Pharmaceutical Excipients and Drug Metabolism: A Mini-Review
Abstract
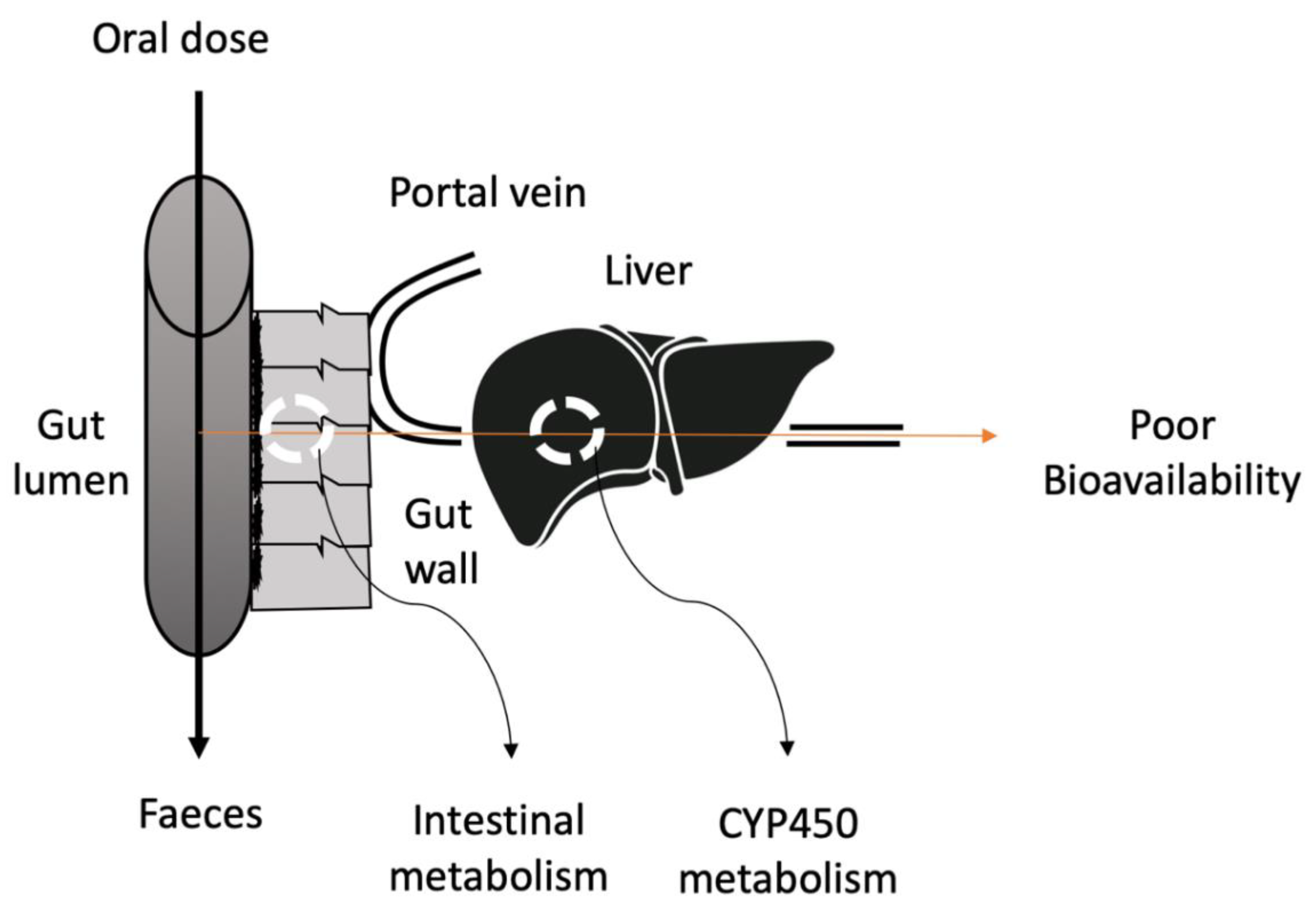
1. Introduction
2. Traditional Strategies to Overcome Pre-Systemic Metabolism
2.1. Prodrug Approaches
2.2. Enzyme Inhibitors
3. Other Approaches
3.1. Novel Approach to Overcome Pre-Systemic Metabolism
3.2. Effect of Excipients
3.3. Surfactants
3.4. Polymers
3.5. Fatty Acids
4. Co-Solvents/Solvents
5. Effect of Excipients on CYP450 Expression
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| AUC | Area Under the Curve |
| CMC | Critical Micellar Concentration |
| CTAB | Cetyltrimethylammonium Bromide |
| CYP450 | Cytochrome P450 |
| DHB | Dihydroxybergamottin |
| DMSO | Dimethyl Sulfoxide |
| FA | Fatty Acids |
| GIT | Gastrointestinal Tract |
| HPC | Hydroxypropyl Cellulose |
| HPMC | Hydroxypropyl Methylcellulose |
| IC50 | Inhibitory Concentration |
| Ki | Inhibitory Constant |
| Clint | Intrinsic Clearance |
| mRNA | Messenger RNA |
| MDZ | Midazolam |
| Mw | Molecular weight |
| MDR1 | Multi-drug Resistance Gene |
| mPEGx-PCLx | Methoxy Poly(ethylene glycol)-poly(ε-caprolactone) (mPEGx-PCLx) |
| ATBC | Acetyl Tributyl Citrate |
| F68 | Pluronic F68 |
| PEG | Polyethylene Glycol |
| PVA | Polyvinyl Acetate |
| PVP | Polyvinyl Pyrrolidone |
| PXR | Pregnane X receptor |
| SDS | Sodium Dodecyl Sulfate |
| SEDSS | Self-emulsifying Drug Delivery System |
| SMEDDS | Self-micro Emulsifying Drug Delivery System |
| NaCMC | Sodium Carboxymethyl Cellulose |
| NI | No Inhibition |
| TPGS | D -α-Tocopherol Polyethylene Glycol 1000 Succinate |
| TX-100 | Triton X-100 |
References
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery—A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef]
- Major Advances in Oral Drug Delivery over the Past 15 Years. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/148747-Major-Advances-in-Oral-Drug-Delivery-over-the-Past-15-Years/ (accessed on 24 July 2020).
