Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence
Abstract
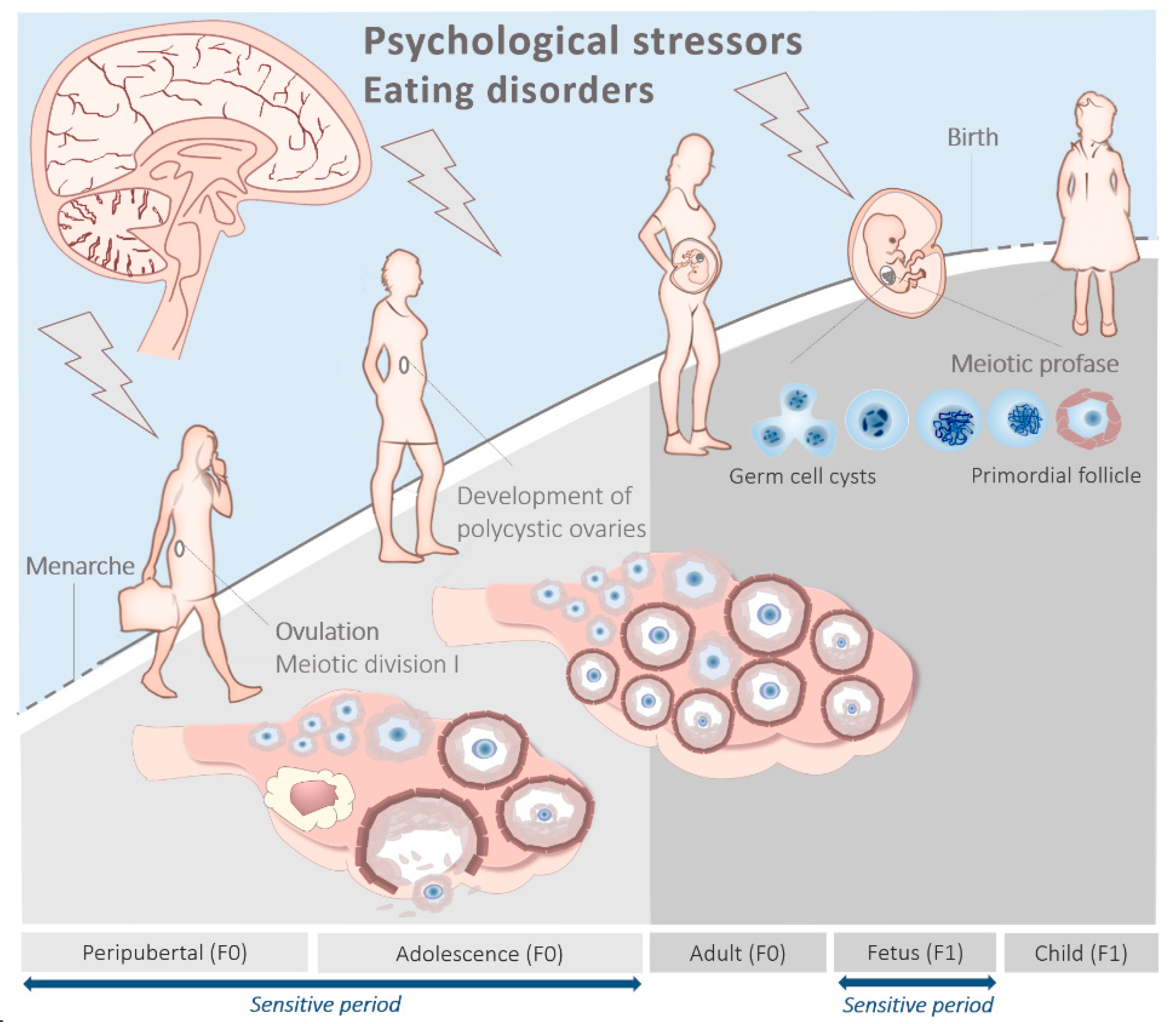
1. Introduction
2. Linking Psychological Distress and Eating Disorders to PCOS
2.1. Eating Disorders and Impaired Neuroendocrine Pathways in PCOS
2.2. Eating Disorders, Gut Microbiota and Intermediary Metabolism
2.3. Eating Disorders and Diet Composition
3. The Peripubertal and Adolescent Origin of PCOS
Peripubertal Metabolism, Stress and Eating Disorders
4. The Prenatal Origin of PCOS
4.1. Anti-Müllerian Hormone
4.2. Prenatal Metabolism and Stress
4.3. Prenatal Stress and the Microbiota
5. Epigenetic Basis of PCOS
5.1. Peripubertal Diet, Epigenetics and PCOS
5.2. Prenatal Diet, Epigenetics and PCOS
6. Hypothesis and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1C | one carbon |
| 5mC | include 5-methylcytosine |
| 5fC | 5-formylcytosine |
| 5hmC | 5-hydroxymethylcytosine |
| 5-HIAA | 5-hydroxyindolacetic acid |
| AG | acylated ghrelin |
| AMH | anti-Müllerian hormone |
| AMHR2 | anti-Müllerian hormone receptor type II |
| AN | anorexia nervosa |
| B12 | cobalamin |
| B2 | riboflavin |
| B9 | folate |
| CYP19A1 | cytochrome P450 family 19 subfamily A member 1 |
| DLK1 | delta-like noncanonical Notch ligand 1 |
| DNMT’s | DNA methyltransferases |
| DOHaD | developmental origins of health and disease |
| EWAS | epigenome-wide association studies |
| FSH | follicle-stimulating hormone |
| GABA | γ-amino butyric acid |
| GLP1 | ghrelin and glucagon-like peptide 1 |
| GnRH | gonadotropin-releasing hormone |
| Gria1 | encoding glutamate receptor 1 |
| H3K9ac | histone 3, Lysine 9 acetylation |
| H3K9me3 | histone 3, lysine 9 trimethylation |
| H3K27ac | histone 3, lysine 27 acetylation |
| HA | hyperandrogenic |
| HAT | histone acetyltransferases |
| HDAC | histone deacetylase |
| HMT | histone methyltransferase |
| HPG | hypothalamic-pituitary-gonadal |
| HSD11B2 | hydroxysteroid 11-beta dehydrogenase 2 |
| IGF2 | insulin-like growth factor 2 |
| KDM | lysine demethylase |
| KDNy | kisspeptin, neurokinin B, and dynorphin neurons |
| KISS1 | kisspeptin |
| KISS1R | kisspeptin receptor |
| LH | luteinizing hormone |
| LHCGR | luteinizing hormone/choriogonadotropin receptors |
| mtDNA | mitochondrial DNA |
| MTR | methionine synthase |
| MKRN3 | makorin ring finger protein 3 |
| NAD+ | nicotinamide adenine dinucleotide |
| OVLT | organum vasculosum laminae terminalis |
| PCOM | polycystic ovary morphology |
| PCOS | polycystic ovary syndrome |
| PMAD | perinatal mood and anxiety disorders |
| SAM | S-adenosylmethionine |
| TCA | tricarboxylic-acid cycle |
| TET | ten-eleven translocation |
| tHcy | total homocysteine |
| UAG | unacylated ghrelin |
| αKG | alpha-ketoglutarate |
References
- ESHRE, T.T.; ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil. Steril. 2008, 89, 505–522. [Google Scholar]
- Blay, S.L.; Aguiar, J.V.; Passos, I.C. Polycystic ovary syndrome and mental disorders: A systematic review and exploratory meta-analysis. Neuropsychiatr. Dis. Treat. 2016, 12, 2895–2903. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Schramm, R.D.; Abbott, D.H. Early origins of polycystic ovary syndrome. Reprod. Fertil. Dev. 2005, 17, 349–360. [Google Scholar] [CrossRef]
- Dunaif, A. Polycystic ovary syndrome in 2011: Genes, aging and sleep apnea in polycystic ovary syndrome. Nat. Rev. Endocrinol. 2011, 8, 72–74. [Google Scholar] [CrossRef]
- Azziz, R. Pcos in 2015: New insights into the genetics of polycystic ovary syndrome. Nat. Rev. Endocrinol. 2016, 12, 74–75. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global who guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef]
- ESHRE/ASRM. Rotterdam sponsored pcos consensus workshop group revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Witchel, S.F.; Roumimper, H.; Oberfield, S. Polycystic ovary syndrome in adolescents. Endocrinol. Metab. Clin. N. Am. 2016, 45, 329–344. [Google Scholar] [CrossRef]
- Ibáñez, L.; Potau, N.; Francois, I.; de Zegher, F. Precocious pubarche, hyperinsulinism, and ovarian hyperandrogenism in girls: Relation to reduced fetal growth. J. Clin. Endocrinol. Metab. 1998, 83, 3558–3562. [Google Scholar] [CrossRef]
