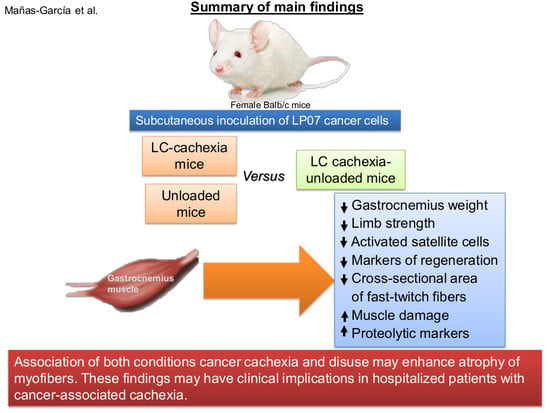
Prolonged Immobilization Exacerbates the Loss of Muscle Mass and Function Induced by Cancer-Associated Cachexia through Enhanced Proteolysis in Mice
Abstract
:1. Introduction
2. Results
2.1. Physiological Characteristics of the Study Animals
2.1.1. Non-Immobilized Controls versus Either Unloading or LC-Cachexia Conditions
2.1.2. LC-Cachexia+Unloading versus Unloading Conditions
2.1.3. LC-Cachexia+Unloading versus LC-Cachexia
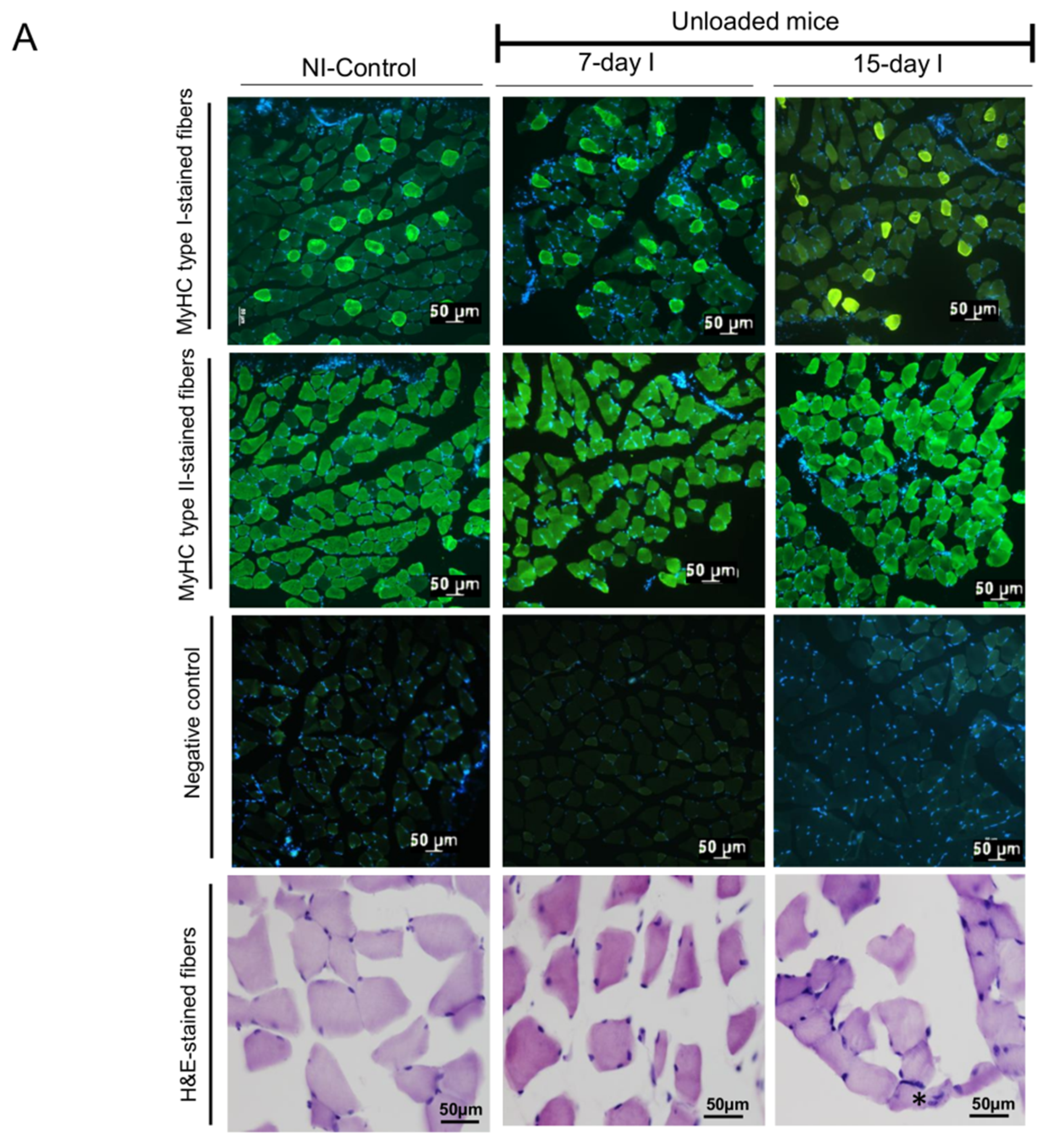
2.2. Structural Phenotypic Characteristics
2.2.1. Non-Immobilized Controls versus Either Unloading or LC-Cachexia Conditions
2.2.2. LC-Cachexia+Unloading versus Unloading Conditions
2.2.3. LC-Cachexia+Unloading versus LC-Cachexia
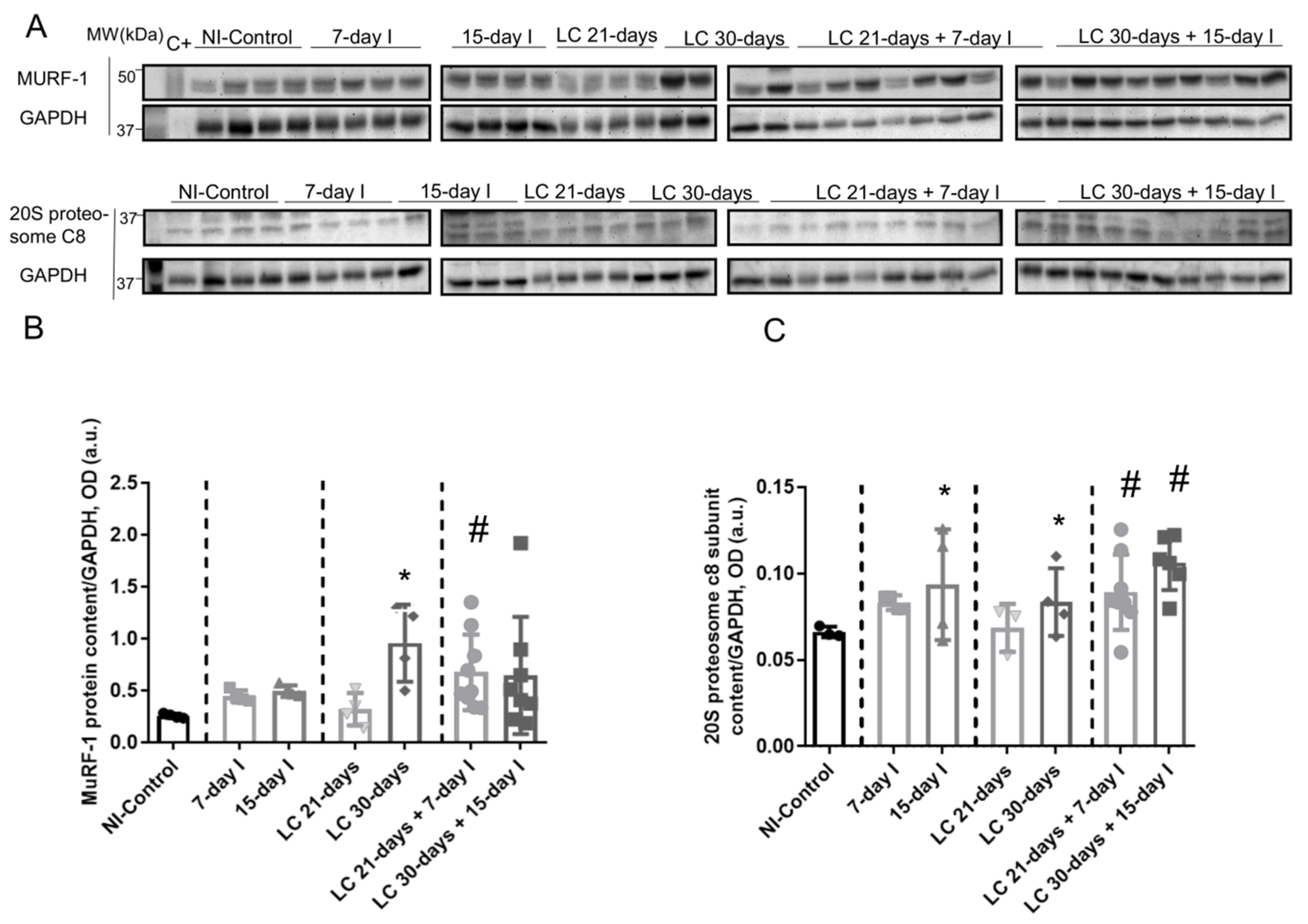
2.3. Muscle Proteolysis and Proteolytic Markers
2.3.1. Non-Immobilized Controls versus Either Unloading or LC-Cachexia Conditions
2.3.2. LC-Cachexia+Unloading versus Unloading Conditions
2.3.3. LC-Cachexia+Unloading versus LC-Cachexia
2.4. Autophagy Markers
2.4.1. Non-Immobilized Controls versus Either Unloading or LC-Cachexia Conditions
2.4.2. LC-Cachexia+Unloading versus Unloading Conditions
2.4.3. LC-Cachexia+Unloading versus LC-Cachexia
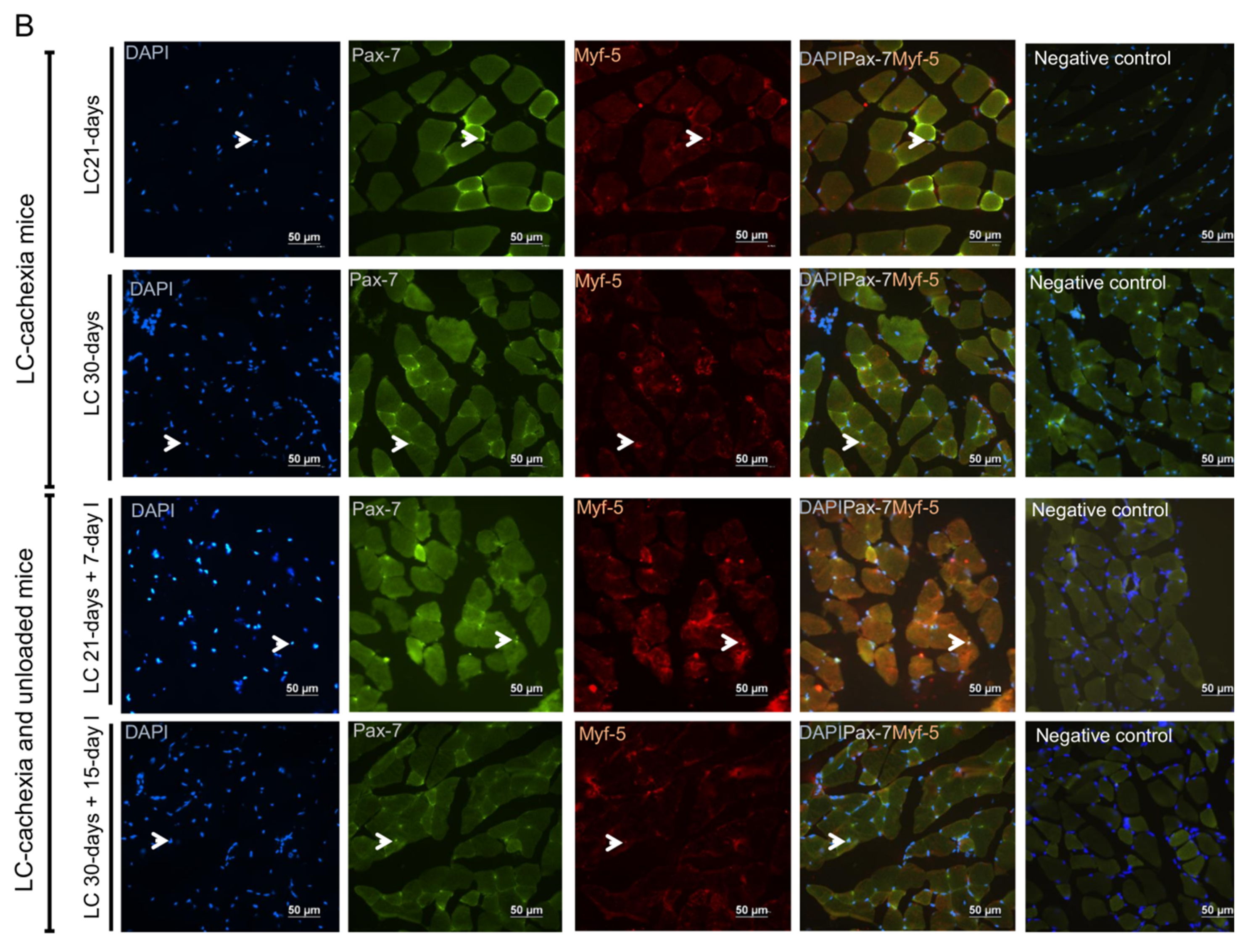
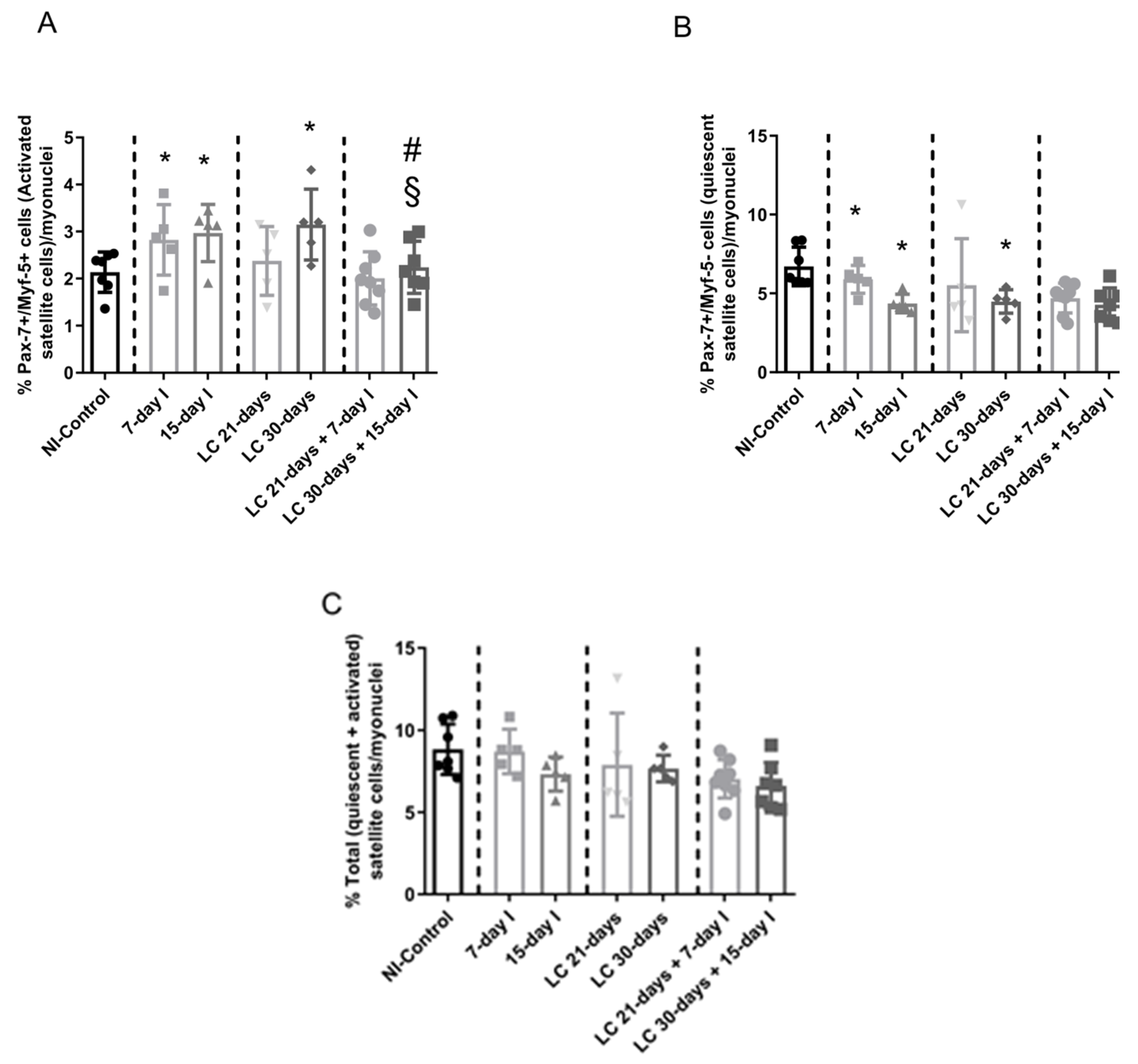
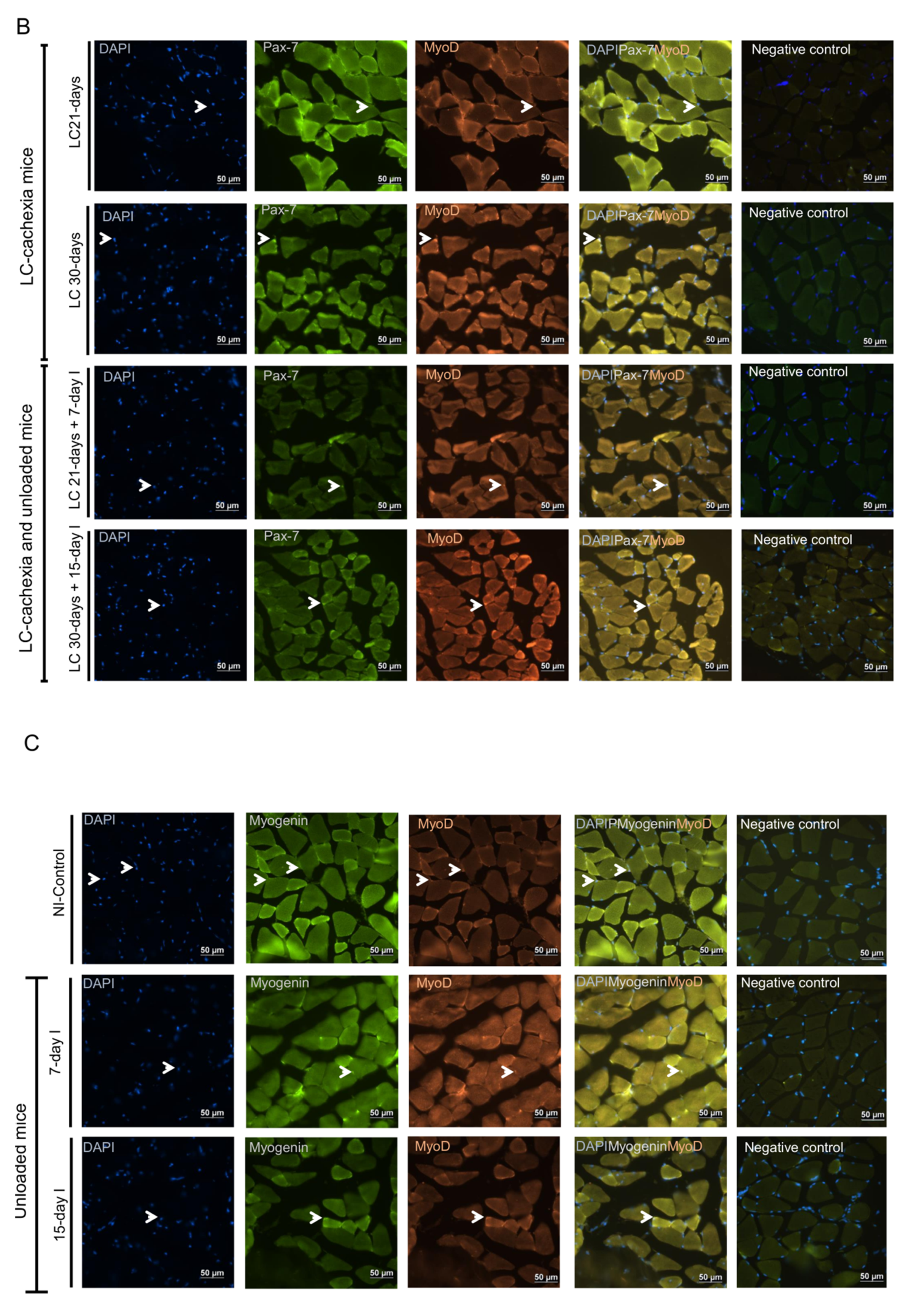
2.5. Satellite Cell Subtypes
2.5.1. Non-Immobilized Controls versus Either Unloading or LC-Cachexia
2.5.2. LC-Cachexia+Unloading versus Unloading Conditions
2.5.3. LC-Cachexia+Unloading versus LC-Cachexia
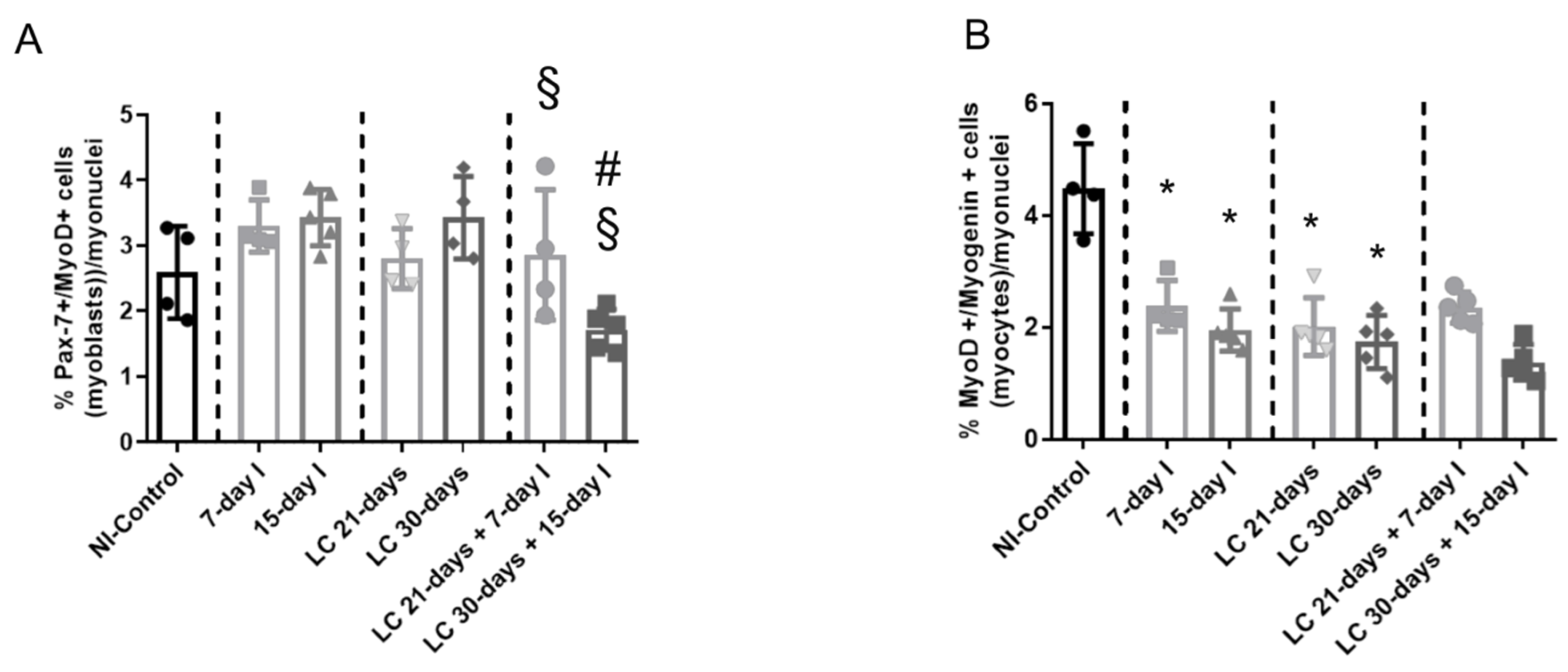
2.6. Profile of Muscle Regeneration Markers
2.6.1. Non-Immobilized Controls versus Either Unloading or LC-Cachexia Conditions
2.6.2. LC-Cachexia+Unloading versus Unloading Conditions
2.6.3. LC-Cachexia+Unloading versus LC-Cachexia
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Animal Experiments
4.1.1. Cells
4.1.2. Animals
4.2. Ethics
4.3. Studies in Mice: In Vivo Measurements
4.4. Mouse Sacrifice and Collection of the Samples
4.5. Tissue Embedding
4.6. Muscle Biology
4.6.1. Muscle Fiber Typing and Morphometrical Analyses
4.6.2. Muscle Structure Abnormalities
4.6.3. Skeletal Muscle Troponin-I Levels in Plasma
4.6.4. Immunoblotting of 1D Electrophoresis
4.6.5. Stripping Methodologies
4.6.6. Satellite Cell Identification Using Immunofluorescence Microscopy
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, J.; Curull, V.; Ramis-Cabrer, D.; Duran, X.; Rodríguez-Fuster, A.; Espases, R.A.; Barreiro, E. EL Peso Corporal Preoperatorio y la Albúmina Predicen la Supervivencia en Pacientes con Neoplasias de Pulmón Resecables: EL Papel de la Epoc. Arch. Bronconeumol. 2020. [Google Scholar] [CrossRef]