- Zhang, W.; Li, Y.; Zou, P.; Wu, M.; Zhang, Z.; Zhang, T. The Effects of Pharmaceutical Excipients on Gastrointestinal Tract Metabolic Enzymes and Transporters—An Update. AAPS PharmSciTech 2016, 18, 830–843. [Google Scholar] [CrossRef]
- Pathak, K.; Raghuvanshi, S. Oral Bioavailability: Issues and Solutions via Nanoformulations. Clin. Pharmacokinet. 2015, 54, 325–357. [Google Scholar] [CrossRef] [PubMed]
- Ezra, A.; Golomb, G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv. Drug Deliv. Rev. 2000, 42, 175–195. [Google Scholar] [CrossRef]
- Cremers, S.; van Hogezand, R.; Bänffer, D.; den Hartigh, J.; Vermeij, P.; Papapoulos, S.E.; Hamdey, N.A.T. Absorption of the oral bisphosphonate alendronate in osteoporotic patients with Crohn’s disease. Osteoporos. Int. 2005, 16, 1727–1730. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.; Christodoulakos, G.; Botsis, D. Women’s health and disease: Gynaecologic, endocrine, and reproductive issues. Ann. N. Y. Acad. Sci. 2006, 1092, 397–402. [Google Scholar] [CrossRef]
- Reinoso, R.F.; Sanchez Navarro, A.; Garcia, M.J.; Prous, J.R. Preclinical pharmacokinetics of statins. Clin. Exp. Pharmacol, Physiol. 2002, 24, 593–613. [Google Scholar]
- Garcia, M.; Reinoso, R.; Sanchez Navarro, A.; Prous, J. Clinical pharmacokinetics of statins. Clin. Exp. Pharmacol. Physiol. 2003, 24, 457–481. [Google Scholar] [CrossRef]
- Buse, J. Statin Treatment in Diabetes Mellitus. Clin. Diabetes 2003, 21, 168–172. [Google Scholar] [CrossRef][Green Version]
- Degim, I.; Acartürk, F.; Erdogan, D.; Demirez Lortlar, N. Transdermal Administration of Bromocriptine. Biol. Pharm. Bull. 2003, 26, 501–505. [Google Scholar] [CrossRef]
- Turner, J.; Glass, B.; Agatonovic-Kustrin, S. Prediction of drug bioavailability based on molecular structure. Anal. Chim. Acta 2003, 485, 89–102. [Google Scholar] [CrossRef]
- Niemi, R.; Turhanen, P.; Vepsäläinen, J.; Taipale, H.; Järvinen, T. Bisphosphonate prodrugs: Synthesis and in vitro evaluation of alkyl and acyloxymethyl esters of etidronic acid as bioreversible prodrugs of etidronate. Eur. J. Pharm. Sci. 2000, 11, 173–180. [Google Scholar] [CrossRef]
- Joseph, N.M.; Sharma, P.K. Cross-linked nanoparticles of cytarabine: Encapsulation, storage and in vivo release. Afr. J. Pharm. Pharmacol. 2007, 1, 10–13. [Google Scholar]
- Ahmad, N.; Keithferris, J.; Gooden, E.; Abell, T. Making a case for domperidone in the treatment of gastrointestinal motility disorders. Curr. Opin. Pharmcol. 2006, 6, 571–576. [Google Scholar] [CrossRef]
- Sturgill, M.; August, D.; Brenner, D. Hepatic Enzyme Induction with Phenobarbital and Doxorubicin Metabolism and Myelotoxicity in the Rabbit. Cancer Investig. 2000, 18, 197–205. [Google Scholar] [CrossRef]
- Dorne, J.; Walton, K.; Renwick, A. Human variability in CYP3A4 metabolism and CYP3A4-related uncertainty factors for risk assessment. Food. Chem. Toxicol. 2003, 41, 201–224. [Google Scholar] [CrossRef]
- Tse, F.; Jaffe, J. Pharmacokinetics of PN 200-110 (isradipine), a new calcium antagonist, after oral administration in man. Eur. J. Clin. Pharmacol. 1987, 32, 361–365. [Google Scholar] [CrossRef]
- Choi, J.; Burm, J. Enhanced nimodipine bioavailability after oral administration of nimodipine with morin, a flavonoid, in rabbits. Arch. Pharm. Res. 2006, 29, 333–338. [Google Scholar] [CrossRef]
- Bennett, M.; Lucas, V.; Brennan, M.; Hughes, A.; O’Donnell, V.; Wee, B. Using anti-muscarinic drugs in the management of death rattle: Evidence-based guidelines for palliative care. Palliat. Med. 2002, 16, 369–374. [Google Scholar] [CrossRef]
- Kharasch, E.; Labroo, R. Metabolism of Ketamine Stereoisomers by Human Liver Microsomes. Anesthesiology 1992, 77, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Manoir, B.; Bourget, P.; Langlois, M.; Szekely, B.; Fischler, M.; Chauvin, M. Evaluation of the pharmacokinetic profile and analgesic efficacy of oral morphine after total hip arthroplasty. Eur. J. Anaesthesiol. 2006, 23, 748–754. [Google Scholar] [CrossRef]
- Aquilonius, S.; Eckernas, S.; Hartvig, P.; Lindstrom, B.; Osterman, P. Pharmacokinetics and oral bioavailability of pyridostigmine in man. Eur. J. Clin. Pharmacol. 1980, 18, 423–428. [Google Scholar] [CrossRef]
- Kleiman-Wexler, R.L.; Adair, C.G.; Ephgrave, K.S. Pharmacokinetics of naloxone: An insight into the locus of effect on stress-ulceration. J. Pharmacol. Exp. Ther. 1989, 251, 435–438. [Google Scholar]
- Finn, A.; Collins, J.; Voyksner, R.; Lindley, C. Bioavailability and Metabolism of Prochlorperazine Administered via the Buccal and Oral Delivery Route. J. Clin. Pharmacol. 2005, 45, 1383–1390. [Google Scholar] [CrossRef]
