Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARS-CoV-2 Infection
Abstract
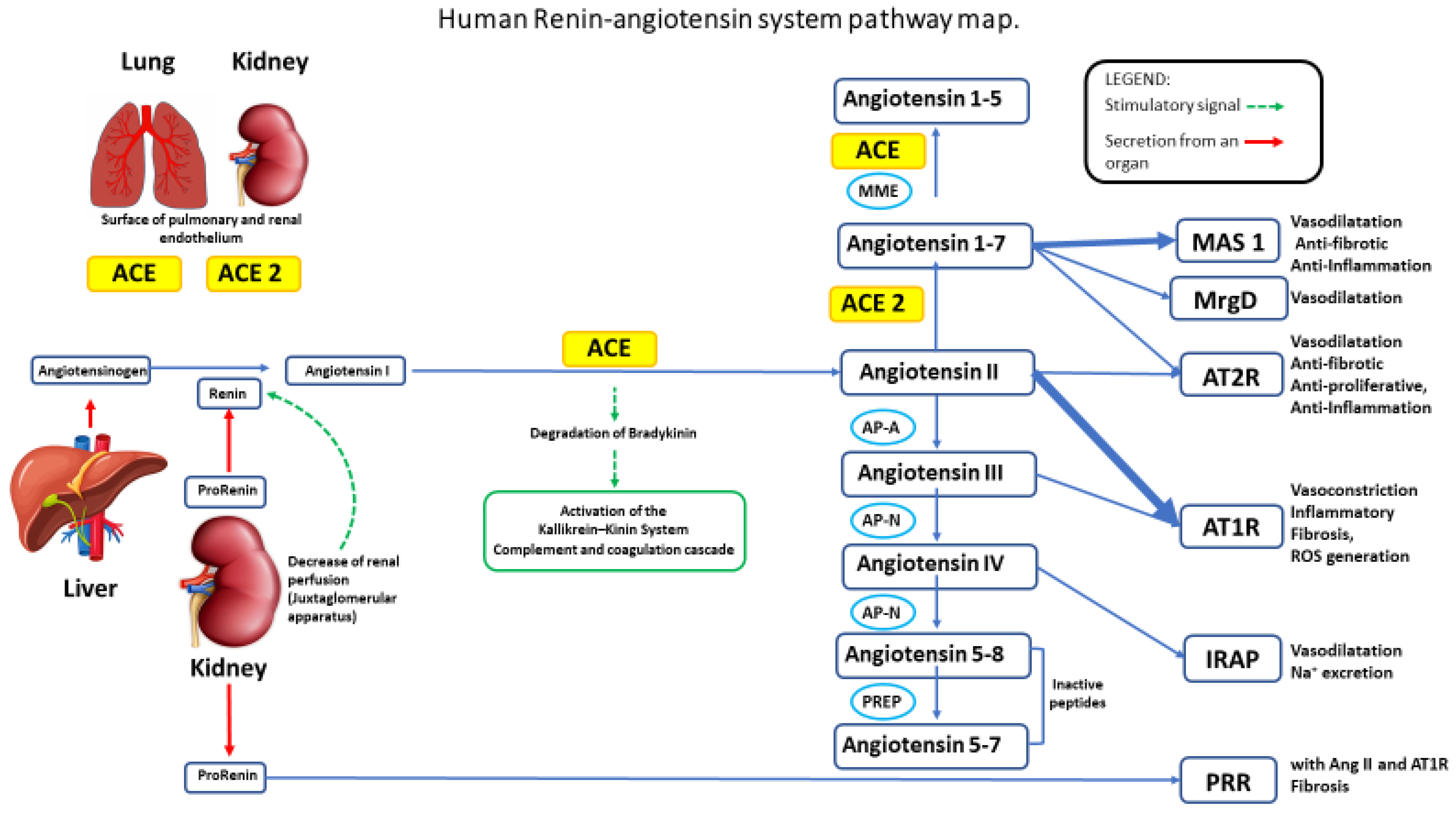
:1. Introduction
2. Hypertension
3. Cardiovascular Active Peptides Endowed with ACE-Mediated Anti-Hypertension Activities
4. ACE2 in Cardiac Cell Homeostasis, Cardiac Remodeling, and Hypertension
ACE2 in Cardiac Remodelling and Hypertension
5. Marine Cardiovascular Active Peptides Targeting the ACE System (ACE 1/ACE 2)
6. ACE2 Inhibitors against SARS-CoV-2
7. Molecular Docking with Human ACE2 and Interferences with SARS-CoV-2 Spike Protein
8. Conclusion and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGT | Angiotensinogen |
| ACE | Angiotensin I converting enzyme |
| ACE2 | Angiotensin I converting enzyme 2 |
| AngA | Angiotensin A |
| AP-A | Glutamyl aminopeptidase |
| AP-N | Alanyl aminopeptidase (membrane) |
| AT1R | Angiotensin II receptor type 1 |
| AT2R | Angiotensin II receptor type 2 |
| CMA1 | Chymase 1 |
| CPA3 | Carboxypeptidase A3 |
| CTSA | Cathepsin A |
| CTSG | Cathepsin G |
| IRAP | Leucyl and cystinyl aminopeptidase |
| MAS | MAS1 proto-oncogene, G protein-coupled receptor |
| MME | Membrane metallo-endopeptidase |
| MRGPRD | MAS related GPR family member D |
| NLN | Neurolysin |
| PRCP | Prolylcarboxypeptidase |
| PREP | Prolyl endopeptidase |
| PRR | ATPase H+ transporting accessory protein 2 |
| RENRBD | ReninReceptor Binding Domain |
| THOP1 | Thimet oligopeptidase |
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautie, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, P.T.; Mackie, A.M. Drugs from the sea-fact or fantasy? Nature 1977, 267, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U. Marine-Derived Pharmaceuticals–Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Tortorella, E.; Tedesco, P.; Palma Esposito, F.; January, G.G.; Fani, R.; Jaspars, M.; de Pascale, D. Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives. Mar. Drugs 2018, 16, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Y.; Lin, S.C.; Feng, C.W.; Chen, P.C.; Su, Y.D.; Li, C.M.; Yang, S.N.; Jean, Y.H.; Sung, P.J.; Duh, C.Y.; et al. Anti-Inflammatory and Analgesic Effects of the Marine-Derived Compound Excavatolide B Isolated from the Culture-Type Formosan Gorgonian Briareum excavatum. Mar. Drugs 2015, 13, 2559–2579. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Giles, T.D.; Berk, B.C.; Black, H.R.; Cohn, J.N.; Kostis, J.B.; Izzo, J.L., Jr.; Weber, M.A. Expanding the definition and classification of hypertension. J. Clin. Hypertens. 2005, 7, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Mourad, J.J.; Levy, B.I. Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat. Rev. Cardiol. 2020, 17, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, M.A.; Oparil, S.; Calhoun, D.A. Drugs targeting the renin-angiotensin-aldosterone system. Nat. Rev. Drug Discov. 2002, 1, 621–636. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Jia, A.; Zhang, Y.; Zhu, H.; Zhang, C.; Sun, Z.; Liu, C. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish Rhopilema esculentum. Food Res. Int. 2013, 50, 339–343. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Caci, G.; Albini, A.; Malerba, M.; Noonan, D.M.; Pochetti, P.; Polosa, R. COVID-19 and Obesity: Dangerous Liaisons. J. Clin. Med. 2020, 9, 2511. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Hypertension, inflammation and atrial fibrillation. J. Hypertens 2014, 32, 480–483. [Google Scholar] [CrossRef]
