The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain
Abstract
1. Introduction
2. Results
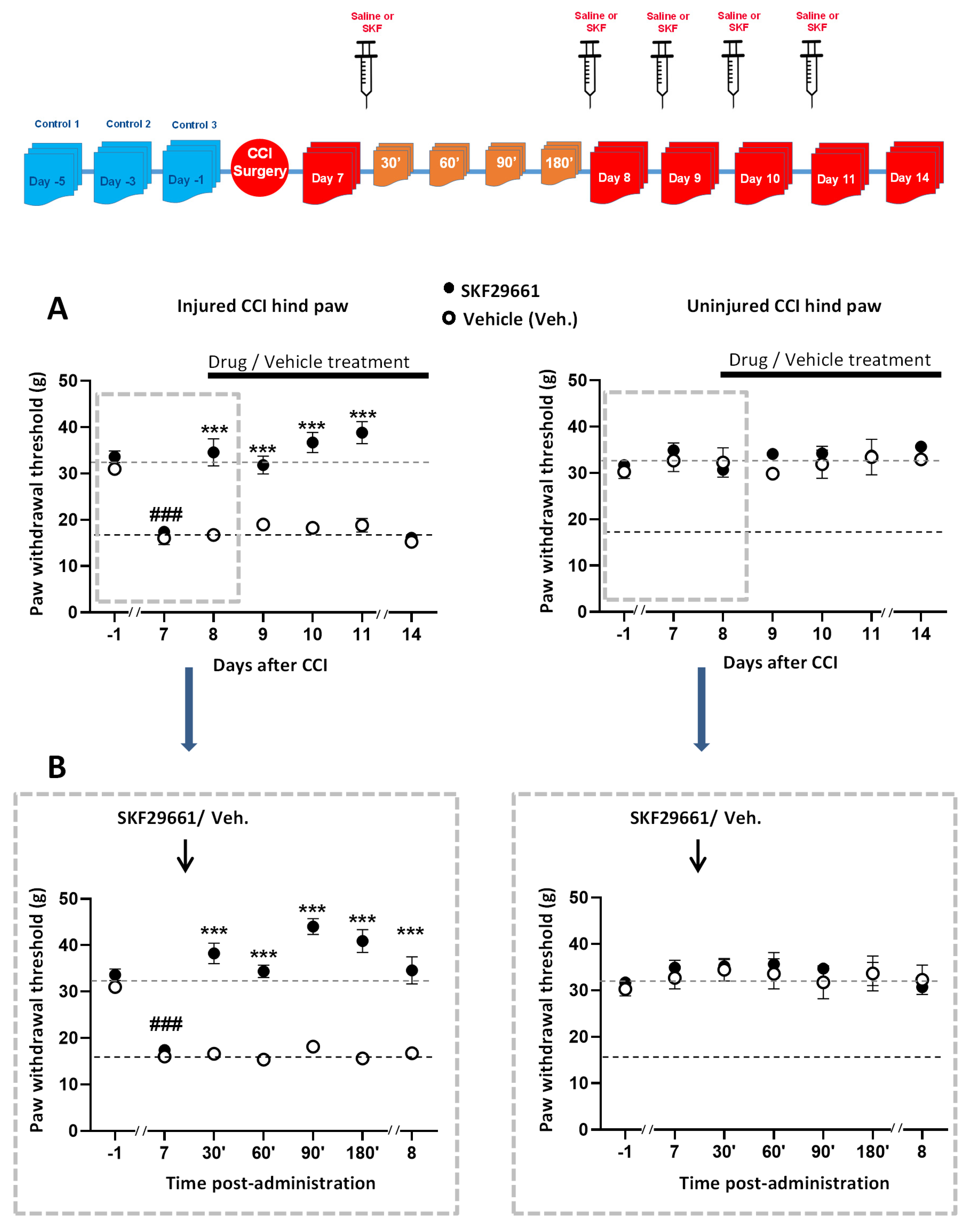
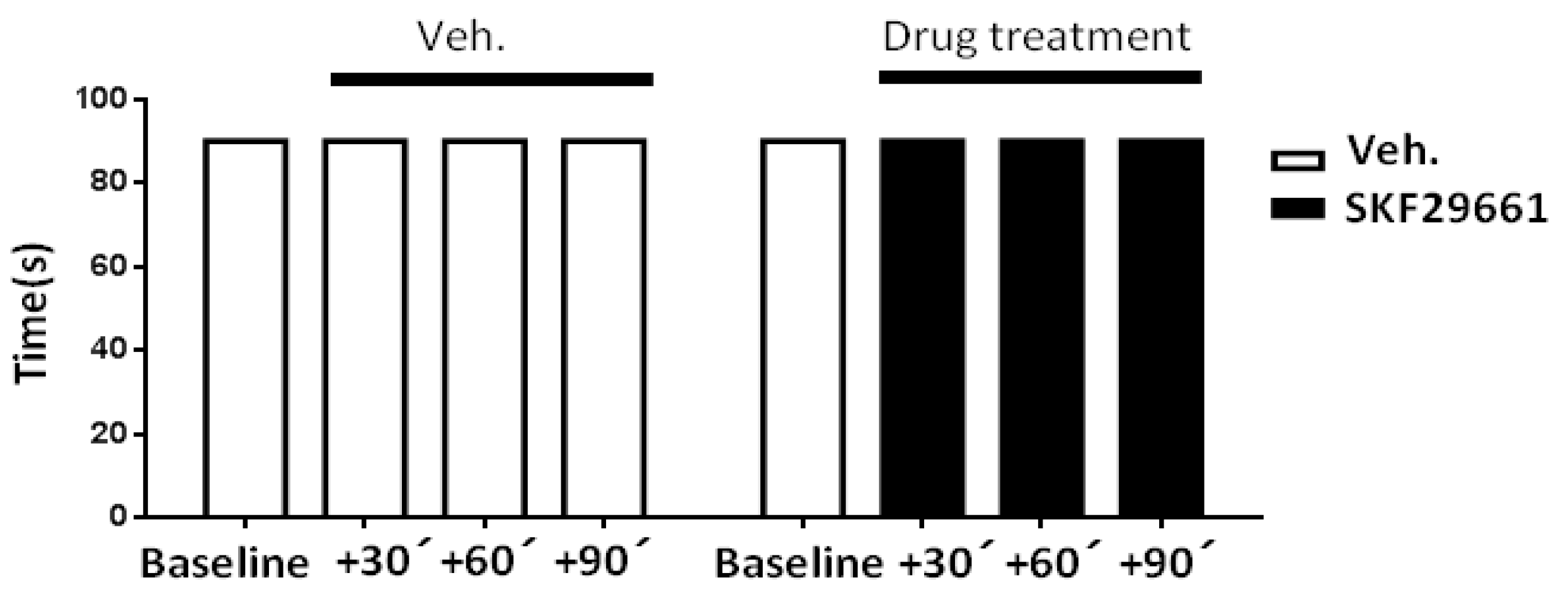
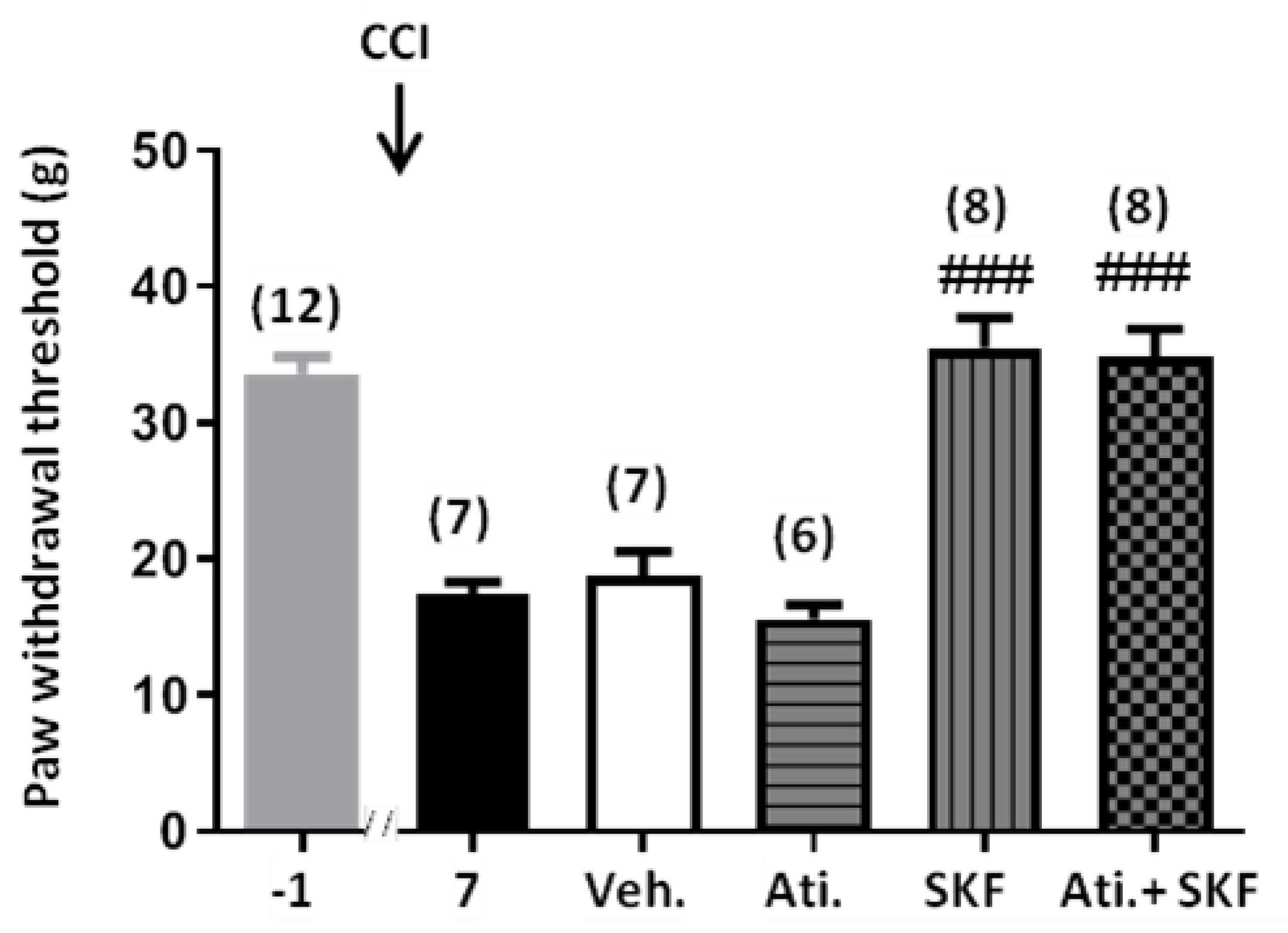
2.1. Effect of SKF29661 on Mechanical Allodynia
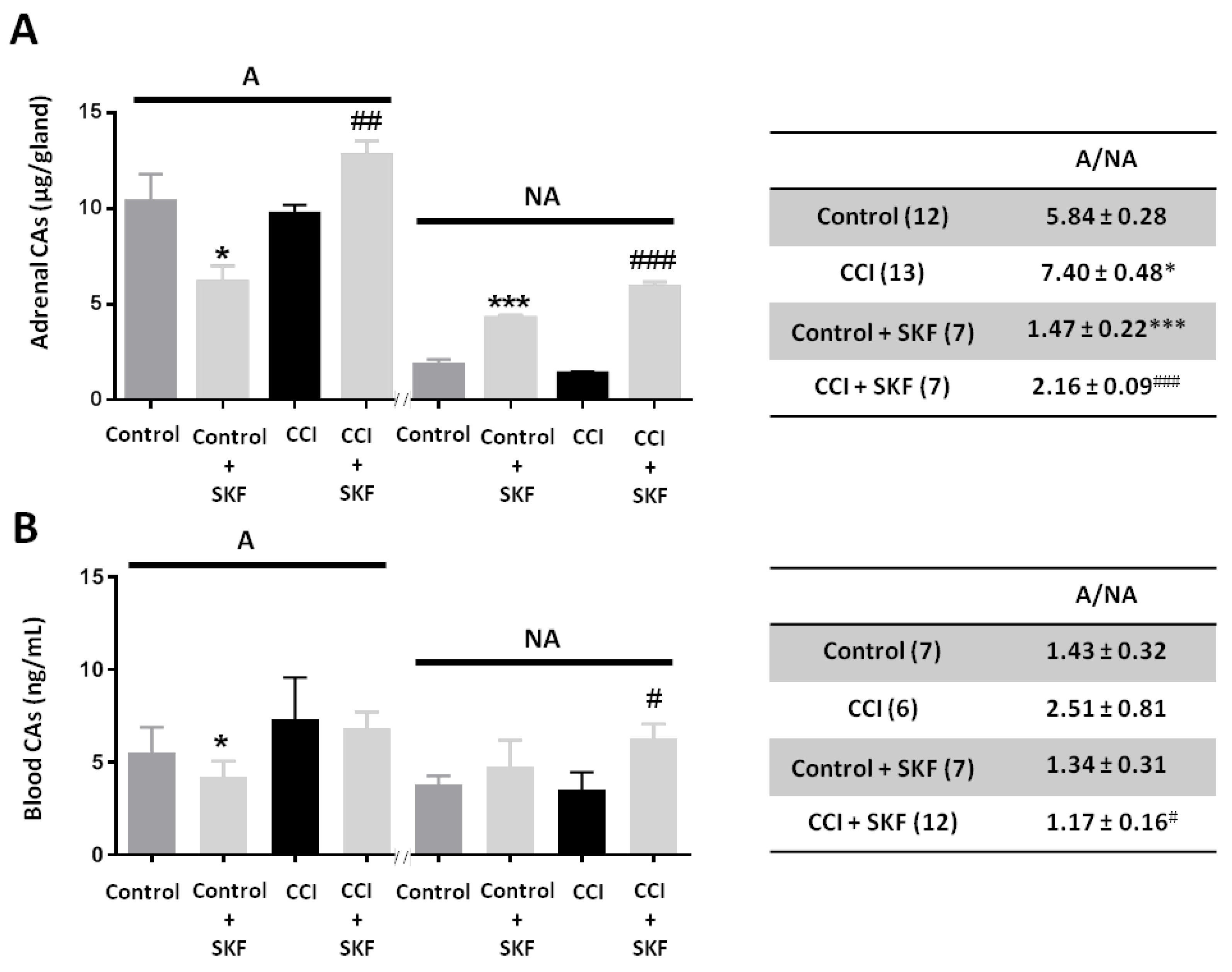
2.2. Effect of SKF29661 on Adrenal and Blood Catecholamines
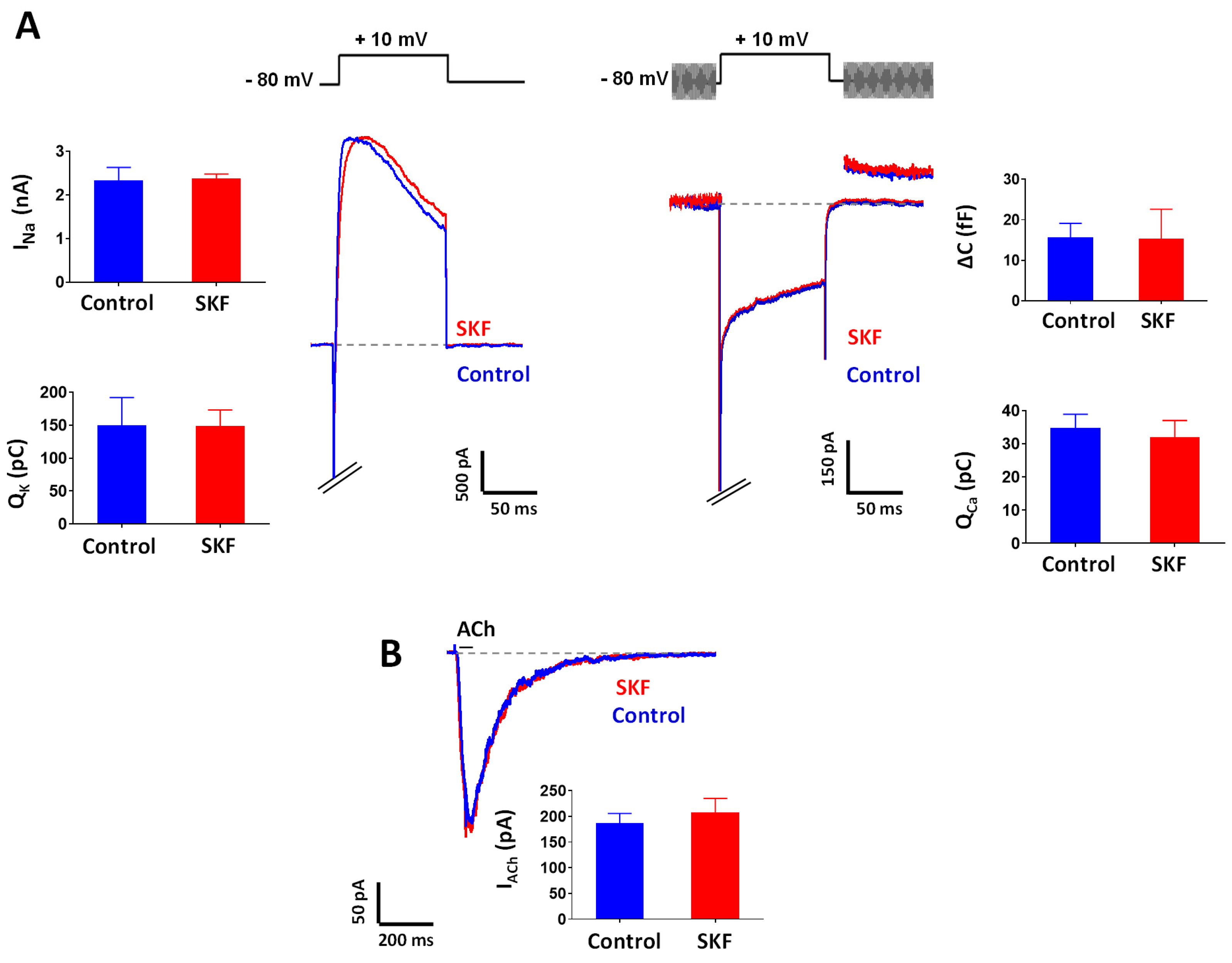
2.3. Effect of SKF29661 on Ionic Conductances and Exocytosis from Chromaffin Cells
2.4. Effect of Adrenal Gland Denervation on Synaptic Activity of Chromaffin Cells and Mechanical Allodynia in CCI Animals
3. Discussion
4. Materials and Methods
4.1. Chronic Constriction Injury of The Sciatic Nerve
4.2. Adrenal Gland Denervation
4.3. Behavioural Testing
4.3.1. Mechanical Allodynia
4.3.2. Motor Coordination
4.4. Adrenal Gland Preparation
4.5. Electrophysiological Recordings
4.6. Determination of Catecholamines
4.6.1. Adrenal Gland Samples
4.6.2. Blood Samples
4.7. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Adrenaline |
| A/NA | A to NA ratio |
| ACh | Acetylcholine |
| ANOVA | Analysis of variance |
| CA | Catecholamine |
| Cav | Voltage-gated calcium channels |
| CCI | Chronic constriction injury |
| ΔC | Capacitance increment |
| DCMB | 2,3-dichloro-α-methylbenzylamine |
| I.P. | Intraperitoneal |
| Kv | Voltage-gated potassium channels |
| LC-MS | Liquid Chromatograph Mass Spectrometer |
| nAChR | Nicotinic acetylcholine receptor |
| NA | Noradrenaline |
| Nav | Voltage-gated sodium channels |
| PNMT | Phenylethanolamine N-methyltransferase |
| PWT | Paw withdrawal threshold |
| sEPSC | Spontaneous excitatory synaptic currents |
| SKF29661 | 1,2,3,4-tetrahydroisoquinoline-7-sulfonamide |
| SNS | Sympathetic nervous system |
| TH | Tyrosine hydroxylase |
| Vh | Holding potential |