- Clarke, A.; Jankovic, J. Selegiline orally disintegrating tablet in the treatment of Parkinson’s disease. Therapy 2006, 3, 349–356. [Google Scholar] [CrossRef]
- Cossons, V.F.; Fuseau, E. Mixed effect modelling of sumatriptan pharmacokinetics during drug development: II. From healthy subjects to phase 2 dose ranging in patients. J. Pharmacokinet. Pharmacodyn. 1999, 27, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Jann, M.; Shirley, K.; Small, G. Clinical Pharmacokinetics and Pharmacodynamics of Cholinesterase Inhibitors. Clin. Pharmacokinet. 2002, 41, 719–739. [Google Scholar] [CrossRef]
- Nakhat, P.; Kondawar, A.; Babla, I.; Rathi, L.; Yeole, P. Studies on buccoadhesive tablets of terbutaline sulphate. Indian J. Pharm. Sci. 2007, 69, 505. [Google Scholar] [CrossRef]
- Isohanni, M.H.; Neuroven, P.J.; Olkkola, K.T. Effect of fluvoxamine and erythromycin on the pharmaco- kinetics of oral lidocaine. Basic Clin. Pharmacol. Toxicol. 2006, 99, 168–172. [Google Scholar] [CrossRef]
- Nelson, D.R. The cytochrome P450 homepage. Hum. Genomics 2009, 4, 59–65. [Google Scholar] [CrossRef]
- Zanger, U.; Turpeinen, M.; Klein, K.; Schwab, M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 2008, 392, 1093–1108. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Keinan, S.; Aponick, A.; Zimmermann, E.; Dahan, A. Lipidic prodrug approach for improved oral drug delivery and therapy. Med. Res. Rev. 2018, 39, 579–607. [Google Scholar] [CrossRef]
- Huttunen, K.; Raunio, H.; Rautio, J. Prodrugs—from Serendipity to Rational Design. Pharmacol. Rev. 2011, 63, 750–771. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Wolk, O.; Agbaria, R. Provisional in-silico biopharmaceutics classification (BCS) to guide oral drug product development. Drug Des. Devel. Ther. 2014, 8, 1563. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Clas, S.; Sanchez, R.; Nofsinger, R. Chemistry-enabled drug delivery (prodrugs): Recent progress and challenges. Drug Discov. Today 2014, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.; Wojcieszak, J.; Olejniczak, A. Prodrugs: A challenge for the drug development. Curr. Pharmacol. Rep. 2013, 65, 1–14. [Google Scholar] [CrossRef]
- Dahan, A.; Zimmermann, E.; Ben-Shabat, S. Modern Prodrug Design for Targeted Oral Drug Delivery. Molecules 2014, 19, 16489–16505. [Google Scholar] [CrossRef]
- Han, H.; Amidon, G. Targeted prodrug design to optimize drug delivery. AAPS PharmSciTech 2000, 2, 48–58. [Google Scholar] [CrossRef]
- Amidon, G.; Leesman, G.; Elliott, R. Improving intestinal absorption of water-insoluble compounds: A membrane metabolism strategy. J. Pharm. Sci. 1980, 69, 1363–1368. [Google Scholar] [CrossRef]
- Stella, V. Prodrugs: Some Thoughts and Current Issues. J. Pharm. Sci. 2010, 99, 4755–4765. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Sousa, I.; Bernkop-Schnürch, A. Pre-systemic metabolism of orally administered drugs and strategies to overcome it. J. Control. Release 2014, 192, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.; Malcolm, J.; Arnold, O.; David Spence, J. Grapefruit juice–drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef]
- Fasinu, P.; Choonara, Y.; Khan, R.; Du Toit, L.; Kumar, P.K.; Ndesendo, V. Flavonoids and Polymer Derivatives as CYP3A4 Inhibitors for Improved Oral Drug Bioavailability. J. Pharm. Sci. 2013, 102, 541–555. [Google Scholar] [CrossRef]
- Lin, H.; Kent, U.; Hollenberg, P. The Grapefruit Juice Effect Is Not Limited to Cytochrome P450 (P450) 3A4: Evidence for Bergamottin-Dependent Inactivation, Heme Destruction, and Covalent Binding to Protein in P450s 2B6 and 3A5. J. Pharmacol. Exp. Ther. 2004, 313, 154–164. [Google Scholar] [CrossRef]
- Tassaneeyakul, W.; Guo, L.; Fukuda, K.; Ohta, T.; Yamazoe, Y. Inhibition Selectivity of Grapefruit Juice Components on Human Cytochromes P450. Arch. Biochem. Biophys. 2000, 378, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Girennavar, B.; Poulose, S.; Jayaprakasha, G.; Bhat, N.; Patil, B. Furocoumarins from grapefruit juice and their effect on human CYP 3A4 and CYP 1B1 isoenzymes. Bioorg. Med. Chem. 2006, 14, 2606–2612. [Google Scholar] [CrossRef]
- Paine, M.; Criss, A.; Watkins, P. Two Major Grapefruit Juice Components Differ in Intestinal CYP3A4 Inhibition Kinetic and Binding Properties. Drug Metab. Dispos. 2004, 32, 1146–1153. [Google Scholar] [CrossRef]
- Paine, M.; Criss, A.; Watkins, P. Two Major Grapefruit Juice Components Differ in Time to Onset of Intestinal CYP3A4 Inhibition. J. Pharmacol. Exp. Ther. 2004, 312, 1151–1160. [Google Scholar] [CrossRef]
- Greenblatt, D. Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin. Pharmacol. Ther. 2003, 74, 121–129. [Google Scholar] [CrossRef]