- Gea, J.; Martínez-Llorens, J. Muscle Dysfunction in Chronic Obstructive Pulmonary Disease: Latest Developments. Arch. Bronconeumol. 2019, 55, 237–238. [Google Scholar] [CrossRef]
- González-Marrón, A.; Martín-Sánchez, J.C.; Garcia-Alemany, F.; Martínez-Martín, E.; Matilla-Santander, N.; Cartanyà-Hueso, À.; Vidal, C.; García, M.; Martínez-Sánchez, J.M. Estimation of the risk of lung cancer in women participating in a population-based breast cancer screening program. Arch. Bronconeumol. 2020, 56, 277–281. [Google Scholar] [CrossRef]
- Rocha, P.; Arriola, E. Immunotherapy is Here to Stay: A New Treatment Paradigm in Lung Cancer. Arch. Bronconeumol. 2019, 55, 124–125. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Berriel Diaz, M. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef]
- Chalela, R.; Gea, J.; Barreiro, E. Immune phenotypes in lung cancer patients with COPD: Potential implications for immunotherapy. J. Thorac. Dis. 2018, 10. [Google Scholar] [CrossRef]
- Barreiro, E. Models of disuse muscle atrophy: Therapeutic implications in critically ill patients. Ann. Transl. Med. 2018, 6, 29. [Google Scholar] [CrossRef]
- Barreiro, E.; Bustamante, V.; Cejudo, P.; Gáldiz, J.B.; Gea, J.; de Lucas, P.; Martínez-Llorens, J.; Ortega, F.; Puente-Maestu, L.; Roca, J.; et al. Guidelines for the Evaluation and Treatment of Muscle Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2015, 51. [Google Scholar] [CrossRef] [Green Version]
- Man, W.D.-C.; Kemp, P.; Moxham, J.; Polkey, M.I. Skeletal muscle dysfunction in COPD: Clinical and laboratory observations. Clin. Sci. 2009, 117, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaŕe, R.; Richard Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189. [Google Scholar] [CrossRef] [Green Version]
- Shrikrishna, D.; Patel, M.; Tanner, R.J.; Seymour, J.M.; Connolly, B.A.; Puthucheary, Z.A.; Walsh, S.L.F.; Bloch, S.A.; Sidhu, P.S.; Hart, N.; et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur. Respir. J. 2012, 40, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Laveneziana, P.; Verges, S.; Barreiro, E. Relevance of Respiratory Muscle Function Assessment in Respiratory Disease. Arch. Bronconeumol. 2020, 56, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2019, 55, 93–99. [Google Scholar] [CrossRef]