- Walters, K.A.; Gilchrist, R.B.; Ledger, W.L.; Teede, H.J.; Handelsman, D.J.; Campbell, R.E. New perspectives on the pathogenesis of pcos: Neuroendocrine origins. Trends Endocrinol. Metab. 2018, 29, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Laven, J.S.; Mulders, A.G.; Visser, J.A.; Themmen, A.P.; De Jong, F.H.; Fauser, B.C. Anti-müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J. Clin. Endocrinol. Metab. 2004, 89, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.; Fauser, B.C.; Hilders, C.G.; Jaddoe, V.W.; Massuger, L.F.; Schoenmakers, S.; van der Post, J.A. Textbook of Obstetrics and Gynaecology: A Life Course Approach; Bohn Stafleu van Loghum: Walmolen, The Netherland, 2019. [Google Scholar]
- Thannickal, A.; Brutocao, C.; Alsawas, M.; Morrow, A.; Zaiem, F.; Murad, M.H.; Javed Chattha, A. Eating, sleeping and sexual function disorders in women with polycystic ovary syndrome (pcos): A systematic review and meta-analysis. Clin. Endocrinol. 2020, 92, 338–349. [Google Scholar] [CrossRef]
- Brutocao, C.; Zaiem, F.; Alsawas, M.; Morrow, A.S.; Murad, M.H.; Javed, A. Psychiatric disorders in women with polycystic ovary syndrome: A systematic review and meta-analysis. Endocrine 2018, 62, 318–325. [Google Scholar] [CrossRef]
- Damone, A.L.; Joham, A.E.; Loxton, D.; Earnest, A.; Teede, H.J.; Moran, L.J. Depression, anxiety and perceived stress in women with and without pcos: A community-based study. Psychol. Med. 2019, 49, 1510–1520. [Google Scholar] [CrossRef]
- Kerchner, A.; Lester, W.; Stuart, S.P.; Dokras, A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: A longitudinal study. Fertil. Steril. 2009, 91, 207–212. [Google Scholar] [CrossRef]
- Himelein, M.J.; Thatcher, S.S. Polycystic ovary syndrome and mental health: A review. Obstet. Gynecol. Surv. 2006, 61, 723–732. [Google Scholar] [CrossRef]
- Murray, K.M.; Byrne, D.G.; Rieger, E. Investigating adolescent stress and body image. J. Adolesc. 2011, 34, 269–278. [Google Scholar] [CrossRef]
- Beck, A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 2008, 165, 969–977. [Google Scholar] [CrossRef]
- Baker, J.H.; Girdler, S.S.; Bulik, C.M. The role of reproductive hormones in the development and maintenance of eating disorders. Expert Rev. Obstet. Gynecol. 2012, 7, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-Z.; Zhou, F.-J.; Sun, Y.-P. Psychological stress is related to a decrease of serum anti-müllerian hormone level in infertile women. Reprod. Biol. Endocrinol. 2017, 15, 51. [Google Scholar] [CrossRef]
- Nestler, J.E.; Jakubowicz, D.J.; de Vargas, A.F.; Brik, C.; Quintero, N.; Medina, F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar]
- Algars, M.; Huang, L.; Von Holle, A.F.; Peat, C.M.; Thornton, L.M.; Lichtenstein, P.; Bulik, C.M. Binge eating and menstrual dysfunction. J. Psychosom. Res. 2014, 76, 19–22. [Google Scholar] [CrossRef]
- Dallman, M.F. Stress-induced obesity and the emotional nervous system. Trends Endocrinol. Metab. 2010, 21, 159–165. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.; Millán, J. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol. Metab. 2007, 18, 266–272. [Google Scholar] [CrossRef]
- Larsen, J.K.; van Ramshorst, B.; van Doornen, L.J.; Geenen, R. Salivary cortisol and binge eating disorder in obese women after surgery for morbid obesity. Int. J. Behav. Med. 2009, 16, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Legro, R.S.; Bixler, E.O.; Grayev, A.; Kales, A.; Chrousos, G.P. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: Role of insulin resistance. J. Clin. Endocrinol. Metab. 2001, 86, 517–520. [Google Scholar]
- El-Haschimi, K.; Pierroz, D.D.; Hileman, S.M.; Bjorbaek, C.; Flier, J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Investig. 2000, 105, 1827–1832. [Google Scholar] [CrossRef]
- Jacobs, H.S.; Conway, G.S. Leptin, polycystic ovaries and polycystic ovary syndrome. Hum. Reprod. Update 1999, 5, 166–171. [Google Scholar] [CrossRef]
- Delhanty, P.J.; Neggers, S.J.; van der Lely, A.J. Mechanisms in endocrinology: Ghrelin: The differences between acyl- and des-acyl ghrelin. Eur. J. Endocrinol. 2012, 167, 601–608. [Google Scholar] [CrossRef]