- Ohnishi, A.; Matsuo, H.; Yamada, S.; Takanaga, H.; Morimoto, S.; Shoyama, Y. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. Br. J. Pharmacol. 2000, 130, 1369–1377. [Google Scholar] [CrossRef]
- Eagling, V.A.; Profit, L.; Back, D.J. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br. J. Clin. Pharmacol. 2001, 48, 543–552. [Google Scholar] [CrossRef]
- Fukuda, K.; Ohta, T.; Yamazoe, Y. Grapefruit Component Interacting with Rat and Human P450 CYP3A: Possible Involvement of Non-Flavonoid Components in Drug Interaction. Biol. Pharm. Bull. 1997, 20, 560–564. [Google Scholar] [CrossRef][Green Version]
- Hanley, M.; Cancalon, P.; Widmer, W.; Greenblatt, D. The effect of grapefruit juice on drug disposition. Expert Opin. Drug Metab. Toxicol. 2011, 7, 267–286. [Google Scholar] [CrossRef]
- Galetin, A.; Gertz, M.; Brian Houston, J. Contribution of Intestinal Cytochrome P450-Mediated Metabolism to Drug-Drug Inhibition and Induction Interactions. Drug Metab. Pharmacokinet. 2010, 25, 28–47. [Google Scholar] [CrossRef]
- Bylund, J.; Bueters, T. Presystemic Metabolism of AZ’0908, A Novel mPGES-1 Inhibitor: An In Vitro and In Vivo Cross-Species Comparison. J. Pharm. Sci. 2013, 102, 1106–1115. [Google Scholar] [CrossRef]
- Zhou, S.; Yung Chan, S.; Cher Goh, B.; Chan, E.; Duan, W.; Huang, M. Mechanism-Based Inhibition of Cytochrome P450 3A4 by Therapeutic Drugs. Clin. Pharmacokinet. 2005, 44, 279–304. [Google Scholar] [CrossRef]
- Horn, J.; Howden, C. Review article: Similarities and differences among delayed-release proton-pump inhibitor formulations. Aliment. Pharm. Ther. 2005, 22, 20–24. [Google Scholar] [CrossRef]
- Lueßen, H.; Rentel, C.; Kotzé, A.; Lehr, C.; de Boer, A.; Verhoef, J. Mucoadhesive polymers in peroral peptide drug delivery. IV. Polycarbophil and chitosan are potent enhancers of peptide transport across intestinal mucosae in vitro. J. Control. Release 1997, 45, 15–23. [Google Scholar] [CrossRef]
- Ren, X.; Mao, X.; Si, L.; Cao, L.; Xiong, H.; Qiu, J. Pharmaceutical excipients inhibit cytochrome P450 activity in cell free systems and after systemic administration. Eur. J. Pharm. Biopharm. 2008, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.; Polson, J.; Fontana, R.; Davern, T.; Lalani, E.; Hynan, L. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology 2005, 42, 1364–1372. [Google Scholar] [CrossRef]
- Marx, J. TOXICOLOGY: Protecting Liver from Painkiller’s Lethal Dose. Science 2002, 298, 341a–342a. [Google Scholar] [CrossRef][Green Version]
- Zaher, H.; Buters, J.; Ward, J.; Bruno, M.; Lucas, A.; Stern, S. Protection against Acetaminophen Toxicity in CYP1A2 and CYP2E1 Double-Null Mice. Toxicol. Appl. Pharm. 1998, 152, 193–199. [Google Scholar] [CrossRef]
- USP A. Acetaminophen Oral Solution USP—PAI. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4383763/ (accessed on 24 July 2020).
- Sekhon, B. Surfactants: Pharmaceutical and Medicinal Aspects. JPTRM 2013, 1, 43–68. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Christiansen, A.; Backensfeld, T.; Denner, K.; Weitschies, W. Effects of non-ionic surfactants on cytochrome P450-mediated metabolism in vitro. Eur. J. Pharm. Biopharm. 2011, 78, 166–172. [Google Scholar] [CrossRef]
- Mudra, D.; Borchardt, R. Absorption Barriers in the Rat Intestinal Mucosa. 3: Effects of Polyethoxylated Solubilizing Agents on Drug Permeation and Metabolism. J. Pharm. Sci. 2010, 99, 1016–1027. [Google Scholar] [CrossRef]
- Hugger, E.; Novak, B.; Burton, P.; Audus, K.; Borchardt, R. A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J. Pharm. Sci. 2002, 91, 1991–2002. [Google Scholar] [CrossRef]
- Hugger, E.; Audus, K.; Borchardt, R. Effects of Poly(ethylene glycol) on Efflux Transporter Activity in Caco-2 Cell Monolayers. J. Pharm. Sci. 2002, 91, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Regev, R.; Assaraf, Y.G.; Eytan, G.D. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. Eur. J. Biochem. 1999, 259, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rege, B.D.; Kao, J.P.Y.; Polli, J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Randall, K.P.; Cheng, S.W.; Kotchevar, A.T. Evaluation of surfactants as solubilizing agents in microsomal metabolism reactions with lipophilic substrates. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 631–639. [Google Scholar] [CrossRef]
- Bravo González, R.C.; Huwyler, J.; Boess, F.; Walter, I.; Bittner, B. In vitro investigation on the impact of the surface-active excipients Cremophor EL, Tween 80 and Solutol HS 15 on the metabolism of midazolam. Biopharm. Drug. Dispos. 2004, 25, 37–49. [Google Scholar] [CrossRef]