References
- Selye, M.H. The general adaptation syndrome and the diseases of adaptation1. J. Clin. Endocrinol. Metab. 1946, 6, 117–230. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Borges, R.; Eiden, L.E.; García, A.G.; Hernández-Cruz, A. Chromaffin Cells of the Adrenal Medulla: Physiology, Pharmacology, and Disease. Compr. Physiol. 2019, 9, 1443–1502. [Google Scholar] [CrossRef] [PubMed]
- Estévez-Herrera, J.; González-Santana, A.; Baz-Dávila, R.; Machado, J.D.; Borges, R. The intravesicular cocktail and its role in the regulation of exocytosis. J. Neurochem. 2016, 137, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Artalejo, A.R. Electrical Properties of Adrenal Chromaffin Cells. In The Electrophysiology of Neuroendocrine Cells; Herscheler, J., Scherubl, H., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 259–299. [Google Scholar]
- Colomer, C.; Ore, L.A.O.; Coutry, N.; Mathieu, M.-N.; Arthaud, S.; Fontanaud, P.; Iankova, I.; Macari, F.; Thouënnon, E.; Yon, L.; et al. Functional Remodeling of Gap Junction-Mediated Electrical Communication between Adrenal Chromaffin Cells in Stressed Rats. J. Neurosci. 2008, 28, 6616–6626. [Google Scholar] [CrossRef]
- Colomer, C.; Olivos-Oré, L.A.; Vincent, A.; McIntosh, J.M.; Artalejo, A.; Guérineau, N.C. Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: Implication in stress-induced functional plasticity. J. Neurosci. 2010, 30, 6732–6742. [Google Scholar] [CrossRef]
- Bernier, L.-P.; Ase, A.R.; Séguéla, P. P2X receptor channels in chronic pain pathways. Br. J. Pharmacol. 2017, 175, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Blázquez, M.; Olivos-Oré, L.A.; Barahona, M.V.; De La Muela, M.S.; Solar, V.; Jiménez, E.; Gualix, J.; McIntosh, J.M.; Ferrer-Montiel, A.; Miras-Portugal, M.-T.; et al. Overexpression of P2X3 and P2X7 Receptors and TRPV1 Channels in Adrenomedullary Chromaffin Cells in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 155. [Google Scholar] [CrossRef]
