz, A.; Steiner, B. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy 2015, 17, 199–214. [Google Scholar] [CrossRef]
- de Melo-Martin, I.; Hellmers, N.; Henchcliffe, C. First-in-human cell transplant trials in Parkinson’s disease: The need for an improved informed consent process. Parkinsonism Relat.Disord. 2015, 21, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M. Towards stem cell based therapies for Parkinson’s disease. Development 2018, 145, dev156117. [Google Scholar] [CrossRef] [Green Version]
- Barazzetti, G.; Hurst, S.A.; Mauron, A. Adapting preclinical benchmarks for first-in-human trials of human embryonic stem cell-based therapies. Stem Cells Transl. Med. 2016, 5, 1058–1066. [Google Scholar] [CrossRef]
- Lindvall, O. Clinical translation of stem cell transplantation in Parkinson’s disease. J. Intern. Med. 2016, 279, 30–40. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Liu, X.; Li, D.; Luo, C.; Fu, B.; Cui, S.; Zhu, F.; Zhao, R.C.; Chen, X. Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats. Stem. Cells Dev. 2013, 22, 3074–3086. [Google Scholar] [CrossRef]
- Munoz, M.F.; Arguelles, S.; Guzman-Chozas, M.; Guillen-Sanz, R.; Franco, J.M.; Pintor-Toro, J.A.; Cano, M.; Ayala, A. Cell tracking, survival, and differentiation capacity of adipose-derived stem cells after engraftment in rat tissue. J. Cell Physiol. 2018, 233, 6317–6328. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yasuhara, T.; Shingo, T.; Kameda, M.; Tajiri, N.; Yuan, W.J.; Kondo, A.; Kadota, T.; Baba, T.; Tayra, J.T.; et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: Focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010, 11, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Siegel, G.J.; Chauhan, N.B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res. Rev. 2000, 33, 199–227. [Google Scholar] [CrossRef]
- Tsukahara, T.; Takeda, M.; Shimohama, S.; Ohara, O.; Hashimoto, N. Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery 1995, 37, 733–739. [Google Scholar] [CrossRef]
- Klein, R.L.; Lewis, M.H.; Muzyczka, N.; Meyer, E.M. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999, 847, 314–320. [Google Scholar] [CrossRef]
- Nam, J.H.; Leem, E.; Jeon, M.T.; Jeong, K.H.; Park, J.W.; Jung, U.J.; Kholodilov, N.; Burke, R.E.; Jin, B.K.; Kim, S.R. Induction of GDNF and BDNF by hRheb(S16H) transduction of SNpc neurons: Neuroprotective mechanisms of hRheb(S16H) in a model of Parkinson’s disease. Mol. Neurobiol. 2015, 51, 487–499. [Google Scholar] [CrossRef]
- Levivier, M.; Przedborski, S.; Bencsics, C.; Kang, U.J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 1995, 15, 7810–7820. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Patschan, D.; Dietz, G.P.; Bahr, M.; Plotkin, M.; Goligorsky, M.S. Glial cell line-derived neurotrophic growth factor increases motility and survival of cultured mesenchymal stem cells and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 2008, 294, F229–F235. [Google Scholar] [CrossRef]
- Wang, F.; Kameda, M.; Yasuhara, T.; Tajiri, N.; Kikuchi, Y.; Liang, H.B.; Tayra, J.T.; Shinko, A.; Wakamori, T.; Agari, T.; et al. GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson’s disease. Neurosci. Res. 2011, 71, 92–98. [Google Scholar] [CrossRef]



| Antibody | Company | Cat No. | Titer | Molecular Weight (kDa) | Source |
|---|---|---|---|---|---|
| TH (Immunohistochemical analysis) | Santa Cruz | sc-14007 | 1:200 | - | Rabbit |
| TH (Western blotting) | Santa Cruz | sc-14007 | 1:2000 | 60 | Rabbit |
| Recombinant Anti-BDNF | Abcam | ab108319 | 1:2000 | 15/37 | Rabbit |
| Anti-GDNF antibody | Abcam | ab18956 | 1:2000 | 25 | Rabbit |
| β-actin antibody | Santa Cruz | sc-47778 | 1:2000 | 43 | Mouse |
| GAPDH (A531) | Bioworld | AP0066 | 1:3000 | 36 | Rabbit |
| Goat anti-mouse IgG (H + L)-HRP conjugate | Bio-rad | #170-6516 | 1:10,000 | - | Goat |
| Goat anti-rabbit IgG (H + L)-HRP conjugate | Bio-rad | #170-6515 | 1:2000~1:10,000 | - | Goat |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Chang, K.-A. Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model. Int. J. Mol. Sci. 2020, 21, 8129. https://doi.org/10.3390/ijms21218129
Park H, Chang K-A. Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model. International Journal of Molecular Sciences. 2020; 21(21):8129. https://doi.org/10.3390/ijms21218129
Chicago/Turabian StylePark, Hyunjun, and Keun-A Chang. 2020. "Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model" International Journal of Molecular Sciences 21, no. 21: 8129. https://doi.org/10.3390/ijms21218129
APA StylePark, H., & Chang, K.-A. (2020). Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model. International Journal of Molecular Sciences, 21(21), 8129. https://doi.org/10.3390/ijms21218129






