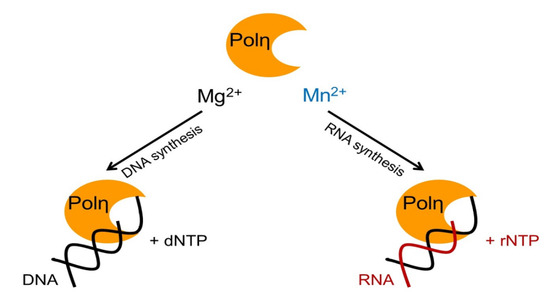
Selective Metal Ion Utilization Contributes to the Transformation of the Activity of Yeast Polymerase η from DNA Polymerization toward RNA Polymerization
Abstract
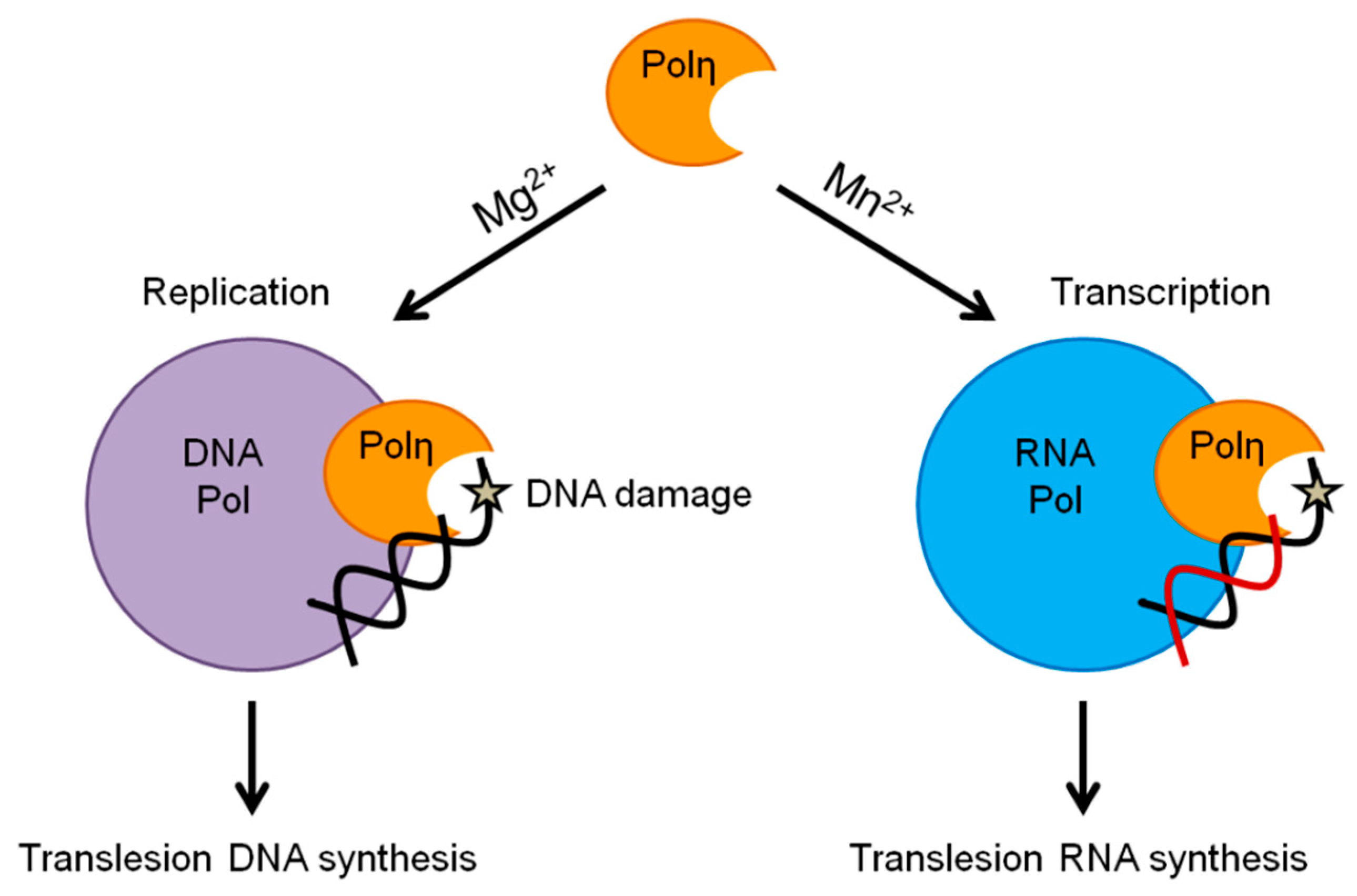
1. Introduction
2. Results
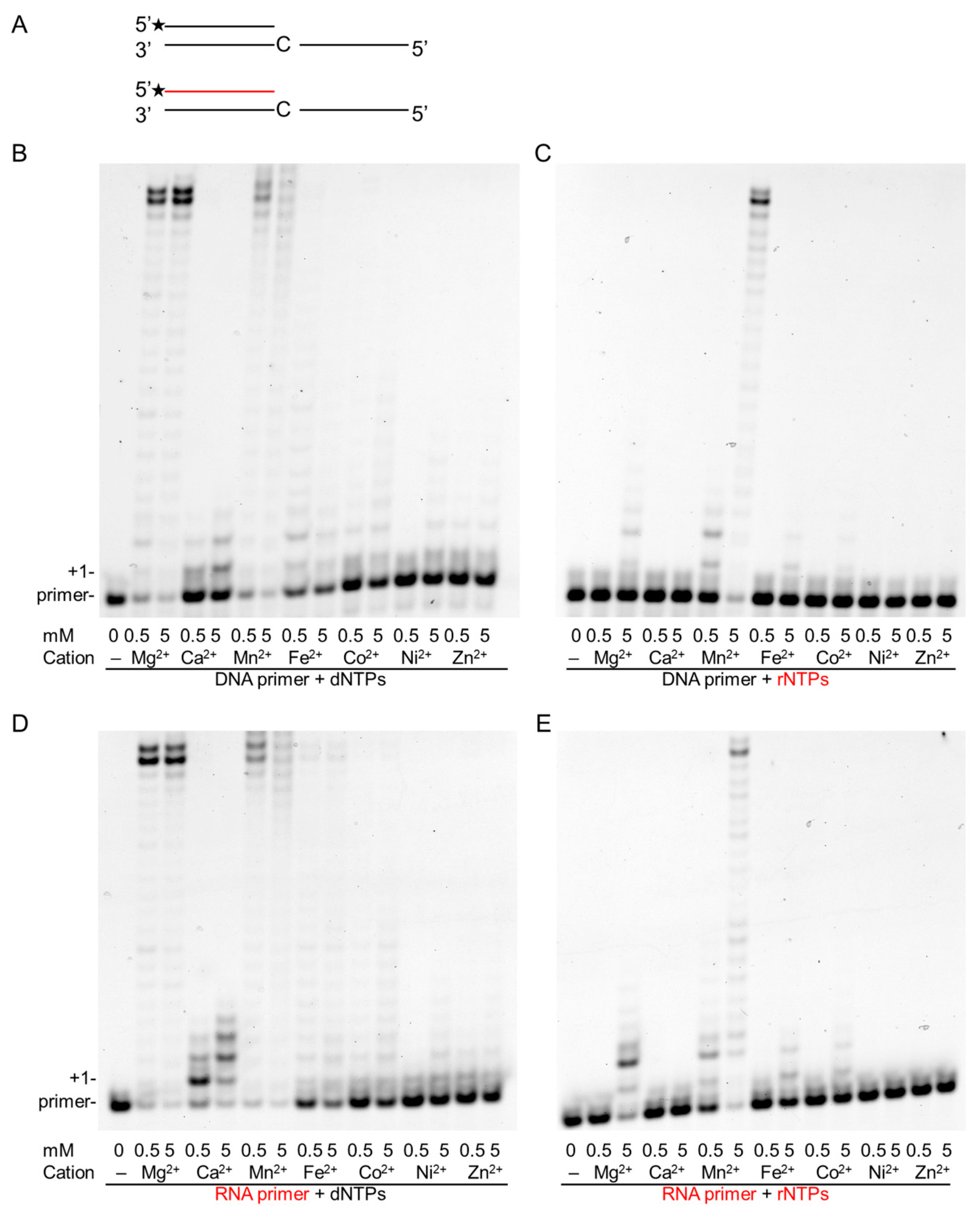
2.1. Effect of Divalent Metal Ions on the Synthetic Activity of Polη
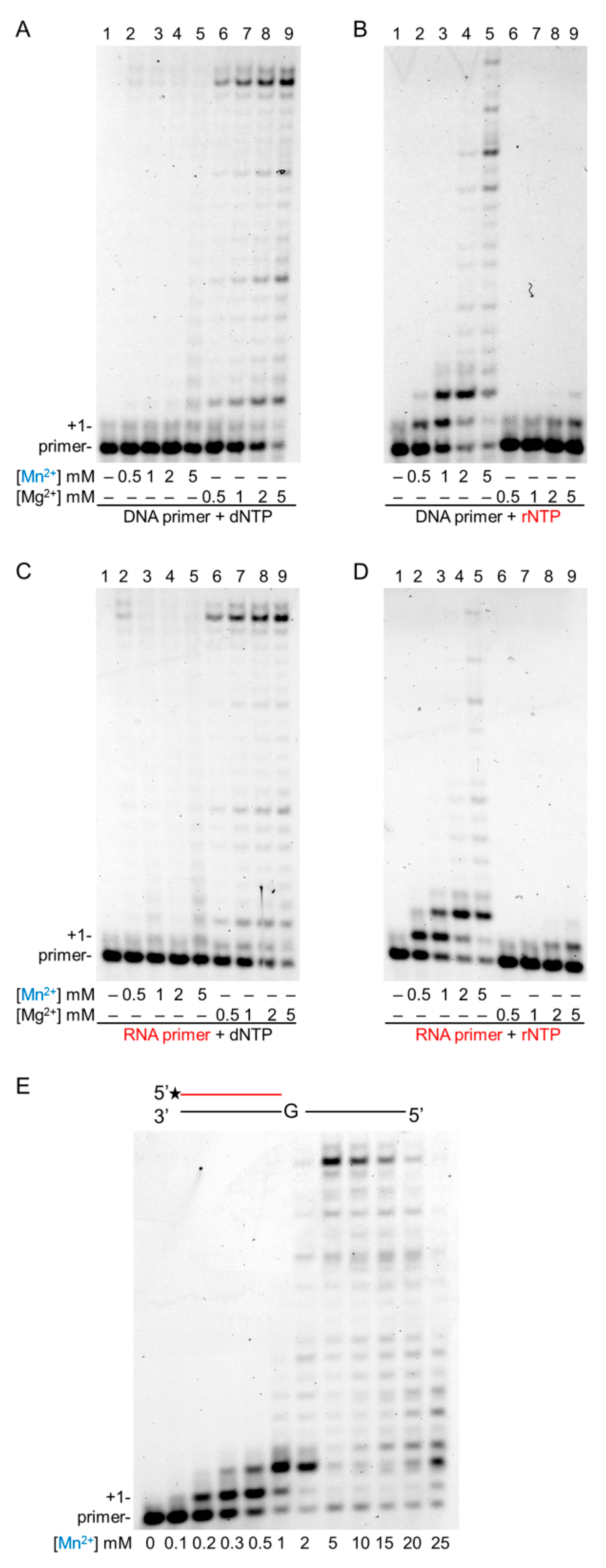
2.2. Mg2+ and Mn2+ Concentration-Dependent Synthesis by Polη
2.3. Kinetics of Correct rNMP Incorporation into RNA in the Presence of Mn2+
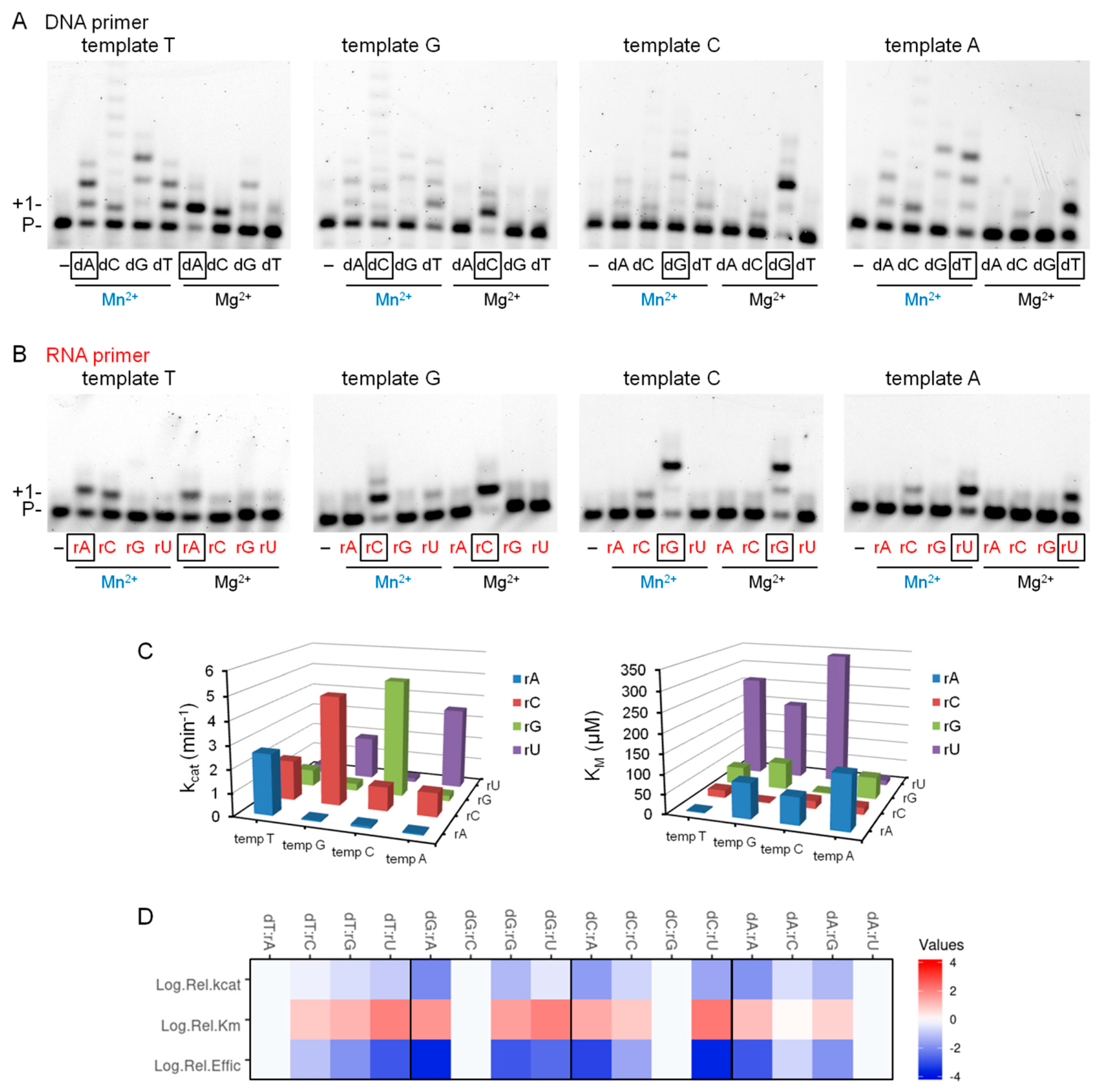
2.4. The Effect of Mn2+ on the Base Selectivity of Polη
2.5. DNA Damage Bypass Activity of Polη with Mn2+
2.6. Metal Preference of Polη during RNA Synthesis
3. Discussion
4. Materials and Methods
4.1. Protein Purification
4.2. Oligonucleotides and Primer Extension Assays
4.3. Determination of Steady-State Kinetic Parameters
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia-Diaz, M.; Bebenek, K. Multiple functions of DNA polymerases. CRC Crit. Rev. Plant Sci. 2007, 26, 105–122. [Google Scholar] [CrossRef]
- Acharya, N.; Khandagale, P.; Thakur, S.; Sahu, J.K.; Utkalaja, B.G. Quaternary structural diversity in eukaryotic DNA polymerases: Monomeric to multimeric form. Curr. Genet. 2020, 66, 635–655. [Google Scholar] [CrossRef] [PubMed]
- Echols, H.; Goodman, M.F. Fidelity Mechanisms In DNA Replication. Annu. Rev. Biochem. 1991, 60, 477–511. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Dixon, N. Replicative DNA polymerases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012799. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Johnson, R.E.; Prakash, L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu. Rev. Biochem. 2005, 74, 317–353. [Google Scholar] [CrossRef] [PubMed]
