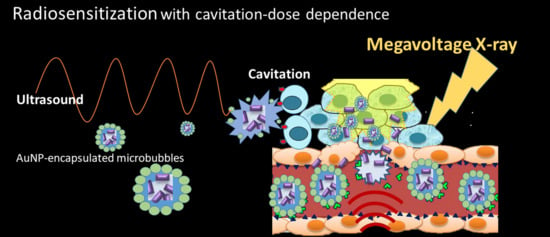
Enhanced Radiosensitization for Cancer Treatment with Gold Nanoparticles through Sonoporation
Abstract
1. Background
2. Methods and Materials
2.1. Hepatocellular Carcinoma Cell Lines
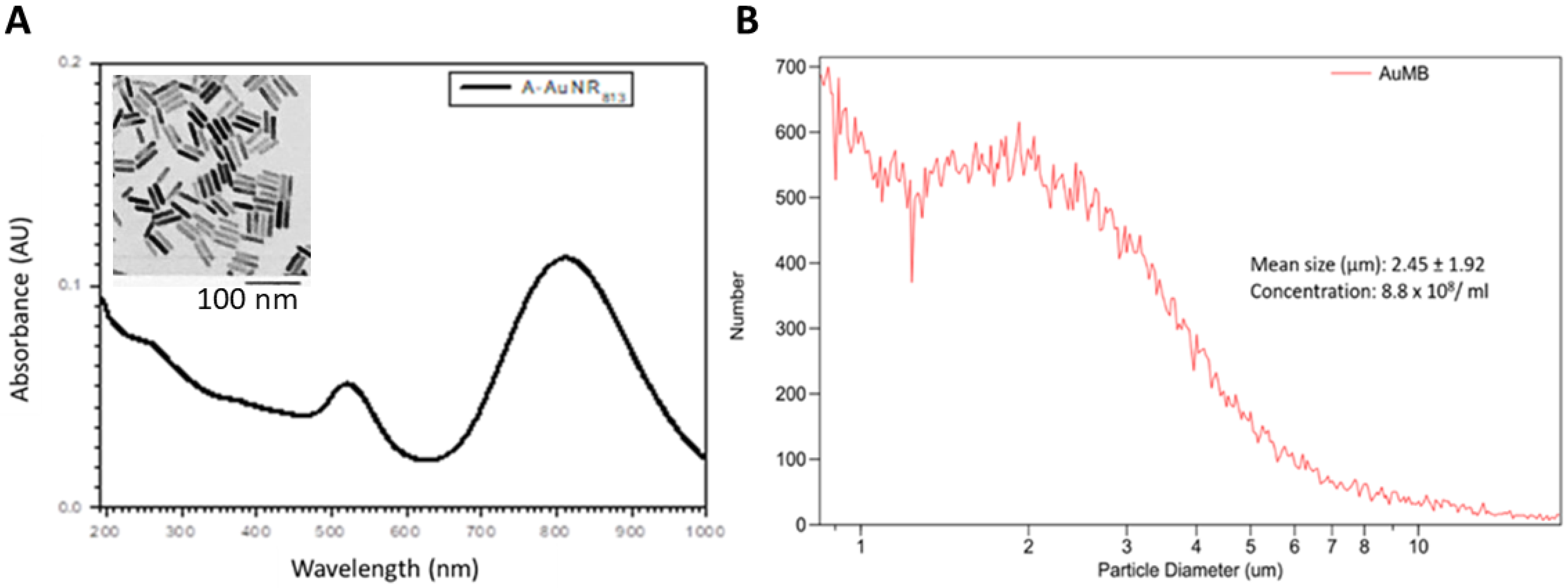