- Jamis-Dow, C.; Klecker, R.; Katki, A.; Collins, J. Metabolism of taxol by human and rat liver in vitro: A screen for drug interactions and interspecies differences. Cancer Chemother. Pharmacol. 1995, 36, 107–114. [Google Scholar] [CrossRef]
- Tayrouz, Y. Pharmacokinetic and pharmaceutic interaction between digoxin and Cremophor RH40. Clin. Pharmacol. Ther. 2003, 73, 397–405. [Google Scholar] [CrossRef]
- Rao, Z.; Si, L.; Guan, Y.; Pan, H.; Qiu, J.; Li, G. Inhibitive effect of cremophor RH40 or tween 80-based self-microemulsifying drug delivery system on cytochrome P450 3A enzymes in murine hepatocytes. J. Huazhong Univ. Sci. Med. 2010, 30, 562–568. [Google Scholar] [CrossRef]
- Lim, Y.P.; Kuo, S.C.; Lai, M.L. Inhibition of CYP3A4 expression by ketoconazole is mediated by the disruption of pregnane X receptor, steroid receptor coactivator-1, and hepatocyte nuclear factor 4alpha interaction. Pharmacogenet. Genom. 2009, 19, 11–24. [Google Scholar] [CrossRef]
- Fujita, T.; Yasuda, S.; Kamata, Y. Contribution of down-regulation of intestinal and hepatic cytochrome P450 3A to increased absorption of cyclosporine A in a rat nephrosis model. J. Pharmacol. Exp. Ther. 2008, 327, 592–599. [Google Scholar] [CrossRef]
- Ren, S.; Park, M.; Kim, A.; Lee, B. In vitro metabolic stability of moisture-sensitive rabeprazole in human liver microsomes and its modulation by pharmaceutical excipients. Arch. Pharm. Res. 2008, 31, 406–413. [Google Scholar] [CrossRef]
- Farsang, E.; Gaál, V.; Horváth, O.; Bárdos, E.; Horváth, K. Analysis of Non-Ionic Surfactant Triton X-100 Using Hydrophilic Interaction Liquid Chromatography and Mass Spectrometry. Molecules 2019, 24, 1223. [Google Scholar] [CrossRef]
- Da Silva, M.; Meirelles, N. Interaction of non-ionic surfactants with hepatic CYP in Prochilodus scrofa. Toxicol. In Vitro 2004, 18, 859–867. [Google Scholar] [CrossRef]
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2016, 24, 525–536. [Google Scholar] [CrossRef]
- Qiu, L.; Li, Q.; Huang, J.; Wu, Q.; Tu, K.; Wu, Y. In vitro effect of mPEG2k-PCLx micelles on rat liver cytochrome P450 enzymes. Int. J. Pharm. 2018, 552, 99–110. [Google Scholar] [CrossRef]
- Martin, P.; Giardiello, M.; McDonald, T.; Rannard, S.; Owen, A. Mediation of in Vitro Cytochrome P450 Activity by Common Pharmaceutical Excipients. Mol. Pharm. 2013, 10, 2739–2748. [Google Scholar] [CrossRef]
- Huang, J.; Si, L.; Jiang, L.; Fan, Z.; Qiu, J.; Li, G. Effect of pluronic F68 block copolymer on P-glycoprotein transport and CYP3A4 metabolism. Int. J. Pharm. 2008, 356, 351–353. [Google Scholar] [CrossRef]
- Johnson, B.; Charman, W.; Porter, C. An in vitro examination of the impact of polyethylene glycol 400, pluronic P85, and vitamin E d-a-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSciTech 2002, 4, 193–205. [Google Scholar] [CrossRef]
- Butt, U.; ElShaer, A.; Snyder, L.; Al-Kinani, A.; Le Gresley, A.; Alany, R. Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Yoon, B.; Jackman, J.; Valle-González, E.; Cho, N. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Kabara, J.; Swieczkowski, D.; Conley, A.; Truant, J. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.; Vrable, R. Antimicrobial lipids: Natural and synthetic fatty acids and monoglycerides. Lipids 1977, 12, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J. Antimicrobial agents derived from fatty acids. J. Am. Oil Chem. Soc. 1984, 61, 397–403. [Google Scholar] [CrossRef]
- Kabara, J.; Conley, A.; Truant, J. Relationship of Chemical Structure and Antimicrobial Activity of Alkyl Amides and Amines. Antimicrob. Agents Chemother. 1972, 2, 492–498. [Google Scholar] [CrossRef]
- Palacharla, R.; Uthukam, V.; Manoharan, A.; Ponnamaneni, R.; Padala, N.; Boggavarapu, R. Inhibition of cytochrome P450 enzymes by saturated and unsaturated fatty acids in human liver microsomes, characterization of enzyme kinetics in the presence of bovine serum albumin (0.1 and 1.0% w/v) and in vitro—in vivo extrapolation of hepatic clearance. Eur. J. Pharm. Sci. 2017, 101, 80–89. [Google Scholar] [CrossRef]
- Schoch, G.; Yano, J.; Wester, M.; Griffin, K.; Stout, C.; Johnson, E. Structure of Human Microsomal Cytochrome P450 2C8. J. Biol. Chem. 2003, 279, 9497–9503. [Google Scholar] [CrossRef] [PubMed]
- Backman, J.; Filppula, A.; Niemi, M.; Neuvonen, P. Role of Cytochrome P450 2C8 in Drug Metabolism and Interactions. Pharmacol. Rev. 2015, 68, 168–241. [Google Scholar] [CrossRef]
- Yao, H.; Chang, Y.; Lan, S.; Chen, C.; Hsu, J.; Yeh, T. The inhibitory effect of polyunsaturated fatty acids on human CYP enzymes. Life Sci. 2006, 79, 2432–2440. [Google Scholar] [CrossRef]