- Rhudy, J.L.; Meagher, M.W. Fear and anxiety: Divergent effects on human pain thresholds. Pain 2000, 84, 65–75. [Google Scholar] [CrossRef]
- Olango, W.M.; Finn, D.P. Neurobiology of Stress-Induced Hyperalgesia. Behav. Neurobiol. PTSD 2014, 20, 251–280. [Google Scholar] [CrossRef]
- Yamamotová, A. Endogenous antinociceptive system and potential ways to influence it. Physiol. Res. 2019, 68, S195–S205. [Google Scholar] [CrossRef]
- Imbe, H. Stress-induced hyperalgesia: Animal models and putative mechanisms. Front. Biosci. 2006, 11, 2179–2192. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.M.; Okine, B.N.; Roche, M.; Finn, D.P. Stress-induced hyperalgesia. Prog. Neurobiol. 2014, 121, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jänig, W. Pain and the Sympathetic Nervous System. In Encyclopedia of Neuroscience; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 371–383. [Google Scholar]
- Bomholt, S.F.; Harbuz, M.S.; Blackburn-Munro, G.; Blackburn-Munro, R.E. Involvement and Role of the Hypothalamo-pituitary-adrenal (HPA) Stress Axis in Animal Models of Chronic Pain and Inflammation. Stress 2004, 7, 1–14. [Google Scholar] [CrossRef]
- Knudsen, L.F.; Terkelsen, A.J.; Drummond, P.D.; Birklein, F. Complex regional pain syndrome: A focus on the autonomic nervous system. Clin. Auton. Res. 2019, 29, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Khasar, S.G.; McCarter, G.; Levine, J.D. Epinephrine Produces a β-Adrenergic Receptor-Mediated Mechanical Hyperalgesia and In Vitro Sensitization of Rat Nociceptors. J. Neurophysiol. 1999, 81, 1104–1112. [Google Scholar] [CrossRef]