- Chockalingam, A.; Campbell, N.R.; Fodor, J.G. Worldwide epidemic of hypertension. Can. J. Cardiol. 2006, 22, 553–555. [Google Scholar] [CrossRef] [Green Version]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef]
- Grobe, J.L.; Mecca, A.P.; Lingis, M.; Shenoy, V.; Bolton, T.A.; Machado, J.M.; Speth, R.C.; Raizada, M.K.; Katovich, M.J. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H736–H742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, R.; Kaur, M.; Singh, J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: Molecular insights and therapeutic strategies. Cardiovasc. Diabetol. 2018, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Baci, D.; Bosi, A.; Parisi, L.; Buono, G.; Mortara, L.; Ambrosio, G.; Bruno, A. Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 21, 7165. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Matyas, C.; Hasko, G.; Liaudet, L.; Trojnar, E.; Pacher, P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Drapala, A.; Sikora, M.; Ufnal, M. Statins, the renin-angiotensin-aldosterone system and hypertension— A tale of another beneficial effect of statins. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Cardin, S.; Li, D.; Thorin-Trescases, N.; Leung, T.K.; Thorin, E.; Nattel, S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: Angiotensin-dependent and -independent pathways. Cardiovasc. Res. 2003, 60, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daliri, E.B.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. J. Sci. Food Agric. 2017, 97, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Pingitore, E.V.; Mozzi, F.; Saavedra, L.; Villegas, J.M.; Hebert, E.M. Lactic Acid Bacteria as Cell Factories for the Generation of Bioactive Peptides. Protein Pept. Lett. 2017, 24, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides 2009, 30, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Li, L.; Liu, G.; Hu, S.Q. Inhibition mechanism and model of an angiotensin I-converting enzyme (ACE)-inhibitory hexapeptide from yeast (Saccharomyces cerevisiae). PLoS ONE 2012, 7, e37077. [Google Scholar] [CrossRef] [Green Version]
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [Green Version]
- Barnett, A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006, 60, 1454–1470. [Google Scholar] [CrossRef]