2.2. AuNPs
2.3. AuMBs
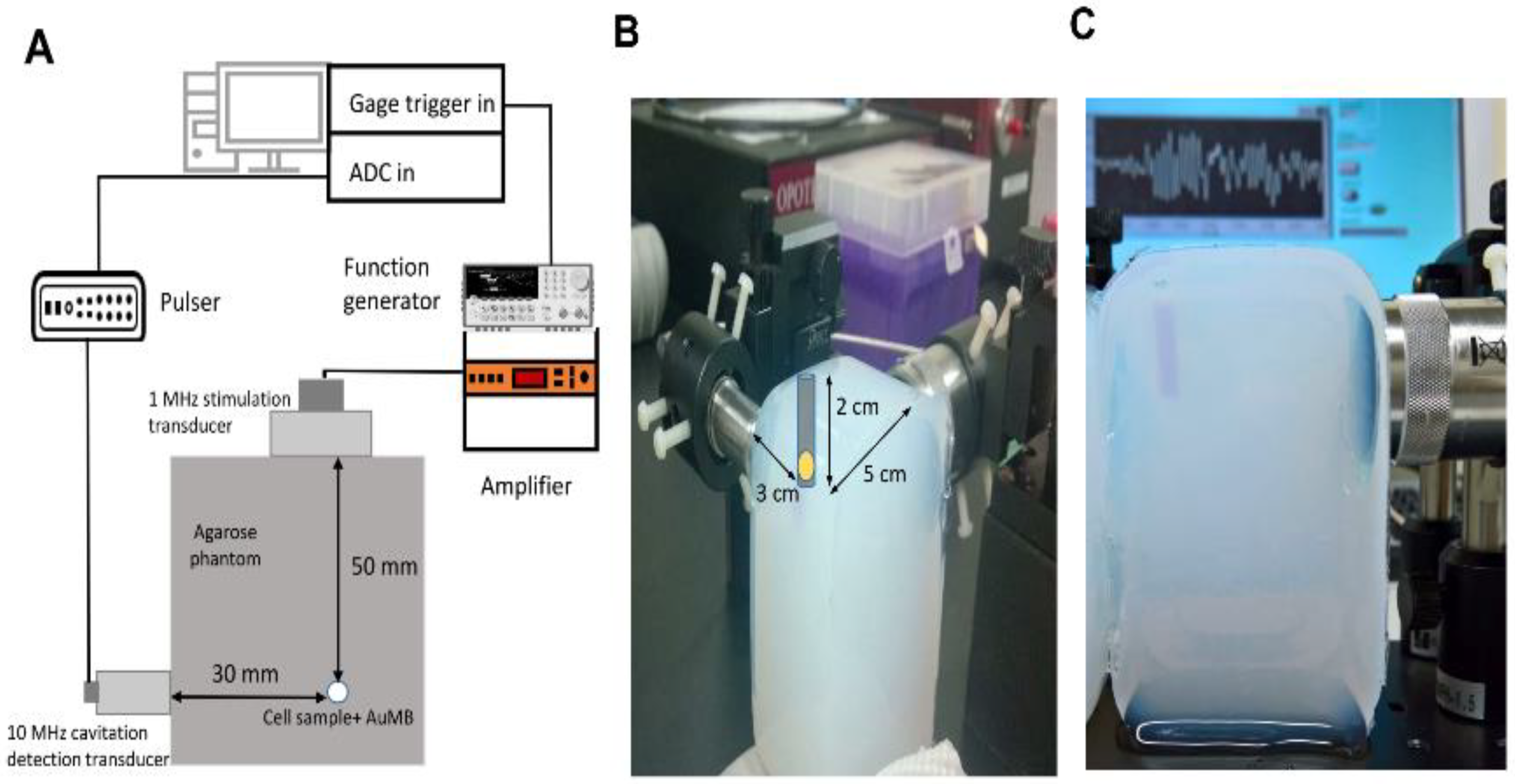
2.4. In Vitro Sonoporation System Setup
2.5. Cavitation Measurements
2.6. Assessment of Intracellular AuNPs
2.7. Colony Formation Assay
2.8. γ-H2AX Immunofluorescence Microscopy
2.9. Western Blot Analysis