- Jaitovich, A.; Barreiro, E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease what we know and can do for our patients. Am. J. Respir. Crit. Care Med. 2018, 198. [Google Scholar] [CrossRef]
- Barreiro, E. Impact of Physical Activity and Exercise on Chronic Obstructive Pulmonary Disease Phenotypes: The Relevance of Muscle Adaptation. Arch. Bronconeumol. 2019, 55, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Bustamante, V.; Curull, V.; Gea, J.; López-Campos, J.L.; Munoz, X. Relationships between chronic obstructive pulmonary disease and lung cancer: Biological insights. J. Thorac. Dis. 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Biol. 2013, 45, 2147–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacon-Cabrera, A.; Fermoselle, C.; Salmela, I.; Yelamos, J.; Barreiro, E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1-/-and Parp-2-/-mice with lung cancer cachexia. Biochim. Biophys. Acta Gen. Subj. 2015, 1850. [Google Scholar] [CrossRef] [Green Version]
- Chacon-Cabrera, A.; Mateu-Jimenez, M.; Langohr, K.; Fermoselle, C.; García-Arumí, E.; Andreu, A.L.; Yelamos, J.; Barreiro, E. Role of PARP activity in lung cancer-induced cachexia: Effects on muscle oxidative stress, proteolysis, anabolic markers, and phenotype. J. Cell. Physiol. 2017, 232. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. DMM Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Chacon-Cabrera, A.; Fermoselle, C.; Urtreger, A.J.; Mateu-Jimenez, M.; Diament, M.J.; de Kier Joffé, E.D.B.; Sandri, M.; Barreiro, E. Pharmacological Strategies in Lung Cancer-Induced Cachexia: Effects on Muscle Proteolysis, Autophagy, Structure, and Weakness. J. Cell. Physiol. 2014, 229, 1660–1672. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Degracia, A.; Blanco, D.; Vilà-Ubach, M.; de Biurrun, G.; de Solórzano, C.O.; Montuenga, L.M.; Barreiro, E. Phenotypic and metabolic features of mouse diaphragm and gastrocnemius muscles in chronic lung carcinogenesis: Influence of underlying emphysema. J. Transl. Med. 2016, 14, 244. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.M.; Kazi, A.A.; Hong-Brown, L.; Lang, C.H. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS ONE 2012, 7, e38910. [Google Scholar] [CrossRef] [Green Version]
- Chacon-Cabrera, A.; Lund-Palau, H.; Gea, J.; Barreiro, E. Time-Course of muscle mass loss, damage, and proteolysis in gastrocnemius following unloading and reloading: Implications in chronic diseases. PLoS ONE 2016, 11, e0164951. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Gea, J.; Barreiro, E. Short- and Long-Term Hindlimb Immobilization and Reloading: Profile of Epigenetic Events in Gastrocnemius. J. Cell. Physiol. 2017, 232. [Google Scholar] [CrossRef] [Green Version]