- Cheon, S.; Dean, M.; Chahrour, M. The ubiquitin proteasome pathway in neuropsychiatric disorders. Neurobiol. Learn. Mem. 2019, 165, 106791. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzon, A.G.; Martinez-Augustin, O.; Botto, C.C.; Drago, S.R. Antithrombotic Activity of Brewers′ Spent Grain Peptides and their Effects on Blood Coagulation Pathways. Plant. Foods Hum. Nutr. 2018, 73, 241–246. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Kim, S.K. Bioactive Peptide of Marine Origin for the Prevention and Treatment of Non-Communicable Diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Shuib, A.S.; Abdullah, N. Structural Characteristics and Antihypertensive Effects of Angiotensin-I-Converting Enzyme Inhibitory Peptides in the Renin-Angiotensin and Kallikrein Kinin Systems. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.L. Marine Organisms as Potential Sources of Bioactive Peptides that Inhibit the Activity of Angiotensin I-Converting Enzyme: A Review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Dong, Z.; Zhen, Y.; Di, B.; Shi, Y.; Fearns, P.; Shi, P. Recurrence of the world′s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar. Pollut. Bull. 2010, 60, 1423–1432. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O′Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef]
- He, H.L.; Wu, H.; Chen, X.L.; Shi, M.; Zhang, X.Y.; Sun, C.Y.; Zhang, Y.Z.; Zhou, B.C. Pilot and plant scaled production of ACE inhibitory hydrolysates from Acetes chinensis and its in vivo antihypertensive effect. Bioresour. Technol. 2008, 99, 5956–5959. [Google Scholar] [CrossRef] [PubMed]
- Nii, Y.; Fukuta, K.; Yoshimoto, R.; Sakai, K.; Ogawa, T. Determination of antihypertensive peptides from an izumi shrimp hydrolysate. Biosci. Biotechnol. Biochem. 2008, 72, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada fucata martensii) Meat Protein Hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Fang, M.; Wu, H.; Xie, J.; Wu, Y.; Li, P.; Zhang, D.; Huang, Z.; Xia, Y.; Zhou, L.; et al. A novel angiotensin I-converting enzyme inhibitory peptide from Phascolosoma esculenta water-soluble protein hydrolysate. J. Funct. Foods 2013, 5, 475–483. [Google Scholar] [CrossRef]
- Yokoyama, K.; Chiba, H.; Yoshikawa, M. Peptide inhibitors for angiotensin I-converting enzyme from thermolysin digest of dried bonito. Biosci. Biotechnol. Biochem. 1992, 56, 1541–1545. [Google Scholar] [CrossRef]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Huentelman, M.J.; Zubcevic, J.; Hernandez Prada, J.A.; Xiao, X.; Dimitrov, D.S.; Raizada, M.K.; Ostrov, D.A. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension 2004, 44, 903–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hum. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Matsufuji, H.; Matsui, T.; Ohshige, S.; Kawasaki, T.; Osajima, K.; Osajima, Y. Antihypertensive effects of angiotensin fragments in SHR. Biosci. Biotechnol. Biochem. 1995, 59, 1398–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohama, Y.; Matsumoto, S.; Oka, H.; Teramoto, T.; Okabe, M.; Mimura, T. IkeIsolation of angiotensin-converting enzyme inhibitor from tuna muscle. Biochem. Biophys. Res. Commun. 1988, 155, 332–337. [Google Scholar] [CrossRef]