- Jänig, W.; Häbler, H.-J. Sympathetic Nervous System: Contribution to Chronic Pain; Elsevier BV: Amsterdam, The Netherlands, 2000; Volume 129, pp. 451–468. [Google Scholar]
- Häbler, H.-J.; Jänig, W.; Koltzenburg, M. Activation of unmyelinated afferents in chronically lesioned nerves by adrenaline and excitation of sympathetic efferents in the cat. Neurosci. Lett. 1987, 82, 35–40. [Google Scholar] [CrossRef]
- Khasar, S.G.; Miao, F.J.-P.; Jänig, W.; Levine, J.D. Modulation of bradykinin-induced mechanical hyperalgesia in the rat by activity in abdominal vagal afferents. Eur. J. Neurosci. 1998, 10, 435–444. [Google Scholar] [CrossRef]
- Schlereth, T.; Drummond, P.D.; Birklein, F. Inflammation in CRPS: Role of the sympathetic supply. Auton. Neurosci. 2014, 182, 102–107. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 1–13. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Strong, J.A.; Zhang, J.-M.; Schaible, H.G. The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Khasar, S.G.; Dina, O.A.; Green, P.G.; Levine, J.D. Sound Stress–Induced Long-Term Enhancement of Mechanical Hyperalgesia in Rats Is Maintained by Sympathoadrenal Catecholamines. J. Pain 2009, 10, 1073–1077. [Google Scholar] [CrossRef]
- Khasar, S.G.; Miao, F.J.-P.; Jänig, W.; Levine, J.D. Vagotomy-Induced Enhancement of Mechanical Hyperalgesia in the Rat Is Sympathoadrenal-Mediated. J. Neurosci. 1998, 18, 3043–3049. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, R.G.; Gessner, G.; Weiner, G.; Jenkins, B.; Sawyer, J.; Bondinell, W.; Intoccia, A. Studies on SK&F 29661, an organ-specific inhibitor of phenylethanolamine N-methyltransferase. J. Pharmacol. Exp. Ther. 1979, 208, 24–30. [Google Scholar] [PubMed]
- Pendleton, R.G.; Gessner, G.; Sawyer, J.; Hillegass, L.; Miller, D.A. Studies on the long term effects of SK&F 29661 upon adrenal catecholamines. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1982, 319, 22–28. [Google Scholar] [CrossRef]