- Arentson-Lantz, E.J.; English, K.L.; Paddon-Jones, D.; Fry, C.S. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J. Appl. Physiol. 2016, 120, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Guitart, M.; Lloreta, J.; Mañas-Garcia, L.; Barreiro, E. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J. Cell. Physiol. 2018, 233. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Degracia, A.; Granado-Martínez, P.; Millán-Sánchez, A.; Tang, J.; Pons-Carreto, A.; Barreiro, E. Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice. J. Cell. Physiol. 2019, 234, 18041–18052. [Google Scholar] [CrossRef]
- Aulino, P.; Berardi, E.; Cardillo, V.M.; Rizzuto, E.; Perniconi, B.; Ramina, C.; Padula, F.; Spugnini, E.P.; Baldi, A.; Faiola, F.; et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer 2010, 10. [Google Scholar] [CrossRef]
- Puig-Vilanova, E.; Rodriguez, D.A.; Lloreta, J.; Ausin, P.; Pascual-Guardia, S.; Broquetas, J.; Roca, J.; Gea, J.; Barreiro, E. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic. Biol. Med. 2015, 79. [Google Scholar] [CrossRef]
- Penna, F.; Costamagna, D.; Pin, F.; Camperi, A.; Fanzani, A.; Chiarpotto, E.M.; Cavallini, G.; Bonelli, G.; Baccino, F.M.; Costelli, P. Autophagic degradation contributes to muscle wasting in cancer cachexia. Am. J. Pathol. 2013, 182, 1367–1378. [Google Scholar] [CrossRef]
- Toledo, M.; Busquets, S.; Penna, F.; Zhou, X.; Marmonti, E.; Betancourt, A.; Massa, D.; López-Soriano, F.J.; Han, H.Q.; Argilés, J.M. Complete reversal of muscle wasting in experimental cancer cachexia: Additive effects of activin type II receptor inhibition and β-2 agonist. Int. J. Cancer 2016, 138, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Degracia, A.; Busquets, S.; Argilés, J.M.; Bargalló-Gispert, N.; López-Soriano, F.J.; Barreiro, E. Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia. Biochimie 2018, 149, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Degracia, A.; Busquets, S.; Argilés, J.M.; López-Soriano, F.J.; Barreiro, E. Formoterol attenuates increased oxidative stress and myosin protein loss in respiratory and limb muscles of cancer cachectic rats. PeerJ 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Busquets, S.; Figueras, M.T.; Fuster, G.; Almendro, V.; Moore-Carrasco, R.; Ametller, E.; Argilés, J.M.; López-Soriano, F.J. Anticachectic effects of formoterol: A drug for potential treatment of muscle wasting. Cancer Res. 2004, 64, 6725–6731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossola, M.; Marzetti, E.; Rosa, F.; Pacelli, F. Skeletal muscle regeneration in cancer cachexia. Clin. Exp. Pharmacol. Physiol. 2016, 43, 522–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef] [Green Version]