- Qian, Z.J.; Je, J.Y.; Kim, S.K. Antihypertensive effect of angiotensin i converting enzyme-inhibitory peptide from hydrolysates of Bigeye tuna dark muscle, Thunnus obesus. J. Agric. Food Chem. 2007, 55, 8398–8403. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Antihypertensive effect of novel angiotensin I converting enzyme inhibitory peptide from chum salmon (Oncorhynchus keta) skin in spontaneously hypertensive rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Ma, Q.; Zhuang, Y. Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen. Mar. Drugs 2016, 14, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aissaoui, N.; Abidi, F.; Hardouin, J.; Abdelkafi, Z.; Marrakchi, N.; Jouenne, T.; Marzouki, M.N. ACE Inhibitory and Antioxidant Activities of Novel Peptides from Scorpaena notata By-product Protein Hydrolysate. Int. J. Pept. Res. Ther. 2017, 23, 13–23. [Google Scholar] [CrossRef]
- Ferrario, C.M. Cardiac remodelling and RAS inhibition. Ther. Adv. Cardiovasc. Dis. 2016, 10, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salampessy, J.; Reddy, N.; Kailasapathy, K.; Phillips, M. Functional and potential therapeutic ACE-inhibitory peptides derived from bromelain hydrolysis of trevally proteins. J. Funct. Foods 2015, 14, 716–725. [Google Scholar] [CrossRef]
- Sun, L.; Wu, S.; Zhou, L.; Wang, F.; Lan, X.; Sun, J.; Tong, Z.; Liao, D. Separation and Characterization of Angiotensin I Converting Enzyme (ACE) Inhibitory Peptides from Saurida elongata Proteins Hydrolysate by IMAC-Ni(2). Mar. Drugs 2017, 15, 29. [Google Scholar] [CrossRef] [Green Version]
- Rella, M.; Rushworth, C.A.; Guy, J.L.; Turner, A.J.; Langer, T.; Jackson, R.M. Structure-based pharmacophore design and virtual screening for novel angiotensin converting enzyme 2 inhibitors. J. Chem. Inf. Model. 2006, 46, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Ryu, B.; Kim, S.K. Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem. 2014, 143, 246–255. [Google Scholar] [CrossRef]
- Nomura, A.; Noda, N.; Maruyama, S. Purification of angiotensin I-converting enzyme inhibitors in pelagic thresher Alopias pelagicus muscle hydrolysate and viscera extracts. Fish. Sci. 2002, 68, 954–956. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Perez-Martin, R.I. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wang, G.; Sun, S.; Liu, R.; Hong, B.; Gao, R.; Bai, K. Processing Optimization and Characterization of Angiotensin-Iota-Converting Enzyme Inhibitory Peptides from Lizardfish (Synodus macrops) Scale Gelatin. Mar. Drugs 2018, 16, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ryu, B.; Zhang, Y.; Liang, P.; Li, C.; Zhou, C.; Yang, P.; Hong, P.; Qian, Z.J. Comparison of an angiotensin-I-converting enzyme inhibitory peptide from tilapia (Oreochromis niloticus) with captopril: Inhibition kinetics, in vivo effect, simulated gastrointestinal digestion and a molecular docking study. J. Sci. Food Agric. 2020, 100, 315–324. [Google Scholar] [CrossRef]