- Menzies, J.R.; Skibicka, K.P.; Leng, G.; Dickson, S.L. Ghrelin, reward and motivation. Endocr. Dev. 2013, 25, 101–111. [Google Scholar] [PubMed]
- Perello, M.; Dickson, S.L. Ghrelin signalling on food reward: A salient link between the gut and the mesolimbic system. J. Neuroendocrinol. 2015, 27, 424–434. [Google Scholar] [CrossRef]
- Valdivia, S.; Cornejo, M.P.; Reynaldo, M.; De Francesco, P.N.; Perello, M. Escalation in high fat intake in a binge eating model differentially engages dopamine neurons of the ventral tegmental area and requires ghrelin signaling. Psychoneuroendocrinology 2015, 60, 206–216. [Google Scholar] [CrossRef]
- King, S.J.; Rodrigues, T.; Watts, A.; Murray, E.; Wilson, A.; Abizaid, A. Investigation of a role for ghrelin signaling in binge-like feeding in mice under limited access to high-fat diet. Neuroscience 2016, 319, 233–245. [Google Scholar] [CrossRef]
- Kuppens, R.J.; Diene, G.; Bakker, N.E.; Molinas, C.; Faye, S.; Nicolino, M.; Bernoux, D.; Delhanty, P.J.; van der Lely, A.J.; Allas, S.; et al. Elevated ratio of acylated to unacylated ghrelin in children and young adults with prader-willi syndrome. Endocrine 2015, 50, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.; Neggers, S.J.; van der Lely, A.J. Des-acyl ghrelin: A metabolically active peptide. Endocr. Dev. 2013, 25, 112–121. [Google Scholar]
- Shi, X.; Zhang, L.; Fu, S.; Li, N. Co-involvement of psychological and neurological abnormalities in infertility with polycystic ovarian syndrome. Arch. Gynecol. Obstet. 2011, 284, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Dawalbhakta, M.; Nampoothiri, L. Gnrh dysregulation in polycystic ovarian syndrome (pcos) is a manifestation of an altered neurotransmitter profile. Reprod. Biol. Endocrinol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Kaye, W.H.; Frank, G.K.; Bailer, U.F.; Henry, S.E.; Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Wagner, A. Serotonin alterations in anorexia and bulimia nervosa: New insights from imaging studies. Physiol. Behav. 2005, 85, 73–81. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Maguire, S.; Palacios, T.; Caterson, I.D. Are the gut bacteria telling us to eat or not to eat? Reviewing the role of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients 2017, 9, 602. [Google Scholar] [CrossRef]
- Rackers, H.S.; Thomas, S.; Williamson, K.; Posey, R.; Kimmel, M.C. Emerging literature in the microbiota-brain axis and perinatal mood and anxiety disorders. Psychoneuroendocrinology 2018, 95, 86–96. [Google Scholar] [CrossRef]
- He, F.-F.; Li, Y.-M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef]
- Qi, X.; Chuyu, Y.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the relationship between gut microbiota and polycystic ovary syndrome (pcos): A review. Geburtshilfe Frauenheilkd 2020, 80, 161–171. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond B Biol. Sci. 2006, 361, 1251–1263. [Google Scholar] [CrossRef]
- Sundblad, C.; Bergman, L.; Eriksson, E. High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr. Scand. 1994, 90, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, N.A.; Laven, J.S.; Labee, C.T.; Louwers, Y.V.; Willemsen, S.P.; Steegers-Theunissen, R.P. Are dieting and dietary inadequacy a second hit in the association with polycystic ovary syndrome severity? PLoS ONE 2015, 10, e0142772. [Google Scholar] [CrossRef]
- Huijgen, N.A.; Louwers, Y.V.; Willemsen, S.P.; de Vries, J.H.M.; Steegers-Theunissen, R.P.M.; Laven, J.S.E. Dietary patterns and the phenotype of polycystic ovary syndrome: The chance of ongoing pregnancy. Reprod. Biomed. Online 2017, 34, 668–676. [Google Scholar] [CrossRef]
- Barr, S.; Hart, K.; Reeves, S.; Sharp, K.; Jeanes, Y.M. Habitual dietary intake, eating pattern and physical activity of women with polycystic ovary syndrome. Eur. J. Clin. Nutr. 2011, 65, 1126–1132. [Google Scholar] [CrossRef]
- Douglas, C.C.; Gower, B.A.; Darnell, B.E.; Ovalle, F.; Oster, R.A.; Azziz, R. Role of diet in the treatment of polycystic ovary syndrome. Fertil. Steril. 2006, 85, 679–688. [Google Scholar] [CrossRef]