- Inaba, S.; Hinohara, A.; Tachibana, M.; Tsujikawa, K.; Fukada, S. Muscle regeneration is disrupted by cancer cachexia without loss of muscle stem cell potential. PLoS ONE 2018, 13, e0205467. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2020. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Shin, H.R.; Lee, B.; Shin, A.; Jung, K.W.; Jee, S.H.; Kim, D.H.; Yun, Y.H.; Park, S.K.; et al. Population-attributable causes of cancer in Korea: Obesity and physical inactivity. PLoS ONE 2014, 9, e90871. [Google Scholar] [CrossRef] [Green Version]
- Paxton, R.J.; Jung, S.Y.; Vitolins, M.Z.; Fenton, J.; Paskett, E.; Pollak, M.; Hays-Grudo, J.; Hursting, S.D.; Chang, S. Associations between time spent sitting and cancer-related biomarkers in postmenopausal women: An exploration of effect modifiers. Cancer Causes Control 2014, 25, 1427–1437. [Google Scholar] [CrossRef]
- Yamada, T.; Ashida, Y.; Tatebayashi, D.; Abe, M.; Himori, K. Cancer Cachexia Induces Preferential Skeletal Muscle Myosin Loss When Combined with Denervation. Front. Physiol. 2020, 11, 445. [Google Scholar] [CrossRef]
- Xiao, J.; Caan, B.J.; Weltzien, E.; Cespedes Feliciano, E.M.; Kroenke, C.H.; Meyerhardt, J.A.; Baracos, V.E.; Kwan, M.L.; Castillo, A.L.; Prado, C.M. Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 654–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, J.; Conboy, J.J. Sirt1-Independent Rescue of Muscle Regeneration by Resveratrol in Type I Diabetes. J. Diabetes Metab. 2013, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Talbert, E.E.; Guttridge, D.C. Impaired regeneration: A role for the muscle microenvironment in cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 82–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mañas-garcía, L.; Bargalló, N.; Gea, J.; Barreiro, E. Muscle phenotype, proteolysis, and atrophy signaling during reloading in mice: Effects of curcumin on the gastrocnemius. Nutrients 2020, 12, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desgeorges, T.; Liot, S.; Lyon, S.; Bouvière, J.; Kemmel, A.; Trignol, A.; Rousseau, D.; Chapuis, B.; Gondin, J.; Mounier, R.; et al. Open-CSAM, a new tool for semi-automated analysis of myofiber cross-sectional area in regenerating adult skeletal muscle. Skelet. Muscle 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Urtreger, A.J.; Diament, M.J.; Ranuncolo, S.M.; Del C Vidal, M.; Puricelli, L.I.; Klein, S.M.; De Kier Joffe, E.D. New murine cell line derived from a spontaneous lung tumor induces paraneoplastic syndromes. Int. J. Oncol. 2001, 18, 639–647. [Google Scholar] [CrossRef]