- Vaisman, A.; Woodgate, R. Translesion DNA polymerases in eukaryotes: What makes them tick? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 274–303. [Google Scholar] [CrossRef]
- Johnson, R.E.; Prakash, S.; Prakash, L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science 1999, 283, 1001–1004. [Google Scholar] [CrossRef]
- Johnson, R.E.; Kondratick, C.M.; Prakash, S.; Prakash, L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 1999, 285, 263–265. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Haracska, L.; Yu, S.L.; Johnson, R.E.; Prakash, L.; Prakash, S. Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nat. Genet. 2000, 25, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Nick McElhinny, S.A.; Watts, B.E.; Kumar, D.; Watt, D.L.; Lundström, E.B.; Burgers, P.M.J.; Johansson, E.; Chabes, A.; Kunkel, T.A. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Natl. Acad. Sci. USA 2010, 107, 4949–4954. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.S.; Walder, R.Y.; Walder, J.A. Substrate specificity of human RNase H1 and its role in excision repair of ribose residues misincorporated in DNA. Biochimie 1993, 75, 123–126. [Google Scholar] [CrossRef]
- Rydberg, B.; Game, J. Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. Proc. Natl. Acad. Sci. USA 2002, 99, 16654–16659. [Google Scholar] [CrossRef]
- Joyce, C.M. Choosing the right sugar: How polymerases select a nucleotide substrate. Proc. Natl. Acad. Sci. USA 1997, 94, 1619–1622. [Google Scholar] [CrossRef]
- Vaisman, A.; Woodgate, R. Ribonucleotide discrimination by translesion synthesis DNA polymerases. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 382–402. [Google Scholar] [CrossRef]