- Rifkind, A.; Lee, C.; Chang, T.; Waxman, D. Arachidonic acid metabolism by human cytochrome P450s 2C8, 2C9, 2E1, and 1A2: Regioselective oxygenation and evidence for a role for CYP2C enzymes in arachidonic acid epoxygenation in human liver microsomes. Arch. Biochem. Biophys. 1995, 320, 380–389. [Google Scholar] [CrossRef]
- Yamazaki, H. Effects of arachidonic acid, prostaglandins, retinol, retinoic acid and cholecalciferol on xenobiotic oxidations catalysed by human cytochrome P450 enzymes. Xenobiotica 1999, 29, 231–241. [Google Scholar] [CrossRef]
- Iwase, M.; Kurata, N.; Ehana, R.; Nishimura, Y.; Masamoto, T.; Yasuhara, H. Evaluation of the effects of hydrophilic organic solvents on CYP3A-mediated drug-drug interaction in vitro. Hum. Exp. Toxicol. 2006, 25, 715–721. [Google Scholar] [CrossRef]
- Cotreau-Bibbo, M.M.; Von Moltke, L.L.; Greenblatt, D.J. Influence of polyethylene glycol and acetone on the in vitro biotransformation of tamoxifen and alprazolam by human liver microsomes. J. Pharm. Sci. 1996, 85, 1180–1185. [Google Scholar] [CrossRef]
- Draper, A.J.; Madan, A.; Parkinson, A. Inhibition of coumarin 7-hydroxylase activity in human liver microsomes. Arch. Biochem. Biophys. 1997, 341, 47–61. [Google Scholar] [CrossRef]
- Chauret, N.; Gauthier, A.; Nicoll-Griffith, D.A. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab. Dispos. 1998, 26, 1–4. [Google Scholar]
- Hickman, D.; Wang, J.P.; Wang, Y.; Unadkat, J.D. Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab. Dispos. 1998, 26, 207–215. [Google Scholar]
- Busby, W.F., Jr.; Ackermann, J.M.; Crespi, C.L. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab. Dispos. 1999, 27, 246–249. [Google Scholar]
- Coller, J.K.; Somogyi, A.A.; Bochner, F. Flunitrazepam oxidative metabolism in human liver microsomes: Involvement of CYP2C19 and CYP3A4. Xenobiotica 1999, 29, 973–986. [Google Scholar] [CrossRef]
- Palamanda, J.; Feng, W.W.; Lin, C.C.; Nomeir, A.A. Stimulation of tolbutamide hydroxylation by acetone and acetonitrile in human liver microsomes and in a cytochrome P-450 2C9-reconstituted system. Drug Metab. Dispos. 2000, 28, 38–43. [Google Scholar]
- Tolonen, A.; Petsalo, A.; Turpeinen, M.; Uusitalo, J.; Pelkonen, O. In vitro interaction cocktail assay for nine major cytochrome P450 enzymes with 13 probe reactions and a single LC/MSMS run: Analytical validation and testing with monoclonal anti-CYP antibodies. J. Mass Spectrom. 2007, 42, 960–966. [Google Scholar] [CrossRef]
- Youdim, K.; Lyons, R.; Payne, L.; Jones, B.; Saunders, K. An automated, high-throughput, 384 well Cytochrome P450 cocktail IC50 assay using a rapid resolution LC–MS/MS end-point. J. Pharmaceut. Biomed. 2008, 48, 92–99. [Google Scholar] [CrossRef]
- Otten, J.; Hingorani, G.P.; Hartley, D.; Kragerud, S.; B. Franklin, R. An In Vitro, High Throughput, Seven CYP Cocktail Inhibition Assay for the Evaluation of New Chemical Entities Using LC-MS/MS. Drug Metab. Lett. 2011, 5, 17–24. [Google Scholar] [CrossRef]
- Kozakai, K. Reliable high-throughput method for inhibition assay of 8 cytochrome P450 isoforms using cocktail of probe substrates and stable isotope-labeled internal standard. Drug Metab. Pharmacokinet. 2012, 27, 520–529. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Long, N.; Lin, L.; Chen, T.; Zhang, F. An improved substrate cocktail for assessing direct inhibition and time-dependent inhibition of multiple cytochrome P450s. Acta Pharmacol. Sin. 2016, 37, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, Y.; Meng, X.; Sun, X.; Yu, Q.; Li, Y. Effect of Regular Organic Solvents on Cytochrome P450-Mediated Metabolic Activities in Rat Liver Microsomes: Fig. 1. Drug Metab. Dispos. 2010, 38, 1922–1925. [Google Scholar] [CrossRef]
- Nishiya, Y.; Nakamura, K.; Okudaira, N.; Abe, K.; Kobayashi, N.; Okazaki, O. Effects of organic solvents on the time-dependent inhibition of CYP3A4 by diazepam. Xenobiotica 2009, 40, 1–8. [Google Scholar] [CrossRef]
- Tatsumi, A.; Ikegami, Y.; Morii, R.; Sugiyama, M.; Kadobayashi, M.; Iwakawa, S. Effect of Ethanol on S-Warfarin and Diclofenac Metabolism by Recombinant Human CYP2C9.1. Biol. Pharm. Bull. 2009, 32, 517–519. [Google Scholar] [CrossRef]
- Tompkins, L.; Lynch, C.; Haidar, S.; Polli, J.; Wang, H. Effects of Commonly Used Excipients on the Expression of CYP3A4 in Colon and Liver Cells. Pharm. Res. 2010, 27, 1703–1712. [Google Scholar] [CrossRef]
- Klaassen, C.; Slitt, A. Regulation of Hepatic Transporters by Xenobiotic Receptors. Curr. Drug Metab. 2005, 6, 309–328. [Google Scholar] [CrossRef]