- Diament, M.J.; Garcìa, C.; Stillitani, I.; Saavedra, V.M.; Manzur, T.; Vauthay, L.; Klein, S. Spontaneous murine lung adenocarcinoma (P07): A new experimental model to study paraneoplastic syndromes of lung cancer. Int. J. Mol. Med. 1998, 2, 45–50. [Google Scholar] [CrossRef]
- Barthel, L.K.; Raymond, P.A. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J. Histochem. Cytochem. 1990, 38, 1383–1388. [Google Scholar] [CrossRef] [Green Version]
- Busquets, S.; Pérez-Peiró, M.; Salazar-Degracia, A.; Argilés, J.M.; Serpe, R.; Rojano-Toimil, A.; López-Soriano, F.J.; Barreiro, E. Differential structural features in soleus and gastrocnemius of carnitine-treated cancer cachectic rats. J. Cell. Physiol. 2020, 235, 526–537. [Google Scholar] [CrossRef]
- Barreiro, E.; Puig-Vilanova, E.; Marin-Corral, J.; Chacón-Cabrera, A.; Salazar-Degracia, A.; Mateu, X.; Puente-Maestu, L.; García-Arumí, E.; Andreu, A.L.; Molina, L. Therapeutic Approaches in Mitochondrial Dysfunction, Proteolysis, and Structural Alterations of Diaphragm and Gastrocnemius in Rats with Chronic Heart Failure. J. Cell. Physiol. 2016, 231, 1495–1513. [Google Scholar] [CrossRef] [Green Version]
- Macgowan, N.A.; Evans, K.G.; Road, J.D.; Reid, W.D. Diaphragm injury in individuals with airflow obstruction. Am. J. Respir. Crit. Care Med. 2001, 163, 1654–1659. [Google Scholar] [CrossRef]
- Chapman, D.W.; Simpson, J.A.; Iscoe, S.; Robins, T.; Nosaka, K. Changes in serum fast and slow skeletal troponin I concentration following maximal eccentric contractions. J. Sci. Med. Sport 2013, 16, 82–85. [Google Scholar] [CrossRef]
- Foster, G.E.; Nakano, J.; Sheel, A.W.; Simpson, J.A.; Road, J.D.; Reid, W.D. Serum skeletal troponin I following inspiratory threshold loading in healthy young and middle-aged men. Eur. J. Appl. Physiol. 2012, 112, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.A.; Labugger, R.; Collier, C.; Brison, R.J.; Iscoe, S.; Van Eyk, J.E. Fast and slow skeletal troponin I in serum from patients with various skeletal muscle disorders: A pilot study. Clin. Chem. 2005, 51, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, Y. lncRNA ZFAS1 Improves Neuronal Injury and Inhibits Inflammation, Oxidative Stress, and Apoptosis by Sponging miR-582 and Upregulating NOS3 Expression in Cerebral Ischemia/Reperfusion Injury. Inflammation 2020, 43, 1337–1350. [Google Scholar] [CrossRef]