- Makarova, A.V.; McElhinny, S.A.N.; Watts, B.E.; Kunkel, T.A.; Burgers, P.M. Ribonucleotide incorporation by yeast DNA polymerase ζ. DNA Repair 2014, 18, 63–67. [Google Scholar] [CrossRef][Green Version]
- Sassa, A.; Çaǧlayan, M.; Rodriguez, Y.; Beard, W.A.; Wilson, S.H.; Nohmi, T.; Honma, M.; Yasui, M. Impact of ribonucleotide backbone on translesion synthesis and repair of 7,8-Dihydro-8-oxoguanine. J. Biol. Chem. 2016, 291, 24314–24323. [Google Scholar] [CrossRef]
- Vaisman, A.; Kuban, W.; McDonald, J.P.; Karata, K.; Yang, W.; Goodman, M.F.; Woodgate, R. Critical amino acids in Escherichia coli UmuC responsible for sugar discrimination and base-substitution fidelity. Nucleic Acids Res. 2012, 40, 6144–6157. [Google Scholar] [CrossRef]
- Ordonez, H.; Uson, M.L.; Shuman, S. Characterization of three mycobacterial DinB (DNA polymerase IV) paralogs highlights DinB2 as naturally adept at ribonucleotide incorporation. Nucleic Acids Res. 2014, 42, 11056–11070. [Google Scholar] [CrossRef]
- Nick McElhinny, S.A.; Ramsden, D.A. Polymerase Mu Is a DNA-Directed DNA/RNA Polymerase. Mol. Cell. Biol. 2003, 23, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Gali, V.K.; Balint, E.; Serbyn, N.; Frittmann, O.; Stutz, F.; Unk, I. Translesion synthesis DNA polymerase η exhibits a specific RNA extension activity and a transcription-associated function. Sci. Rep. 2017, 7, 13055. [Google Scholar] [CrossRef]
- Donigan, K.A.; Cerritelli, S.M.; McDonald, J.P.; Vaisman, A.; Crouch, R.J.; Woodgate, R. Unlocking the steric gate of DNA polymerase η leads to increased genomic instability in Saccharomyces cerevisiae. DNA Repair 2015, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Egli, M.; Guengerich, F.P. Human DNA polymerase η accommodates RNA for strand extension. J. Biol. Chem. 2017, 292, 18044–18051. [Google Scholar] [CrossRef]