2.10. Targeted AuMBs with VEGFR2 Conjugation
2.11. Targeted AuMB-Enhanced Ultrasound Imaging in Mice
2.12. AuMB-Sonoporation-Mediated Radiosensitization
2.13. Statistical Analysis
3. Results
3.1. Characterization of AuNPs and AuMBs
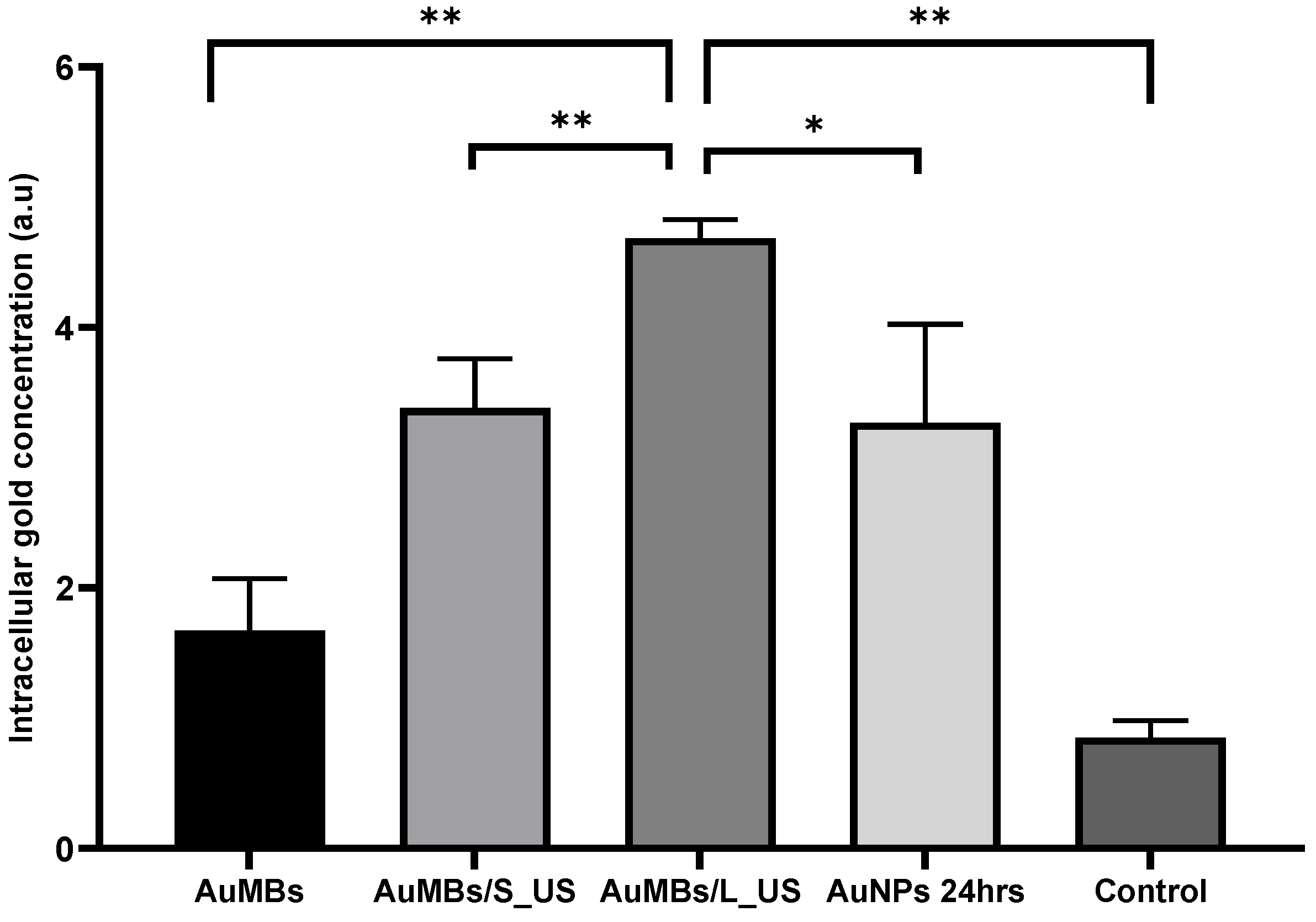