- Moran, L.J.; Ko, H.; Misso, M.; Marsh, K.; Noakes, M.; Talbot, M.; Frearson, M.; Thondan, M.; Stepto, N.; Teede, H.J. Dietary composition in the treatment of polycystic ovary syndrome: A systematic review to inform evidence-based guidelines. Hum. Reprod. Update 2013, 19, 432. [Google Scholar] [CrossRef]
- Moran, L.J.; Grieger, J.A.; Mishra, G.D.; Teede, H.J. The association of a mediterranean-style diet pattern with polycystic ovary syndrome status in a community cohort study. Nutrients 2015, 7, 8553–8564. [Google Scholar] [CrossRef] [PubMed]
- Chiofalo, B.; Laganà, A.S.; Palmara, V.; Granese, R.; Corrado, G.; Mancini, E.; Vitale, S.G.; Ban Frangež, H.; Vrtačnik-Bokal, E.; Triolo, O. Fasting as possible complementary approach for polycystic ovary syndrome: Hope or hype? Med. Hypotheses 2017, 105, 1–3. [Google Scholar] [CrossRef]
- Facchinetti, F.; Unfer, V.; Dewailly, D.; Kamenov, Z.A.; Diamanti-Kandarakis, E.; Laganà, A.S.; Nestler, J.E.; Soulage, C.O. Inositols in polycystic ovary syndrome: An overview on the advances. Trends Endocrinol. Metab. 2020, 31, 435–447. [Google Scholar] [CrossRef]
- Evans, M.C.; Anderson, G.M. Neuroendocrine integration of nutritional signals on reproduction. J. Mol. Endocrinol. 2017, 58, R107–R128. [Google Scholar] [CrossRef] [PubMed]
- Goss, A.M.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Wright Bates, G.; Gower, B.A. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with pcos. Metabolism 2014, 63, 1257–1264. [Google Scholar] [CrossRef]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G.W. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with pcos. Clin. Endocrinol. 2013, 79, 550–557. [Google Scholar] [CrossRef]
- Mehrabani, H.H.; Salehpour, S.; Amiri, Z.; Farahani, S.J.; Meyer, B.J.; Tahbaz, F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: A randomized controlled intervention study. J. Am. Coll. Nutr. 2012, 31, 117–125. [Google Scholar] [CrossRef]
- Sorensen, L.B.; Soe, M.; Halkier, K.H.; Stigsby, B.; Astrup, A. Effects of increased dietary protein-to-carbohydrate ratios in women with polycystic ovary syndrome. Am. J. Clin. Nutr. 2012, 95, 39–48. [Google Scholar] [CrossRef]
- Stamets, K.; Taylor, D.S.; Kunselman, A.; Demers, L.M.; Pelkman, C.L.; Legro, R.S. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil. Steril. 2004, 81, 630–637. [Google Scholar] [CrossRef]
- Toscani, M.K.; Mario, F.M.; Radavelli-Bagatini, S.; Wiltgen, D.; Matos, M.C.; Spritzer, P.M. Effect of high-protein or normal-protein diet on weight loss, body composition, hormone, and metabolic profile in southern brazilian women with polycystic ovary syndrome: A randomized study. Gynecol. Endocrinol. 2011, 27, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.L.; Almario, R.U.; Buchan, W.; Kim, K.; Karakas, S.E. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metabolism 2011, 60, 1711–1718. [Google Scholar] [CrossRef]
- Schaumberg, K.; Welch, E.; Breithaupt, L.; Hübel, C.; Baker, J.H.; Munn-Chernoff, M.A.; Yilmaz, Z.; Ehrlich, S.; Mustelin, L.; Ghaderi, A.; et al. The science behind the academy for eating disorders’ nine truths about eating disorders. Eur. Eat. Disord. Rev. 2017, 25, 432–450. [Google Scholar] [CrossRef]
- Klump, K.L.; Gobrogge, K.L.; Perkins, P.S.; Thorne, D.; Sisk, C.L.; Breedlove, S.M. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol. Med. 2006, 36, 539–546. [Google Scholar] [CrossRef]
- Klump, K.L.; Keel, P.K.; Sisk, C.; Burt, S.A. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol. Med. 2010, 40, 1745–1753. [Google Scholar] [CrossRef]
- Kaltiala-Heino, R.; Rimpelä, M.; Rissanen, A.; Rantanen, P. Early puberty and early sexual activity are associated with bulimic-type eating pathology in middle adolescence. J. Adolesc. Health 2001, 28, 346–352. [Google Scholar] [CrossRef]
- Kelly, Y.; Zilanawala, A.; Sacker, A.; Hiatt, R.; Viner, R. Early puberty in 11-year-old girls: Millennium cohort study findings. Arch. Dis. Child. 2017, 102, 232–237. [Google Scholar] [CrossRef]
- Lian, Q.; Mao, Y.; Luo, S.; Zhang, S.; Tu, X.; Zuo, X.; Lou, C.; Zhou, W. Puberty timing associated with obesity and central obesity in chinese han girls. BMC Pediatr. 2019, 19, 1. [Google Scholar] [CrossRef]