- Meyer, U. Overview of enzymes of drug metabolism. J. Pharmacokinet. Phar. 1996, 24, 449–459. [Google Scholar] [CrossRef]
- Lin, J. Sense and Nonsense in the Prediction of Drug-Drug Interactions. Curr. Drug Metab. 2000, 1, 305–331. [Google Scholar] [CrossRef]
- Wang, H.; LeCluyse, E. Role of Orphan Nuclear Receptors in the Regulation of Drug-Metabolising Enzymes. Clin. Pharmacokinet. 2003, 42, 1331–1357. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.; Moore, J.; Wade, L.; Staudinger, J.; Watson, M.; Jones, S. An Orphan Nuclear Receptor Activated by Pregnanes Defines a Novel Steroid Signaling Pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Lehmann, J.; McKee, D.; Watson, M.; Willson, T.; Moore, J.; Kliewer, S. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investig. 1998, 102, 1016–1023. [Google Scholar] [CrossRef]
- Takeshita, A.; Igarashi-Migitaka, J.; Nishiyama, K.; Takahashi, H.; Takeuchi, Y.; Koibuchi, N. Acetyl Tributyl Citrate, the Most Widely Used Phthalate Substitute Plasticizer, Induces Cytochrome P450 3A through Steroid and Xenobiotic Receptor. Toxicol. Sci. 2011, 123, 460–470. [Google Scholar] [CrossRef]


| Drugs | Pharmacological Class | Bioavailability (%) | Reasons | References |
|---|---|---|---|---|
| Alendronate | Bisphosphonates | 0.59–0.78 | Poor solubility and absorption | [5,6,7] |
| Atorvastatin | Statins | 14 | P-gp and CYP450 activities | [8,9,10] |
| Bromocriptine | Dopamine receptor agonists | 5–10 | Extensive first-pass effect | [11,12,13] |
| Clodronate | Bisphosphonates | 1 | Poor solubility and absorption | [5,6,7] |
| Cytarabine | Antimetabolites | 20 | Intestinal and hepatic first-pass | [14] |
| Domperidone | D2 receptor antagonists | 15 | Gut and liver first-pass | [15] |
| Doxorubicin | Anthracycline antibiotics | 5 | Hepatic and intestinal metabolism | [16] |
| Budesonide | Corticosteroids | 11 | Hepatic first-pass effect | [17] |
| Etidronate | Bisphosphonates | 5 | Poor solubility and absorption | [6,7,13] |
| Felodipine | Calcium channel blockers | 15 | P-gp and CYP450 activities | [17] |
| Isradipine | Calcium channel blockers | 15 | P-gp and CYP450 activities | [18] |
| Fluvastatin | Statins | 20 | P-gp and CYP450 activities | [8,9,10] |
| Nimodipine | Calcium Channel blockers | 13 | P-gp and CYP450 activities | [19] |
| Hyoscine | Antispasmodics | 20 | Hepatic metabolism | [20] |
| Ketamine | Dissociative anesthetics | 20 | Hepatic and intestinal metabolism | [21] |
| Lovastatin | Statins | <5 | P-gp and CYP450 activities | [8,9,10] |
| Morphine | Opioids | 20–33 | Gut and liver first-pass | [22] |
| Pyridostigmine | Acetylcholinesterase inhibitors | 14 | Poor absorption | [23] |
| Naloxone | Opioid antagonists | 2–10 | Extensive first-pass but 90% absorption | [24] |
| Naltrexone | Opiate antagonists | 5–40 | First-pass, enterohepatic recycling | [10] |
| Pamidronate | Bisphosphonates | 1 | Poor solubility and absorption | [5,6,7] |
| Pravastatin | Statins | 17–34 | P-gp and CYP450 activities | [8,9,10] |
| Prochlorperazine | Phenothiazines | 20 | Intestinal and hepatic first-pass | [25] |
| Risedronate | Bisphosphonates | <1 | Poor solubility and absorption | [5,6,7] |
| Selegiline | Monoamine oxidase type B inhibitors | 20 | Extensive first-pass | [26] |
| Simvastatin | Statins | 5–48 | P-gp and CYP450 activities | [8,9,10] |
| Sumatriptan | Serotonin receptor agonists | 20 | Hepatic first-pass | [27] |
| Tacrine | Cholinesterase inhibitors | 10–30 | Hepatic first-pass | [28] |
| Terbutaline | Adrenergic receptor agonists | 9–21 | Extensive first-pass and poor absorption | [29] |
| Lidocaine | Local anesthetics | 3 | Hepatic first-pass effect | [30] |
| Tiludronate | Bisphosphonates | 6 | Poor solubility and absorption | [5,6,7] |
| Surfactants | Substrates | Mechanism of Action | Structures | Type | References |
|---|---|---|---|---|---|
| Brij 35 | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Non-ionic | [74] |
| Brij 58 | Rabeprazole | Significant inhibition of drug degradation by CYP enzymes |  | Non-ionic | [81] |
| CTAB | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Cationic | [74] |
| Kollidon 12 PF | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Non-ionic | [74] |
| Lutrol F68 NF | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Non-ionic | [74] |
| Octyl-B-D-glucopyranoside | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Non-ionic | [74] |
| SDS | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Anionic | [74] |
| Solutol HS 15 | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Non-ionic | [74] |
| Triton X-100 reduced | 7-ethoxycoumarin | Increased CYP3A4 inhibition with increased surfactant concentration |  | Non-ionic | [74] |