- Oscar, M.A.; Barak, S.; Winters, G. The Tropical Invasive Seagrass, Halophila stipulacea, Has a Superior Ability to Tolerate Dynamic Changes in Salinity Levels Compared to Its Freshwater Relative, Vallisneria americana. Front. Plant. Sci. 2018, 9, 950. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, C.; Rudemiller, N.P.; Abais, J.M.; Mattson, D.L. Inflammation and hypertension: New understandings and potential therapeutic targets. Curr. Hypertens. Rep. 2015, 17, 507. [Google Scholar] [CrossRef] [Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. J. Am. Med. Assoc. 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Clark, A.L. Catabolism in chronic heart failure. Eur. Heart J. 2000, 21, 521–532. [Google Scholar] [CrossRef]
- Nguyen, Q.; Dominguez, J.; Nguyen, L.; Gullapalli, N. Hypertension management: An update. Am. Health Drug Benefits 2010, 3, 47–56. [Google Scholar] [PubMed]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Hernandez-Ledesma, B.; del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Martinez-Maqueda, D.; Miralles, B.; Recio, I.; Hernandez-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef]
- Nakamura, A.; Osonoi, T.; Terauchi, Y. Relationship between urinary sodium excretion and pioglitazone-induced edema. J. Diabetes Investig. 2010, 1, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Geng, M.; Liu, C.; Wang, J.; Min, W.; Liu, J. Structural and molecular basis of angiotensin-converting enzyme by computational modeling: Insights into the mechanisms of different inhibitors. PLoS ONE 2019, 14, e0215609. [Google Scholar] [CrossRef] [PubMed]
- Chamsi-Pasha, M.A.; Shao, Z.; Tang, W.H. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Curr. Heart Fail. Rep. 2014, 11, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef] [Green Version]
- Albini, A.; Di Guardo, G.; Noonan, D.M.; Lombardo, M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020, 15, 759–766. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, Y.; Gallagher, P.E.; Averill, D.B.; Tallant, E.A.; Brosnihan, K.B.; Ferrario, C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004, 43, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Ocaranza, M.P.; Godoy, I.; Jalil, J.E.; Varas, M.; Collantes, P.; Pinto, M.; Roman, M.; Ramirez, C.; Copaja, M.; Diaz-Araya, G.; et al. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 2006, 48, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Iwata, M.; Silva Enciso, J.E.; Greenberg, B.H. Selective and specific regulation of ectodomain shedding of angiotensin-converting enzyme 2 by tumor necrosis factor alpha-converting enzyme. Am. J. Physiol. Cell Physiol. 2009, 297, C1318–C1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; MacDonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005, 26, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.A.; Yang, D.; Kida, K.; Molotkova, N.; Yeo, S.J.; Varki, N.; Iwata, M.; Dalton, N.D.; Peterson, K.L.; Siems, W.E.; et al. Effects of ACE2 inhibition in the post-myocardial infarction heart. J. Card Fail. 2010, 16, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef]
- Evans, S.; Weinheimer, C.J.; Kovacs, A.; Williams, J.W.; Randolph, G.J.; Jiang, W.; Barger, P.M.; Mann, D.L. Ischemia reperfusion injury provokes adverse left ventricular remodeling in dysferlin-deficient hearts through a pathway that involves TIRAP dependent signaling. Sci. Rep. 2020, 10, 14129. [Google Scholar] [CrossRef]