- Nguyen, N.T.K.; Fan, H.Y.; Tsai, M.C.; Tung, T.H.; Huynh, Q.T.V.; Huang, S.Y.; Chen, Y.C. Nutrient intake through childhood and early menarche onset in girls: Systematic review and meta-analysis. Nutrients 2020, 12, 2544. [Google Scholar] [CrossRef]
- Rothenberg, S.S.; Beverley, R.; Barnard, E.; Baradaran-Shoraka, M.; Sanfilippo, J.S. Polycystic ovary syndrome in adolescents. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 103–114. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarząbek-Bielecka, G.; Opydo-Szymaczek, J.; Wendland, N.; Więckowska, B.; Kędzia, W. Risk factors of overweight and obesity related to diet and disordered eating attitudes in adolescent girls with clinical features of polycystic ovary syndrome. J. Clin. Med. 2020, 9, 3041. [Google Scholar] [CrossRef]
- Eskandari, Z.; Sadrkhanlou, R.-A.; Nejati, V.; Tizro, G. Pcos women show significantly higher homocysteine level, independent to glucose and e2 level. Int. J. Reprod. Biomed. 2016, 14, 495–500. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, X.; Peng, Z.; Liu, X.; Sun, Y.; Dai, S. Association between high serum homocysteine levels and biochemical characteristics in women with polycystic ovarian syndrome: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0157389. [Google Scholar] [CrossRef]
- Must, A.; Jacques, P.F.; Rogers, G.; Rosenberg, I.H.; Selhub, J. Serum total homocysteine concentrations in children and adolescents: Results from the third national health and nutrition examination survey (nhanes iii). J. Nutr. 2003, 133, 2643–2649. [Google Scholar] [CrossRef]
- Chung, K.H.; Chiou, H.Y.; Chen, Y.H. Associations between serum homocysteine levels and anxiety and depression among children and adolescents in taiwan. Sci. Rep. 2017, 7, 8330. [Google Scholar] [CrossRef] [PubMed]
- Esnafoglu, E.; Ozturan, D.D. The relationship of severity of depression with homocysteine, folate, vitamin b12, and vitamin d levels in children and adolescents. Child Adolesc. Ment. Health 2020. [Google Scholar] [CrossRef]
- Loria-Kohen, V.; Gómez-Candela, C.; Palma-Milla, S.; Amador-Sastre, B.; Hernanz, A.; Bermejo, L.M. A pilot study of folic acid supplementation for improving homocysteine levels, cognitive and depressive status in eating disorders. Nutr. Hosp. 2013, 28, 807–815. [Google Scholar]
- Schiuma, N.; Costantino, A.; Bartolotti, T.; Dattilo, M.; Bini, V.; Aglietti, M.C.; Renga, M.; Favilli, A.; Falorni, A.; Gerli, S. Micronutrients in support to the one carbon cycle for the modulation of blood fasting homocysteine in pcos women. J. Endocrinol. Investig. 2020, 43, 779–786. [Google Scholar] [CrossRef]
- Sharma, U.; Rando, O.J. Metabolic inputs into the epigenome. Cell Metab. 2017, 25, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; Bannister, A.J. Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways. Mol. Metab. 2020, 38, 100942. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Patel, D. A structural perspective on readout of epigenetic histone and DNA methylation marks. Cold Spring Harb. Perspect. Biol. 2016, 8, a018754. [Google Scholar] [CrossRef]
- Hu, M.; Richard, J.E.; Maliqueo, M.; Kokosar, M.; Fornes, R.; Benrick, A.; Jansson, T.; Ohlsson, C.; Wu, X.; Skibicka, K.P.; et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc. Natl. Acad. Sci. USA 2015, 112, 14348–14353. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr. Rev. 2020, 41, 538–576. [Google Scholar] [CrossRef]
- Sir-Petermann, T.; Hitchsfeld, C.; Maliqueo, M.; Codner, E.; Echiburú, B.; Gazitúa, R.; Recabarren, S.; Cassorla, F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum. Reprod. 2005, 20, 2122–2126. [Google Scholar] [CrossRef]
- Nicandri, K.F.; Hoeger, K. Diagnosis and treatment of polycystic ovarian syndrome in adolescents. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 497–504. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, J.; Wang, Y.; Li, W.; Katzmarzyk, P.T.; Chaput, J.P.; Fogelholm, M.; Johnson, W.D.; Kuriyan, R.; Kurpad, A.; et al. Birth weight and childhood obesity: A 12-country study. Int. J. Obes. Suppl. 2015, 5, S74–S79. [Google Scholar] [CrossRef]