- Goldstein, M.; Saito, M.; Lew, J.Y.; Hieble, J.P.; Pendleton, R.G. The blockade of α2-adrenoceptors by the PNMT inhibitor SK&F 64139. Eur. J. Pharmacol. 1980, 67, 305–308. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef]
- Pendleton, R.G.; Kaiser, C.; Gessner, G. Studies on adrenal phenylethanolamine N-methyltransferase (PNMT) with SK&F 64139, a selective inhibitor. J. Pharmacol. Exp. Ther. 1976, 197, 623–632. [Google Scholar]
- Coupland, R.E. Electron microscopic observations on the structure of the rat adrenal medulla. I. The ultrastructure and organization of chromaffin cells in the normal adrenal medulla). J. Anat. 1965, 99, 231–254. [Google Scholar]
- Cooper, B.R.; Wightman, R.M.; Jorgenson, J.W. Quantitation of epinephrine and norepinephrine secretion from individual adrenal medullary cells by microcolumn high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1994, 653, 25–34. [Google Scholar] [CrossRef]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Adrenal Responses to Stress. Cell. Mol. Neurobiol. 2010, 30, 1433–1440. [Google Scholar] [CrossRef]
- Vollmer, R.R.; Baruchin, A.; Kolibal-Pegher, S.S.; Corey, S.P.; Stricker, E.M.; Kaplan, B.B. Selective activation of norepinephrine- and epinephrine-secreting chromaffin cells in rat adrenal medulla. Am. J. Physiol. Integr. Comp. Physiol. 1992, 263, R716–R721. [Google Scholar] [CrossRef]
- Morrison, S.F.; Cao, W.-H. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am. J. Physiol. Integr. Comp. Physiol. 2000, 279, R1763–R1775. [Google Scholar] [CrossRef]