- Gallagher, P.E.; Ferrario, C.M.; Tallant, E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2373–H2379. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid Med. Cell Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Myocardial repair/remodelling following infarction: Roles of local factors. Cardiovasc. Res. 2009, 81, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, D.; Riad, A.; Lettau, O.; Roks, A.; Savvatis, K.; Becher, P.M.; Escher, F.; Jan Danser, A.H.; Schultheiss, H.P.; Tschope, C. Renin inhibition improves cardiac function and remodeling after myocardial infarction independent of blood pressure. Hypertension 2008, 52, 1068–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabelo, L.A.; Todiras, M.; Nunes-Souza, V.; Qadri, F.; Szijarto, I.A.; Gollasch, M.; Penninger, J.M.; Bader, M.; Santos, R.A.; Alenina, N. Genetic Deletion of ACE2 Induces Vascular Dysfunction in C57BL/6 Mice: Role of Nitric Oxide Imbalance and Oxidative Stress. PLoS ONE 2016, 11, e0150255. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.; Velkoska, E.; Freeman, M.; Wai, B.; Lancefield, T.F.; Burrell, L.M. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front. Physiol. 2014, 5, 227. [Google Scholar] [CrossRef]
- Soro-Paavonen, A.; Gordin, D.; Forsblom, C.; Rosengard-Barlund, M.; Waden, J.; Thorn, L.; Sandholm, N.; Thomas, M.C.; Groop, P.H.; FinnDiane Study, G. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Diez-Freire, C.; Vazquez, J.; Correa de Adjounian, M.F.; Ferrari, M.F.; Yuan, L.; Silver, X.; Torres, R.; Raizada, M.K. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol. Genom. 2006, 27, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Rentzsch, B.; Todiras, M.; Iliescu, R.; Popova, E.; Campos, L.A.; Oliveira, M.L.; Baltatu, O.C.; Santos, R.A.; Bader, M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 2008, 52, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Wysocki, J.; Ye, M.; Rodriguez, E.; Gonzalez-Pacheco, F.R.; Barrios, C.; Evora, K.; Schuster, M.; Loibner, H.; Brosnihan, K.B.; Ferrario, C.M.; et al. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: Prevention of angiotensin II-dependent hypertension. Hypertension 2010, 55, 90–98. [Google Scholar] [CrossRef]
- Ikeda, A.; Ichino, H.; Kiguchiya, S.; Chigwechokha, P.; Komatsu, M.; Shiozaki, K. Evaluation and Identification of Potent Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Dwarf Gulper Shark (C entrophorus atromarginatus). J. Food Process. Preserv. 2015, 39, 107–115. [Google Scholar] [CrossRef]
- Ko, J.Y.; Lee, J.H.; Samarakoon, K.; Kim, J.S.; Jeon, Y.J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. J. Food Drug Anal. 2018, 26, 696–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, Z.; Zhao, Y.; Zhu, X.; Yu, R.; Dong, S.; Wu, H. Novel Natural Angiotensin Converting Enzyme (ACE)-Inhibitory Peptides Derived from Sea Cucumber-Modified Hydrolysates by Adding Exogenous Proline and a Study of Their Structure(-)Activity Relationship. Mar. Drugs 2018, 16, 271. [Google Scholar] [CrossRef] [Green Version]