- Robinson, S.L.; Ghassabian, A.; Sundaram, R.; Trinh, M.H.; Bell, E.M.; Mendola, P.; Yeung, E.H. The associations of maternal polycystic ovary syndrome and hirsutism with behavioral problems in offspring. Fertil. Steril. 2020, 113, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kong, L.; Piltonen, T.T.; Gissler, M.; Lavebratt, C. Association of polycystic ovary syndrome or anovulatory infertility with offspring psychiatric and mild neurodevelopmental disorders: A finnish population-based cohort study. Hum. Reprod. 2020, 35, 2336–2347. [Google Scholar] [CrossRef]
- Piltonen, T.T.; Giacobini, P.; Edvinsson, Å.; Hustad, S.; Lager, S.; Morin-Papunen, L.; Tapanainen, J.S.; Sundström-Poromaa, I.; Arffman, R.K. Circulating antimüllerian hormone and steroid hormone levels remain high in pregnant women with polycystic ovary syndrome at term. Fertil. Steril. 2019, 111, 588–596. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; et al. Anti-müllerian hormone attenuates the effects of fsh on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Lesnick, T.G.; Stassart, J.P.; Ball, G.D.; Wong, A.; Abbott, D.H. Intrafollicular antimüllerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (fsh) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human fsh therapy for in vitro fertilization and embryo transfer. Fertil. Steril. 2009, 92, 217–221. [Google Scholar]
- Prevot, V.; Dehouck, B.; Sharif, A.; Ciofi, P.; Giacobini, P.; Clasadonte, J. The versatile tanycyte: A hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 2018, 39, 333–368. [Google Scholar] [CrossRef] [PubMed]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-müllerian hormone in the regulation of gnrh neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.A.; Papadakis, G.E.; Messina, A.; Mimouni, N.E.H.; Trova, S.; Imbernon, M.; Allet, C.; Cimino, I.; Acierno, J.; Cassatella, D.; et al. Defective amh signaling disrupts gnrh neuron development and function and contributes to hypogonadotropic hypogonadism. eLife 2019, 8, e47198. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.L.; Siow, Y.; Brenner, A.G.; Fallat, M.E. Relationship between serum müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil. Steril. 2002, 77, 141–146. [Google Scholar] [CrossRef]
- Barbotin, A.L.; Peigné, M.; Malone, S.A.; Giacobini, P. Emerging roles of anti-müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology 2019, 109, 218–229. [Google Scholar] [CrossRef]
- Silva, M.S.B.; Giacobini, P. New insights into anti-müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol. Life Sci. 2020, 1–16. [Google Scholar] [CrossRef]
- Alder, J.; Fink, N.; Bitzer, J.; Hösli, I.; Holzgreve, W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J. Matern Fetal Neonatal Med. 2007, 20, 189–209. [Google Scholar] [CrossRef]
- Hoirisch-Clapauch, S.; Brenner, B.; Nardi, A.E. Adverse obstetric and neonatal outcomes in women with mental disorders. Thromb. Res. 2015, 135 (Suppl. 1), S60–S63. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Hoyos, L.R.; Chazenbalk, G.D.; Naik, R.; Padmanabhan, V.; Abbott, D.H. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction 2020, 159, R1–R13. [Google Scholar] [CrossRef]
- Hansen, N.S.; Strasko, K.S.; Hjort, L.; Kelstrup, L.; Houshmand-Øregaard, A.; Schrölkamp, M.; Schultz, H.S.; Scheele, C.; Pedersen, B.K.; Ling, C.; et al. Fetal hyperglycemia changes human preadipocyte function in adult life. J. Clin. Endocrinol. Metab. 2017, 102, 1141–1150. [Google Scholar] [CrossRef]
- Filippou, P.; Homburg, R. Is foetal hyperexposure to androgens a cause of pcos? Hum. Reprod. Update 2017, 23, 421–432. [Google Scholar] [CrossRef]
- Siemienowicz, K.J.; Coukan, F.; Franks, S.; Rae, M.T.; Duncan, W.C. Aberrant subcutaneous adipogenesis precedes adult metabolic dysfunction in an ovine model of polycystic ovary syndrome (pcos). Mol. Cell. Endocrinol. 2020, 519, 111042. [Google Scholar] [CrossRef]
- Chakraborty, P.; Goswami, S.K.; Rajani, S.; Sharma, S.; Kabir, S.N.; Chakravarty, B.; Jana, K. Recurrent pregnancy loss in polycystic ovary syndrome: Role of hyperhomocysteinemia and insulin resistance. PLoS ONE 2013, 8, e64446. [Google Scholar] [CrossRef]