- Shao, Z.; Ma, X.; Zhang, Y.; Sun, Y.; Lv, W.; He, K.; Xia, R.; Wang, P.; Gao, X. CPNE1 predicts poor prognosis and promotes tumorigenesis and radioresistance via the AKT singling pathway in triple-negative breast cancer. Mol. Carcinog. 2020, 59, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [Green Version]










| Physiological Parameters | NI-Control | 7-Day I | 15-Day I | LC 21-Days | LC 30-Days | LC 21-Days + 7-Day I | LC 30-Days + 15-Day I |
|---|---|---|---|---|---|---|---|
| Total body weight gain (%) | +7.38 (1.36) | −3.47 (1.90) * | −10.01 (1.74) ** | −2.12 (1.93) * | −11.79 (2.83) ** | −4.83 (1.87) | −14.53 (3.52) |
| Unloaded limb muscle weights (g) | |||||||
| Gastrocnemius | 0.125 (0.014) | 0.106 (0.004) * | 0.094 (0.002) ** | 0.115 (0.017) * | 0.090 (0.017) ** | 0.089 (0.009) #,§§ | 0.086 (0.016) |
| Tibialis anterior | 0.045 (0.005) | 0.042 (0.002) | 0.040 (0.007) | 0.044 (0.005) | 0.042 (0.005) | 0.041 (0.004) | 0.038 (0.002) |
| Contralateral-control limb muscle weights (g) | |||||||
| Gastrocnemius | 0.112 (0.002) | 0.108 (0.011) | 0.110 (0.006) | 0.110 (0.008) | 0.085 (0.018) | 0.095 (0.007) | 0.079 (0.007) § |
| Tibialis anterior | 0.046 (0.009) | 0.045 (0.008) | 0.047 (0.005) | 0.047 (0.005) | 0.042 (0.013) | 0.047 (0.008) | 0.039 (0.007) § |
| Limb strenght gain (%) | +7.24 (1.89) | −4.41 (1.95) ** | −7.62 (1.80) ** | −2.45 (1.56) * | −12.83 (3.21) ** | −11.89 (3.84) #,§ | −20.04 (4.70) #,§ |
| Tumor weight (g) | NA | NA | NA | 1.46 (0.68) | 1.98 (0.10) | 1.73 (0.59) | 3.08 (1.13) # |
| Other organ weights (g) | |||||||
| Diaphragm | 0.070 (0.006) | 0.068 (0.008) | 0.067 (0.011) | 0.070 (0.004) | 0.060 (0.010) * | 0.064 (0.012) | 0.058 (0.020) |
| Spleen | 0.111 (0.007) | 0.093 (0.010) | 0.088 (0.001) | 0.321 (0.090) * | 0.531 (0.140) ** | 0.379 (0.090) § | 0.555 (0.130) § |
| Lungs | 0.167 (0.017) | 0.162 (0.009) | 0.158 (0.015) | 0.186 (0.008) | 0.220 (0.030) | 0.173 (0.016) | 0.225 (0.02) |
| Heart | 0.115 (0.011) | 0.107 (0.010) | 0.108 (0.010) | 0.100 (0.020) | 0.106 (0.010) | 0.108 (0.010) | 0.106 (0.020) |
| Structural Characteristics | NI-Control | 7-Day I | 15-Day I | LC 21-Days | LC 30-Days | LC 21-Days + 7-Day I | LC 30-Days + 15-Day I |
|---|---|---|---|---|---|---|---|
| Muscle fiber type, % | |||||||
| Type I fibers | 12.50 (5.29) | 15.91 (1.61) | 14.04 (3.88) | 14.87 (3.60) | 17.01 (4.26) | 19.04 (4.11) | 15.35 (1.95) |