- Agirbasli, Z.; Cavas, L. In silico evaluation of bioactive peptides from the green algae Caulerpa. Environ. Boil. Fishes 2017, 29, 1635–1646. [Google Scholar] [CrossRef]
- Bitam, F.; Ciavatta, M.L.; Carbone, M.; Manzo, E.; Mollo, E.; Gavagnin, M. Chemical analysis of flavonoid constituents of the seagrass Halophila stipulacea: First finding of malonylated derivatives in marine phanerogams. Biochem. Syst. Ecol. 2010, 38, 689–690. [Google Scholar] [CrossRef]
- Barbosa, E.A.; Oliveira, A.; Placido, A.; Socodato, R.; Portugal, C.C.; Mafud, A.C.; Ombredane, A.S.; Moreira, D.C.; Vale, N.; Bessa, L.J.; et al. Structure and function of a novel antioxidant peptide from the skin of tropical frogs. Free Radic. Biol. Med. 2018, 115, 68–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [Green Version]
- Dales, N.A.; Gould, A.E.; Brown, J.A.; Calderwood, E.F.; Guan, B.; Minor, C.A.; Gavin, J.M.; Hales, P.; Kaushik, V.K.; Stewart, M.; et al. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J. Am. Chem. Soc. 2002, 124, 11852–11853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.Y. HPEPDOCK: A web server for blind peptide-protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]



| Phylum | Species | Amino Acid Sequence | IC50 (μM) | Ref. |
|---|---|---|---|---|
| Ochrophyta | Undaria pinnatifida (Harvey) Suringar | IY | 2.7 | [45] |
| IW | 1.5 | |||
| VY | 35.2 | |||
| IY | 6.1 | |||
| AW | 18.8 | |||
| FY | 42.3 | |||
| VW | 3.3 | |||
| IW | 1.5 | |||
| Rhodophyta | Neopyropia yezoensis (Ueda) Yang & Brodie (as Porphyra yezoensis) | LW | 23.6 | [46] |
| Rhodophyta | Palmaria palmata (Linnaeus) Weber & Mohr | LMPIIRLIIVLMA | 3.344 × 103 | [47] |
| Arthropoda | Acetes chinensis Hansen, 1919 | IFVPAFDP | 3.4 | [48] |
| LHP | 2.15 | |||
| Arthropoda | Plesionika izumiae Omori, 1971 | ST | 4.03 | [49] |
| Mollusca | Pinctada imbricata fucata (Gould, 1850) (as Pinctada fucata martensii) | ALAPE | 167.5 | [50] |
| Cnidaria | Rhopilema esculentum Kishinouye, 1891 | QPGPT | 80.67 | [14] |
| Annelida (Sipuncula) | Phascolosoma arcuatum (Gray, 1828) (as Phascolosoma esculenta) | AWLHPGAPKVF | 135 | [51] |
| Chordata (Pisces) | Katsuwonus pelamis (Linnaeus, 1758) | IKPLNY | 43 | [52] |
| IVGRPRHQG | 2.4 | |||
| IWHHT | 5.8 | |||
| ALPHA | 10 | |||
| FQP | 12 | |||
| LKPNM | 2.4 | [52,53] | ||
| IY | 2.31 | [52,53] | ||
| DYGP | 62 | [52] | ||
| LKP | 0.32 | [53] | ||
| IWHHT | 3.5 | |||
| IKP | 6.9 | |||
| IVGRPR | 300 | [54] | ||
| Chordata (Pisces) | Sarda lineolata (Girard, 1858) | MF | 44.7 | [55] |
| RY | 51 | |||
| MY | 193 | |||
| LY | 38.5 | |||
| YL | 82 | |||
| IY | 10.5 | |||
| VF | 43.7 | |||
| GRP | 20 | |||
| RFP | 330 | |||
| AKK | 3.13 | |||
| RVY | 205.6 | |||
| GWAP | 3.86 | |||
| KY | 1.63 | |||
| VY | 10 | [55,56] | ||
| Chordata (Pisces) | Thunnus obesus (Lowe, 1839) | GDLGKTTTVSNWSPPKYKDTP | 11.28 | [57] |
| WPEAAELMMEVDP | 21.6 | [58] | ||
| Chordata (Pisces) | Limanda aspera (Pallas, 1814) | MIFPGAGGPEL | 28.7 | [59] |
| Chordata (Pisces) | Gadus chalcogrammus Pallas, 1814 (as Theragra chalcogramma) | GPM | 17.13 | [60] |