- Kvetňanský, R.; Pacák, K.; Sabban, E.; Kopin, I.; Goldstein, D. Stressor specificity of peripheral catecholaminergic activation. Adv. Pharmacol. 1998, 42, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.C.; Claycomb, R.; Siddall, B.J.; Bell, R.A.; Kvetnansky, R.; Wong, D.L. Stress-induced changes in epinephrine expression in the adrenal medulla in vivo. J. Neurochem. 2007, 101, 1108–1118. [Google Scholar] [CrossRef]
- Kvetnanský, R.; Gewirtz, G.; Weise, V.; Kopin, I. Catecholamine-synthesizing enzymes in the rat adrenal gland during exposure to cold. Am. J. Physiol. Content 1971, 220, 928–931. [Google Scholar] [CrossRef]
- Sabban, E.L.; Kvetňanský, R. Stress-triggered activation of gene expression in catecholaminergic systems: Dynamics of transcriptional events. Trends Neurosci. 2001, 24, 91–98. [Google Scholar] [CrossRef]
- Wong, D.L. Epinephrine Biosynthesis: Hormonal and Neural Control During Stress. Cell. Mol. Neurobiol. 2006, 26, 889–898. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic Systems in Stress: Structural and Molecular Genetic Approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef]
- Duncan, R.R.; Greaves, J.; Wiegand, U.K.; Matskevich, I.; Bodammer, G.; Apps, D.K.; Shipston, M.J.; Chow, R.H. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nat. Cell Biol. 2003, 422, 176–180. [Google Scholar] [CrossRef]
- Estévez-Herrera, J.; Domínguez, N.; Pardo, M.R.; González-Santana, A.; Westhead, E.W.; Borges, R.; Machado, J.D. ATP: The crucial component of secretory vesicles. Proc. Natl. Acad. Sci. USA 2016, 113, E4098–E4106. [Google Scholar] [CrossRef]
- Araki, T.; Ito, K.; Kurosawa, M.; Sato, A. Responses of adrenal sympathetic nerve activity and catecholamine secretion to cutaneous stimulation in anesthetized rats. Neuroscience 1984, 12, 289–299. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z.; Xu, X.J.; Langel, U.; Bedecs, K.; Hokfelt, T.; Bartfai, T. Galanin-mediated control of pain: Enhanced role after nerve injury. Proc. Natl. Acad. Sci. USA 1992, 89, 3334–3337. [Google Scholar] [CrossRef]