| Polysorbate 80 | Testosterone Diclofenac | Increased CYP3A4 and CYP2C9 inhibition in concentration-dependent manner |  | Non-ionic | [68] |
| TPGS | Testosterone Diclofenac | Increased CYP3A4 and CYP2C9 inhibition in concentration-dependent manner |  | Non-ionic | [68] |
| Sucrose laurate | Testosterone Diclofenac | Increased CYP3A4 and CYP2C9 inhibition in concentration-dependent manner |  | Non-ionic | [68] |
| Gelucire 44/14 | Rabeprazole | Significant inhibition of drug degradation by CYP enzymes | Lauroyl polyoxyl-32 glycerides (C9H14N2) | Non-ionic | [81] |
| Polyoxyl 40 Stearate | Midazolam | Strong inhibition of rCYP3A4 |  | Non-ionic | [61] |
| Pluronic F68 | Midazolam | Strong inhibition of rCYP3A4 |  | Non-ionic | [61] |
| Polymers | Examples |
|---|---|
| Natural | Sodium alginate Gelatin Chitosan |
| Semi-synthetic | Cellulose derivatives |
| Synthetic | Polyethylene glycols Poloxamers Polyactides Polyamides Acrylic acid polymers |
| Fermentation products | Xanthan gum |
| Polymer | IC50 Values (μM) | ||||||
|---|---|---|---|---|---|---|---|
| CYP2E1 | CYP3A4 | CYP3A5 | CYP2C9 | CYP2C19 | CYP1A2 | CYP2D6 | |
| PEG | 75.3 ± 2.1 | - | 78.0 ± 17.8 | 365.6 ± 32.8 | 139.0 ± 22.4 | - | 409.6 ± 34.5 |
| F68 | 203.7 ± 48.3 | 59.1 ± 13.6 | 209.9 ± 29.7 | 244.8 ± 13.2 | - | - | - |
| F127 | 218.9 ± 13.3 | - | - | - | - | - | - |
| NaCMC | - | - | - | - | - | 224.7 ± 14.8 | |
| HPC | - | - | - | - | - | - | - |
| HPMC | 253.5 ± 17.9 | - | 19.4 ± 0.6 | - | - | - | - |
| PVA | 548.9 ± 30.4 | - | - | - | - | - | - |
| Kollicoat | 598.1 ± 26.1 | - | - | - | - | 10.0 ± 3.9 | 89.9 ± 2.9 |
| HG | 141.2 ± 14.1 | - | - | - | - | 40.9 ± 8.4 | - |
| PVP | - | 107.3 ± 11.2 | - | - | - | 78.3 ± 4.2 | - |
| Fatty Acid | Absolute IC50 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1A2 | 2A6 | 2B6 | 2C8 | 2C9 | 2C19 | 2D6 | 2E1 | 3A4 | |
| Arachidonic acid | 9.7 | 21.4 | 4.6 | 1.3 | 3.3 | 23.2 | 18.3 | 61.7 | 11.7 |
| Behenic acid | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 | >30 |
| Cervonic acid | 6.3 | 11.7 | 6.7 | 1.2 | 2.6 | 15.8 | 5.6 | 44.4 | 7.5 |
| Gondoic acid | 16.2 | 81.9 | 16.6 | 6.0 | 17 | >100 | >100 | >100 | >100 |
| Lauric acid | >100 | >100 | 21.5 | 42.9 | >100 | >100 | >100 | >100 | >100 |
| Linoleic acid | 13.3 | 28.9 | 7.1 | 1.0 | 7.4 | 55.8 | 17.5 | 58.9 | 18.5 |
| α-Linolenic acid | 8.8 | 13.5 | 9.7 | 4.4 | 10.6 | 53.3 | 34.3 | 67.2 | 36.9 |
| Myristic acid | 15.8 | >100 | 10.7 | 13.3 | 36.1 | >100 | >100 | >100 | >100 |
| Nervonic acid | >11.1 | >11.1 | >11.1 | >11.1 | >11.1 | >11.1 | >11.1 | >11.1 | >11.1 |
| Oleic acid | 11.2 | 25 | 8.2 | 4.4 | 5.7 | 98.9 | 18.1 | 83.8 | 11.4 |
| Palmitic acid | >100 | >100 | 90.5 | >100 | >100 | >100 | >100 | >100 | >100 |
| Palmitoleic acid | 7.8 | 36.2 | 8 | 9.7 | 11.9 | 58.1 | 30.3 | 72.1 | 26.5 |
| Stearic acid | >33.3 | >33.3 | >33.3 | >33.3 | >33.3 | >33.3 | >33.3 | >33.3 | >33.3 |
| Timnodonic acid | 8.2 | 17.4 | 5.9 | 1.5 | 3.8 | 13.8 | 5.7 | 77.4 | 16 |
| Fatty Acids | IC50 | |||||
|---|---|---|---|---|---|---|
| CYP1A2 | CYP2C9 | CYP2C19 | CYP2D6 | CYP2E1 | CYP3A4 | |
| Palmitic acid | NI | NI | NI | NI | NI | NI |
| Stearic acid | NI | NI | NI | NI | NI | NI |
| Linoleic acid | 74 | 4.1 | 15 | 192 | 113 | 49 |
| Linolenic acid | 52 | 8.1 | 9.3 | 151 | 82 | 61 |
| Arachidonic acid | 37 | 3.5 | 4.8 | 113 | 67 | 48 |
| Eicosapentaenoic acid | 41 | 4.4 | 4.4 | 127 | 53 | 54 |
| Docosahexaenoic acid | 41 | 2.9 | 6.7 | 122 | 65 | 34 |
| Excipients | Fa2N4 | HPH | LS174T | |||
|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | CYP3A4 | MDR1 | |
| HPMC | ↑ | ↓ | = | x | ↓ | ↓a |
| Pregelatinized starch | = | = | ↓ | x | ↓ | ↓ |
| Croscarmellose sodium | ↑ | = | ↑ | x | ↓a | ↓a |
| Crospovidone | ↑a | ↓ | = | x | ↓ | ↓a |
| Polysorbate-80 | ↓ | ↓ | ↓ | ↓ | = | = |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Barker, J.; ElShaer, A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 8224. https://doi.org/10.3390/ijms21218224
Patel R, Barker J, ElShaer A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. International Journal of Molecular Sciences. 2020; 21(21):8224. https://doi.org/10.3390/ijms21218224
Chicago/Turabian StylePatel, Rahul, James Barker, and Amr ElShaer. 2020. "Pharmaceutical Excipients and Drug Metabolism: A Mini-Review" International Journal of Molecular Sciences 21, no. 21: 8224. https://doi.org/10.3390/ijms21218224
APA StylePatel, R., Barker, J., & ElShaer, A. (2020). Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. International Journal of Molecular Sciences, 21(21), 8224. https://doi.org/10.3390/ijms21218224