- Su, Y.; Egli, M.; Guengerich, F.P. Mechanism of ribonucleotide incorporation by human DNA polymerase η. J. Biol. Chem. 2016, 291, 3747–3756. [Google Scholar] [CrossRef]
- Mentegari, E.; Crespan, E.; Bavagnoli, L.; Kissova, M.; Bertoletti, F.; Sabbioneda, S.; Imhof, R.; Sturla, S.J.; Nilforoushan, A.; Hübscher, U.; et al. Ribonucleotide incorporation by human DNA polymerase η impacts translesion synthesis and RNase H2 activity. Nucleic Acids Res. 2017, 45, 2600–2614. [Google Scholar] [CrossRef]
- Meroni, A.; Nava, G.M.; Bianco, E.; Grasso, L.; Galati, E.; Bosio, M.C.; Delmastro, D.; Muzi-Falconi, M.; Lazzaro, F. RNase H activities counteract a toxic effect of Polymerase η in cells replicating with depleted dNTP pools. Nucleic Acids Res. 2019, 47, 4612–4623. [Google Scholar] [CrossRef]
- Beese, L.S.; Steitz, T.A. Structural basis for the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I: A two metal ion mechanism. EMBO J. 1991, 10, 25–33. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, W. Capture of a third Mg2+ is essential for catalyzing DNA synthesis. Science 2016, 352, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Warshel, A. Simulating the Fidelity And The Three Mg Mechanism of Pol η and clarifying the validity of transition state theory in enzyme catalysis. Proteins 2017, 85, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Hammes-Schiffer, S. Exploring the Role of the Third Active Site Metal Ion in DNA Polymerase η with QM/MM Free Energy Simulations. J. Am. Chem. Soc. 2018, 140, 8965–8969. [Google Scholar] [CrossRef]
- Sirover, M.A.; Loeb, L.A. Metal activation of DNA synthesis. Biochem. Biophys. Res. Commun. 1976, 70, 812–817. [Google Scholar] [CrossRef]
- Sirover, M.A.; Dube, D.K.; Loeb, L.A. On the fidelity of DNA replication. Metal activation of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979, 254, 107–111. [Google Scholar]