- Kazerooni, T.; Ghaffarpasand, F.; Asadi, N.; Dehkhoda, Z.; Dehghankhalili, M.; Kazerooni, Y. Correlation between thrombophilia and recurrent pregnancy loss in patients with polycystic ovary syndrome: A comparative study. J. Chin. Med. Assoc. 2013, 76, 282–288. [Google Scholar] [CrossRef]
- Szafarowska, M.; Segiet, A.; Jerzak, M.M. Methylenotetrahydrololate reductase a1298c and c677t polymorphisms and adverse pregnancy outcome in women with pcos. Neuro Endocrinol. Lett. 2016, 37, 141–146. [Google Scholar] [PubMed]
- Chang, C.L.; Huang, S.Y.; Hsu, Y.C.; Chin, T.H.; Soong, Y.K. The serum level of irisin, but not asprosin, is abnormal in polycystic ovary syndrome patients. Sci. Rep. 2019, 9, 6447. [Google Scholar] [CrossRef]
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Fernandez Gonzalez, S.; Llurba Olivé, E.; García Algar, O.; Solana, M.J.; Cabero Perez, M.J.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef]
- Kaplan, J.L.; Shi, H.N.; Walker, W.A. The role of microbes in developmental immunologic programming. Pediatr. Res. 2011, 69, 465–472. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef]
- Hu, J.; Ly, J.; Zhang, W.; Huang, Y.; Glover, V.; Peter, I.; Hurd, Y.L.; Nomura, Y. Microbiota of newborn meconium is associated with maternal anxiety experienced during pregnancy. Dev. Psychobiol. 2019, 61, 640–649. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Tan, Q. Deciphering the DNA methylome of polycystic ovary syndrome. Mol. Diagn. Ther. 2020, 24, 245–250. [Google Scholar] [CrossRef]
- Shukla, P.; Mukherjee, S. Mitochondrial dysfunction: An emerging link in the pathophysiology of polycystic ovary syndrome. Mitochondrion 2020, 52, 24–39. [Google Scholar] [CrossRef]
- Mao, Z.; Li, T.; Zhao, H.; Qin, Y.; Wang, X.; Kang, Y. Identification of epigenetic interactions between microrna and DNA methylation associated with polycystic ovarian syndrome. J. Hum. Genet. 2020. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Deshmukh, H.; Atkin, S.; Sathyapalan, T. A review of therapeutic options for managing the metabolic aspects of polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938305. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of mirna in polycystic ovary syndrome (pcos). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef]
- Stueve, T.R.; Wolff, M.S.; Pajak, A.; Teitelbaum, S.L.; Chen, J. Cyp19a1 promoter methylation in saliva associated with milestones of pubertal timing in urban girls. BMC Pediatr. 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Peterson, K.E.; Sánchez, B.N.; Dolinoy, D.C.; Mercado-Garcia, A.; Téllez-Rojo, M.M.; Goodrich, J.M. Association of blood leukocyte DNA methylation at line-1 and growth-related candidate genes with pubertal onset and progression. Epigenetics 2018, 13, 1222–1233. [Google Scholar] [CrossRef]
- Roberts, S.A.; Kaiser, U.B. Genetics in endocrinology: Genetic etiologies of central precocious puberty and the role of imprinted genes. Eur. J. Endocrinol. 2020, 183, R107–R117. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Rodriguez, E.; Vargas, F.; Montoro-Molina, S.; Romero, M.; Espejo-Calvo, J.A.; Vilchez, P.; Jaramillo, S.; Olmo-García, L.; Carrasco-Pancorbo, A.; et al. Cardioprotective effect of a virgin olive oil enriched with bioactive compounds in spontaneously hypertensive rats. Nutrients 2019, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, H.; Matsuzawa, D.; Ishii, D.; Matsuda, S.; Kawai, K.; Mashimo, Y.; Sutoh, C.; Shimizu, E. Methyl-donor deficiency in adolescence affects memory and epigenetic status in the mouse hippocampus. Genes Brain Behav. 2015, 14, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, J.; He, B.; Jia, Y.; Niu, Y.; Wang, C.; Zhao, R. Abnormally activated one-carbon metabolic pathway is associated with mtdna hypermethylation and mitochondrial malfunction in the oocytes of polycystic gilt ovaries. Sci. Rep. 2016, 6, 19436. [Google Scholar] [CrossRef]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-carbon metabolism: Linking nutritional biochemistry to epigenetic programming of long-term development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Ibáñez, L.; de Zegher, F. Polycystic ovary syndrome in adolescent girls. Pediatr. Obes. 2020, 15, e12586. [Google Scholar] [CrossRef]