| Type II fibers | 87.50 (5.29) | 84.90 (1.69) | 85.96 (3.88) | 85.13 (3.60) | 82.99 (4.26) | 80.96 (4.11) | 84.65 (1.95) |
| Cross-sectional area, µm2 | |||||||
| type I fibers | 975.10 (169.18) | 820.99 (110.23) | 775.865 (180.30) * | 951.61 (163.37) | 796.24 (189.99) | 766.28 (169.85) | 615.41 (183.06) |
| type II fibers | 1144 (154.25) | 939.38 (159.62) * | 641.36 (133.99) ** | 947.98 (149.49) | 775.30 (56.29) * | 819.46 (185.67) #,§ | 575.05 (113.72) #,§ |
| Total muscle structural abnormalities | 1.44 (0.40) | 3.5 (0.86) ** | 3.82 (3.46) ** | 5.39 (0.80) ** | 5.69 (0.75) ** | 7.04 (0.48) #,§ | 8.06 (2.63) # |
| Internal nuclei, % | 0.65 (0.32) | 1.28 (0.29) * | 1.52 (0.36) * | 2.03 (0.55) * | 1.99 (0.27) * | 2.31 (0.43) § | 3.09 (1.22) #,§ |
| Inflammatory cells, % | 0.73 (0.26) | 1.11 (0.18) * | 1.15 (3.15) * | 2.66 (0.41) * | 3.07 (0.96) * | 3.87 (0.65) ##,§§ | 4.00 (1.45) |
| Other items, % | 0.06 (0.09) | 1.11 (0.55) * | 1.15 (0.48) * | 0.70 (0.22) * | 0.63 (0.48) * | 0.86 (0.45) | 0.97 (0.66) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mañas-García, L.; Penedo-Vázquez, A.; López-Postigo, A.; Deschrevel, J.; Durán, X.; Barreiro, E. Prolonged Immobilization Exacerbates the Loss of Muscle Mass and Function Induced by Cancer-Associated Cachexia through Enhanced Proteolysis in Mice. Int. J. Mol. Sci. 2020, 21, 8167. https://doi.org/10.3390/ijms21218167
Mañas-García L, Penedo-Vázquez A, López-Postigo A, Deschrevel J, Durán X, Barreiro E. Prolonged Immobilization Exacerbates the Loss of Muscle Mass and Function Induced by Cancer-Associated Cachexia through Enhanced Proteolysis in Mice. International Journal of Molecular Sciences. 2020; 21(21):8167. https://doi.org/10.3390/ijms21218167
Chicago/Turabian StyleMañas-García, Laura, Antonio Penedo-Vázquez, Adrián López-Postigo, Jorieke Deschrevel, Xavier Durán, and Esther Barreiro. 2020. "Prolonged Immobilization Exacerbates the Loss of Muscle Mass and Function Induced by Cancer-Associated Cachexia through Enhanced Proteolysis in Mice" International Journal of Molecular Sciences 21, no. 21: 8167. https://doi.org/10.3390/ijms21218167
APA StyleMañas-García, L., Penedo-Vázquez, A., López-Postigo, A., Deschrevel, J., Durán, X., & Barreiro, E. (2020). Prolonged Immobilization Exacerbates the Loss of Muscle Mass and Function Induced by Cancer-Associated Cachexia through Enhanced Proteolysis in Mice. International Journal of Molecular Sciences, 21(21), 8167. https://doi.org/10.3390/ijms21218167