- Pelletier, H.; Sawaya, M.R.; Wolfle, W.; Wilson, S.H.; Kraut, J. A structural basis for metal ion mutagenicity and nucleotide selectivity in human DNA polymerase β. Biochemistry 1996, 35, 12762–12777. [Google Scholar] [CrossRef]
- Blanca, G.; Shevelev, I.; Ramadan, K.; Villani, G.; Spadari, S.; Hübscher, U.; Maga, G. Human DNA polymerase λ diverged in evolution from DNA polymerase β toward specific Mn++ dependence: A kinetic and thermodynamic study. Biochemistry 2003, 42, 7467–7476. [Google Scholar] [CrossRef]
- Martin, M.J.; Garcia-Ortiz, M.V.; Esteban, V.; Blanco, L. Ribonucleotides and manganese ions improve non-homologous end joining by human Polm No Title. EMBO J. 2013, 41, 2428–2436. [Google Scholar]
- Frank, E.G.; Woodgate, R. Increased catalytic activity and altered fidelity of human DNA polymerase iota in the presence of manganese. J. Biol. Chem. 2007, 282, 24689–24696. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.K.; Ketkar, A.; Lodeiro, M.F.; Cameron, C.E.; Eoff, R.L. Kinetic analysis of human PrimPol DNA polymerase activity reveals a generally error-prone enzyme capable of accurately bypassing 7,8-dihydro-8-oxo-2′-deoxyguanosine. Biochemistry 2014, 53, 6584–6594. [Google Scholar] [CrossRef]
- García-Gómez, S.; Reyes, A.; Martínez-Jiménez, M.I.; Chocrón, E.S.; Mourón, S.; Terrados, G.; Powell, C.; Salido, E.; Méndez, J.; Holt, I.J.; et al. PrimPol, an Archaic Primase/Polymerase Operating in Human Cells. Mol. Cell 2013, 52, 541–553. [Google Scholar] [CrossRef]
- Washington, M.T.; Johnson, R.E.; Prakash, S.; Prakash, L. Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase η. J. Biol. Chem. 1999, 274, 36835–36838. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Loria, A.; Zhong, K. Probing of tertiary interactions in RNA: 2′-hydroxyl-base contacts between the RNase P RNA and pre-tRNA. Proc. Natl. Acad. Sci. USA 1995, 92, 12510–12514. [Google Scholar] [CrossRef]