| GPL | 2.6 | |||
| LGP | 0.72 | |||
| GLP | 1.62 | |||
| PLG | 4.74 | |||
| LPG | 5.73 | |||
| PGL | 13.93 | |||
| FGASTRGA | 14.7 | |||
| Chordata (Pisces) | Katsuwonus pelamis (Linnaeus, 1758) | IKPLNY | 43 | [61] |
| DYGLYP | 62 | |||
| LRP | 1 | |||
| LKPNM | 2.4 | |||
| Chordata (Pisces) | Sardinella aurita Valenciennes, 1847 | KW | 1.63 | [62] |
| Chordata (Pisces) | Pseudocaranx sp. | AR | 570.78 | [63] |
| AV | 956.28 | |||
| APER | 530.21 | |||
| EY | 1.98 | |||
| FE | 2.68 | |||
| CF | 1.45 | |||
| Chordata (Pisces) | Saurida elongata (Temminck & Schlegel, 1846) | RVCLP | 175 | [64] |
| GMKCAF | 45.70 | |||
| Chordata (Pisces) | Thunnus albacares (Bonnaterre, 1788) (as Neothunnus macropterus) | WGD | 2 | [57] |
| Chordata (Pisces) | Oncorhynchus keta (Walbaum, 1792) | WA | 277.3 | [65] |
| VW | 2.5 | [65] | ||
| WM | 96.6 | [65] | ||
| MW | 9.9 | [42] | ||
| IW | 4.7 | [65] | ||
| LW | 17.4 | [65] | ||
| Chordata (Pisces) | Oncorhynchus gorbuscha (Walbaum, 1792) | IW | 1.2 | [15] |
| Chordata (Pisces) | Okamejei kenojei (Müller & Henle, 1841) | MVGSAPGVL | 3.09 | [66] |
| Chordata (Pisces) | Alopias pelagicus Nakamura, 1935 | IKW | 0.54 | [67] |
| Chordata (Pisces) | Scyliorhinus canicula (Linnaeus, 1758) | VAMPF | 0.44 | [68] |
| SOURCE | SEQUENCE | MW, g/mol | NET CHARGE pH 7.0 | Average Hydrophilicity | % Hydrophilic Residues |
|---|---|---|---|---|---|
| Thunnus obesus (Lowe, 1839) | GDLGKTTTVSNWSPPKYKDTP | 2292.53 | 1.0 | 0.2 | 38% |
| Undaria pinnatifida (Harvey) Suringar | VW | 303.36 | 0 | −2.5 | 0% |
| Palmaria palmata (Linnaeus) Weber & Mohr | LMPIIRLIIVLMA | 1496.04 | 1.0 | −1.1 | 8% |
| Species | ACE2 | ACE2-RBD |
|---|---|---|
| Thunnus obesus | −246.50 | −223.60 |
| Palmaria palmata | −245.44 | −283.83 |
| Undaria pinnatifida | −117.65 | −123.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festa, M.; Sansone, C.; Brunet, C.; Crocetta, F.; Di Paola, L.; Lombardo, M.; Bruno, A.; Noonan, D.M.; Albini, A. Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARS-CoV-2 Infection. Int. J. Mol. Sci. 2020, 21, 8364. https://doi.org/10.3390/ijms21218364
Festa M, Sansone C, Brunet C, Crocetta F, Di Paola L, Lombardo M, Bruno A, Noonan DM, Albini A. Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARS-CoV-2 Infection. International Journal of Molecular Sciences. 2020; 21(21):8364. https://doi.org/10.3390/ijms21218364
Chicago/Turabian StyleFesta, Marco, Clementina Sansone, Christophe Brunet, Fabio Crocetta, Luisa Di Paola, Michele Lombardo, Antonino Bruno, Douglas M. Noonan, and Adriana Albini. 2020. "Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARS-CoV-2 Infection" International Journal of Molecular Sciences 21, no. 21: 8364. https://doi.org/10.3390/ijms21218364
APA StyleFesta, M., Sansone, C., Brunet, C., Crocetta, F., Di Paola, L., Lombardo, M., Bruno, A., Noonan, D. M., & Albini, A. (2020). Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARS-CoV-2 Infection. International Journal of Molecular Sciences, 21(21), 8364. https://doi.org/10.3390/ijms21218364