- Sagen, J. Chromaffin Cell Transplants for Alleviation of Chronic Pain. ASAIO J. 1992, 38, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.J.; Martinez, M.; Karmally, S.; Lopez, T.; Sagen, J. Initial Characterization of the Transplant of Immortalized Chromaffin Cells for the Attenuation of Chronic Neuropathic Pain. Cell Transplant. 2000, 9, 637–656. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Roh, D.-H.; Kim, H.-W.; Han, H.-J.; Beitz, A.J.; Lee, J.-H. Blockade of Adrenal Medulla-Derived Epinephrine Potentiates Bee Venom-Induced Antinociception in the Mouse Formalin Test: Involvement of Peripheralβ-Adrenoceptors. Evid.-Based Complement. Altern. Med. 2013, 2013, 809062. [Google Scholar] [CrossRef]
- Kang, S.; Roh, D.; Kim, H.-W.; Han, H.J.; Beitz, A.J.; Lee, J.-H. Suppression of adrenal gland-derived epinephrine enhances the corticosterone-induced antinociceptive effect in the mouse formalin test. Eur. J. Pain 2013, 18, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Ulate, G.; Scott, S.R.; González, J.; Gilabert, J.A.; Artalejo, A. Extracellular ATP regulates exocytosis by inhibiting multiple Ca2+ channel types in bovine chromaffin cells. Pflügers Archiv. Eur. J. Physiol. 1999, 439, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.J.-P.; Jänig, W.; Levine, J.D. Nociceptive neuroendocrine negative feedback control of neurogenic inflammation activated by capsaicin in the rat paw: Role of the adrenal medulla. J. Physiol. 2000, 527, 601–610. [Google Scholar] [CrossRef]
- Guneli, E.; Karabayyavasoglu, N.; Apaydin, S.; Uyar, M. Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol. Biochem. Behav. 2007, 88, 9–17. [Google Scholar] [CrossRef]