- Lindqvist, M.; Sarkar, M.; Winqvist, A.; Rozners, E.; Strömberg, R.; Gräslund, A. Optical spectroscopic study of the effects of a single deoxyribose substitution in a ribose backbone: Implications in RNA-RNA interaction. Biochemistry 2000, 39, 1693–1701. [Google Scholar] [CrossRef]
- Fahlman, R.P.; Olejniczak, M.; Uhlenbeck, O.C. Quantitative analysis of deoxynucleotide substitutions in the codon-anticodon helix. J. Mol. Biol. 2006, 355, 887–892. [Google Scholar] [CrossRef]
- Morin, B.; Whelan, S.P.J. Sensitivity of the polymerase of vesicular stomatitis virus to 2′ substitutions in the template and nucleotide triphosphate during initiation and elongation. J. Biol. Chem. 2014, 289, 9961–9969. [Google Scholar] [CrossRef]
- Wang, D.; Bushnell, D.A.; Westover, K.D.; Kaplan, C.D.; Kornberg, R.D. Structural Basis of Transcription: Role of the Trigger Loop in Substrate Specificity and Catalysis. Cell 2006, 127, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, J.L.; Shaw, J.J.; Youngman, E.M.; Green, R. Peptide release on the ribosome depends critically on the 2′ OH of the peptidyl tRNA substrate. RNA 2008, 14, 1526–1531. [Google Scholar] [CrossRef][Green Version]
- Cyert, M.S.; Philpott, C.C. Regulation of cation balance in Saccharomyces cerevisiae. Genetics 2013, 193, 677–713. [Google Scholar] [CrossRef]
- Tottey, S.; Harvie, D.R.; Robinson, N.J. Understanding how cells allocate metals using metal sensors and metallochaperones. Acc. Chem. Res. 2005, 38, 775–783. [Google Scholar] [CrossRef]
- Capdevila, D.A.; Edmonds, K.A.; Giedroc, D.P. Metallochaperones and metalloregulation in bacteria. Essays Biochem. 2017, 61, 177–200. [Google Scholar]