- Xu, N.; Kwon, S.; Abbott, D.H.; Geller, D.H.; Dumesic, D.A.; Azziz, R.; Guo, X.; Goodarzi, M.O. Epigenetic mechanism underlying the development of polycystic ovary syndrome (pcos)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS ONE 2011, 6, e27286. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Abbott, D.H.; Dumesic, D.A.; Levine, J.E. Hyperandrogenic origins of polycystic ovary syndrome—Implications for pathophysiology and therapy. Expert Rev. Endocrinol. Metab. 2019, 14, 131–143. [Google Scholar] [CrossRef]
- Zhang, D.; Cong, J.; Shen, H.; Wu, Q.; Wu, X. Genome-wide identification of aberrantly methylated promoters in ovarian tissue of prenatally androgenized rats. Fertil. Steril. 2014, 102, 1458–1467. [Google Scholar] [CrossRef]
- Sinha, N.; Roy, S.; Huang, B.; Wang, J.; Padmanabhan, V.; Sen, A. Developmental programming: Prenatal testosterone-induced epigenetic modulation and its effect on gene expression in sheep ovary†. Biol. Reprod. 2020, 102, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Ding, L.; Su, J.; Liu, M.; Shi, Q.; Zhou, J.; Sun, H.; Yan, G. Attenuated expression of mtr in both prenatally androgenized mice and women with the hyperandrogenic phenotype of pcos. PLoS ONE 2017, 12, e0187427. [Google Scholar] [CrossRef]
- Lambertini, L.; Saul, S.R.; Copperman, A.B.; Hammerstad, S.S.; Yi, Z.; Zhang, W.; Tomer, Y.; Kase, N. Intrauterine reprogramming of the polycystic ovary syndrome: Evidence from a pilot study of cord blood global methylation analysis. Front. Endocrinol. 2017, 8, 352. [Google Scholar] [CrossRef]
- Echiburú, B.; Milagro, F.; Crisosto, N.; Pérez-Bravo, F.; Flores, C.; Arpón, A.; Salas-Pérez, F.; Recabarren, S.E.; Sir-Petermann, T.; Maliqueo, M. DNA methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with pcos. Epigenetics 2020, 1–17. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- Oostingh, E.C.; Koster, M.P.H.; van Dijk, M.R.; Willemsen, S.P.; Broekmans, F.J.M.; Hoek, A.; Goddijn, M.; Klijn, N.F.; van Santbrink, E.J.P.; Steegers, E.A.P.; et al. First effective mhealth nutrition and lifestyle coaching program for subfertile couples undergoing in vitro fertilization treatment: A single-blinded multicenter randomized controlled trial. Fertil. Steril. 2020. [Google Scholar] [CrossRef]
- Oostingh, E.C.; Hall, J.; Koster, M.P.H.; Grace, B.; Jauniaux, E.; Steegers-Theunissen, R.P.M. The impact of maternal lifestyle factors on periconception outcomes: A systematic review of observational studies. Reprod. Biomed. Online 2019, 38, 77–94. [Google Scholar] [CrossRef]
- Van der Windt, M.; van der Kleij, R.M.; Snoek, K.M.; Willemsen, S.P.; Dykgraaf, R.H.M.; Laven, J.S.E.; Schoenmakers, S.; Steegers-Theunissen, R.P.M. Impact of a blended periconception lifestyle care approach on lifestyle behaviors: Before-and-after study. J. Med. Internet Res. 2020, 22, e19378. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Cooney, L.G.; Saini, S.; Smith, M.E.; Sammel, M.D.; Allison, K.C.; Dokras, A. Increased risk of disordered eating in polycystic ovary syndrome. Fertil. Steril. 2017, 107, 796–802. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steegers-Theunissen, R.P.M.; Wiegel, R.E.; Jansen, P.W.; Laven, J.S.E.; Sinclair, K.D. Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence. Int. J. Mol. Sci. 2020, 21, 8211. https://doi.org/10.3390/ijms21218211
Steegers-Theunissen RPM, Wiegel RE, Jansen PW, Laven JSE, Sinclair KD. Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence. International Journal of Molecular Sciences. 2020; 21(21):8211. https://doi.org/10.3390/ijms21218211
Chicago/Turabian StyleSteegers-Theunissen, Régine P. M., Rosalieke E. Wiegel, Pauline W. Jansen, Joop S. E. Laven, and Kevin D. Sinclair. 2020. "Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence" International Journal of Molecular Sciences 21, no. 21: 8211. https://doi.org/10.3390/ijms21218211
APA StyleSteegers-Theunissen, R. P. M., Wiegel, R. E., Jansen, P. W., Laven, J. S. E., & Sinclair, K. D. (2020). Polycystic Ovary Syndrome: A Brain Disorder Characterized by Eating Problems Originating during Puberty and Adolescence. International Journal of Molecular Sciences, 21(21), 8211. https://doi.org/10.3390/ijms21218211