- Carabelli, V.; Giancippoli, A.; Baldelli, P.; Carbone, E.; Artalejo, A. Distinct Potentiation of L-Type Currents and Secretion by cAMP in Rat Chromaffin Cells. Biophys. J. 2003, 85, 1326–1337. [Google Scholar] [CrossRef]
- Wojnicz, A.; Avendaño-Ortiz, J.; De Pascual, R.; Ruiz-Pascual, L.; García, A.G.; Ruiz-Nuño, A. Simultaneous monitoring of monoamines, amino acids, nucleotides and neuropeptides by liquid chromatography-tandem mass spectrometry and its application to neurosecretion in bovine chromaffin cells. J. Mass Spectrom. 2016, 51, 651–664. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arribas-Blázquez, M.; Olivos-Oré, L.A.; Barahona, M.V.; Wojnicz, A.; De Pascual, R.; Sánchez de la Muela, M.; García, A.G.; Artalejo, A.R. The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 8325. https://doi.org/10.3390/ijms21218325
Arribas-Blázquez M, Olivos-Oré LA, Barahona MV, Wojnicz A, De Pascual R, Sánchez de la Muela M, García AG, Artalejo AR. The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain. International Journal of Molecular Sciences. 2020; 21(21):8325. https://doi.org/10.3390/ijms21218325
Chicago/Turabian StyleArribas-Blázquez, Marina, Luis Alcides Olivos-Oré, María Victoria Barahona, Aneta Wojnicz, Ricardo De Pascual, Mercedes Sánchez de la Muela, Antonio G. García, and Antonio R. Artalejo. 2020. "The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain" International Journal of Molecular Sciences 21, no. 21: 8325. https://doi.org/10.3390/ijms21218325
APA StyleArribas-Blázquez, M., Olivos-Oré, L. A., Barahona, M. V., Wojnicz, A., De Pascual, R., Sánchez de la Muela, M., García, A. G., & Artalejo, A. R. (2020). The Adrenal Medulla Modulates Mechanical Allodynia in a Rat Model of Neuropathic Pain. International Journal of Molecular Sciences, 21(21), 8325. https://doi.org/10.3390/ijms21218325