- Culotta, V.C.; Klomp, L.W.J.; Strain, J.; Casareno, R.L.B.; Krems, B.; Gitlin, J.D. The copper chaperone for superoxide dismutase. J. Biol. Chem. 1997, 272, 23469–23472. [Google Scholar] [CrossRef]
- Moira Glerum, D.; Shtanko, A.; Tzagoloff, A. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 1996, 271, 14504–14509. [Google Scholar] [CrossRef]


| Templating Nucleotide | Incoming Nucleotide | Cation | kcat (min−1) | KM (µM) | kcat/KM (min−1µM−1) | Relative Efficiency a |
|---|---|---|---|---|---|---|
| T | rATP | Mg2+ | 0.24 ± 0.01 b | 466 ± 47.3 b | 5.15 × 10−4 b | |
| Mn2+ | 2.61 ± 0.14 | 2.51 ± 0.64 | 1.04 | 2019 | ||
| G | rCTP | Mg2+ | 2.76 ± 0.06 b | 438 ± 37.5 b | 6.30 × 10−3 b | |
| Mn2+ | 4.68 ± 0.22 | 1.89 ± 0.42 | 2.48 | 394 | ||
| C | rGTP | Mg2+ | 0.45 ± 0.01 b | 394 ± 52 b | 1.14 × 10−3 b | |
| Mn2+ | 5.07 ± 0.27 | 2.55 ± 0.63 | 1.99 | 1746 | ||
| A | rUTP | Mg2+ | 0.10 ± 0.01 b | 423 ± 90.4 b | 2.36 × 10−4 b | |
| Mn2+ | 3.51 ± 0.19 | 12.8 ± 2.25 | 2.74 × 10−1 | 1161 | ||
| 8-oxo-G | rCTP | Mg2+ | 0.034 ± 0.004b | 974 ± 270 b | 3.52 × 10−5 | |
| Mn2+ | 0.275 ± 0.01 | 1.25 ± 0.28 | 2.20 × 10−1 | 6286 | ||
| TT dimer | rATP | Mg2+ | 0.0083 ± 0.001 b | 1678 ± 445 b | 4.94 × 10−6 | |
| Mn2+ | 0.174 ± 0.005 | 11.3 ± 1.35 | 1.54 × 10−2 | 3117 |
| Templating Nucleotide | Incoming Nucleotide | kcat (min−1) | KM (µM) | kcat/KM (min−1µM−1) | Relative Efficiency a | Discrimination1/f b |
|---|---|---|---|---|---|---|
| T | rATP | 2.61 ± 0.14 | 2.51 ± 0.64 | 1.04 | ||
| rCTP | 1.72 ± 0.06 | 19.4 ± 3.14 | 0.09 | 8.7 × 10−2 | 12 | |
| rGTP | 0.72 ± 0.03 | 44.6 ± 7.03 | 0.016 | 1.5 × 10−2 | 67 | |
| rUTP | 0.36 ± 0.02 | 261 ± 50.6 | 0.0014 | 1.3 × 10−3 | 769 | |
| G | rATP | 0.06 ± 0.00 | 89.3 ± 17.7 | 0.0007 | 2.8 × 10−4 | 3571 |
| rCTP | 4.68 ± 0.22 | 1.89 ± 0.42 | 2.48 | |||
| rGTP | 0.31 ± 0.02 | 68.9 ± 14.9 | 0.0045 | 1.8 × 10−3 | 555 | |
| rUTP | 1.86 ± 0.04 | 199 ± 14.5 | 0.0093 | 3.8 × 10−3 | 263 | |
| C | rATP | 0.10 ± 0.00 | 69.2 ± 11.9 | 0.0014 | 7.0 × 10−4 | 1429 |
| rCTP | 1.03 ± 0.04 | 19.9 ± 3.74 | 0.052 | 2.6 × 10−2 | 38 | |
| rGTP | 5.07 ± 0.27 | 2.55 ± 0.63 | 1.99 | |||
| rUTP | 0.16 ± 0.01 | 339 ± 49.4 | 0.0005 | 2.5 × 10−4 | 4000 | |
| A | rATP | 0.05 ± 0.00 | 138 ± 26.2 | 0.0004 | 1.48 × 10−3 | 676 |
| rCTP | 1.03 ± 0.07 | 17.5 ± 4.92 | 0.059 | 2.2 × 10−1 | 5 | |
| rGTP | 0.24 ± 0.01 | 55.9 ± 8.64 | 0.0043 | 1.6 ×10−2 | 63 | |
| rUTP | 3.51 ± 0.19 | 12.8 ± 2.25 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balint, E.; Unk, I. Selective Metal Ion Utilization Contributes to the Transformation of the Activity of Yeast Polymerase η from DNA Polymerization toward RNA Polymerization. Int. J. Mol. Sci. 2020, 21, 8248. https://doi.org/10.3390/ijms21218248
Balint E, Unk I. Selective Metal Ion Utilization Contributes to the Transformation of the Activity of Yeast Polymerase η from DNA Polymerization toward RNA Polymerization. International Journal of Molecular Sciences. 2020; 21(21):8248. https://doi.org/10.3390/ijms21218248
Chicago/Turabian StyleBalint, Eva, and Ildiko Unk. 2020. "Selective Metal Ion Utilization Contributes to the Transformation of the Activity of Yeast Polymerase η from DNA Polymerization toward RNA Polymerization" International Journal of Molecular Sciences 21, no. 21: 8248. https://doi.org/10.3390/ijms21218248
APA StyleBalint, E., & Unk, I. (2020). Selective Metal Ion Utilization Contributes to the Transformation of the Activity of Yeast Polymerase η from DNA Polymerization toward RNA Polymerization. International Journal of Molecular Sciences, 21(21), 8248. https://doi.org/10.3390/ijms21218248