3.2. Acoustic Cavitation Effect and Intracellular Localization of AuNPs
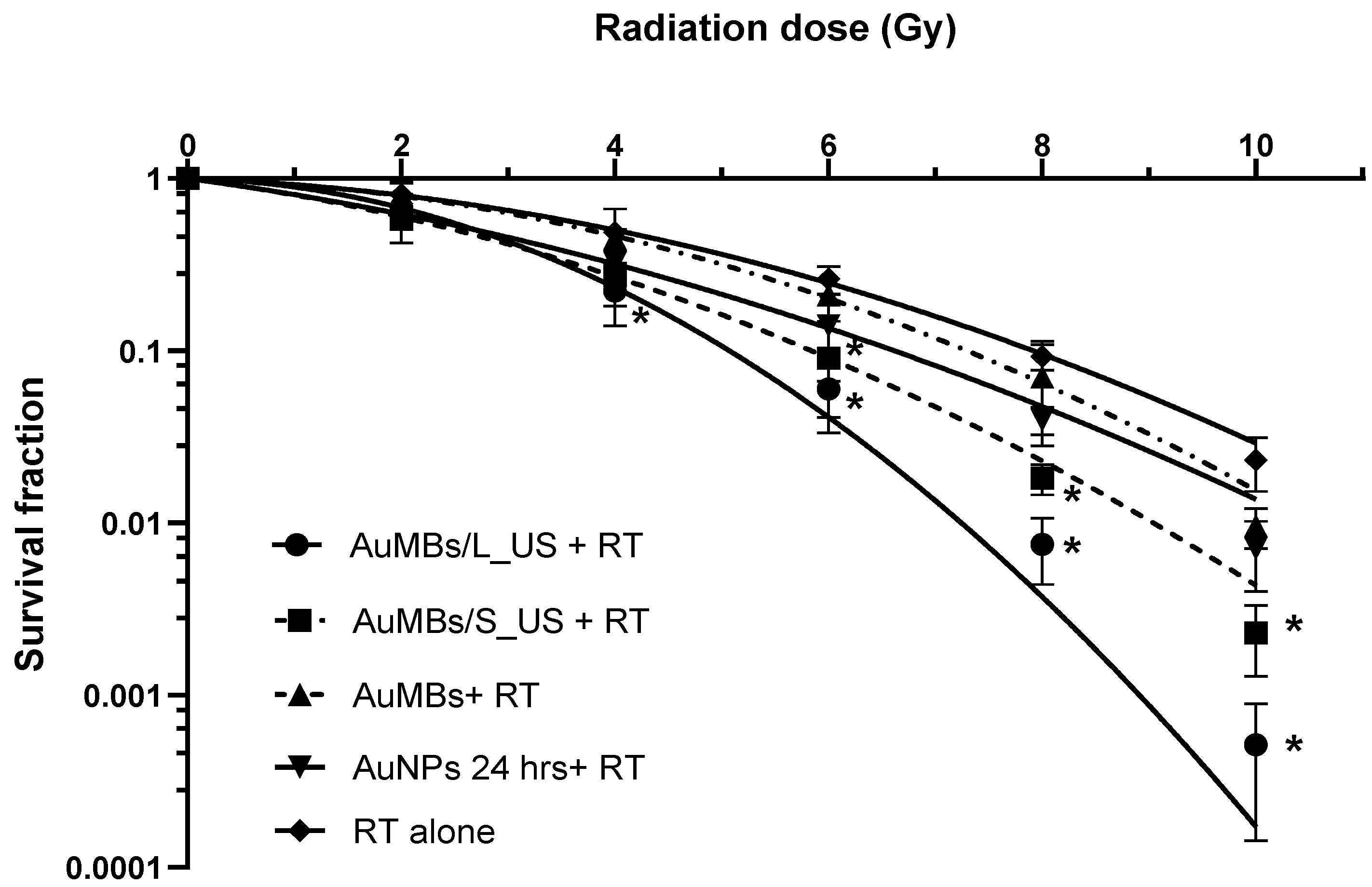
3.3. Clonogenic Assessment of In Vitro Radiosensitization by AuMB Sonoporation
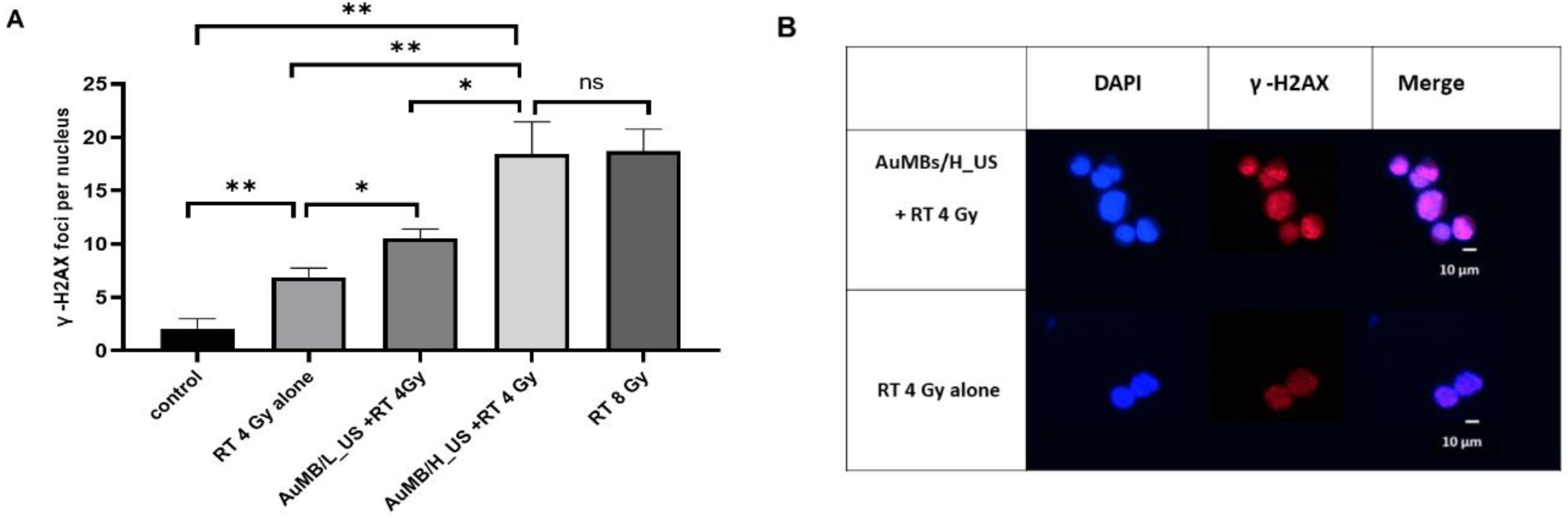
3.4. γ-H2AX Immunofluorescence Staining
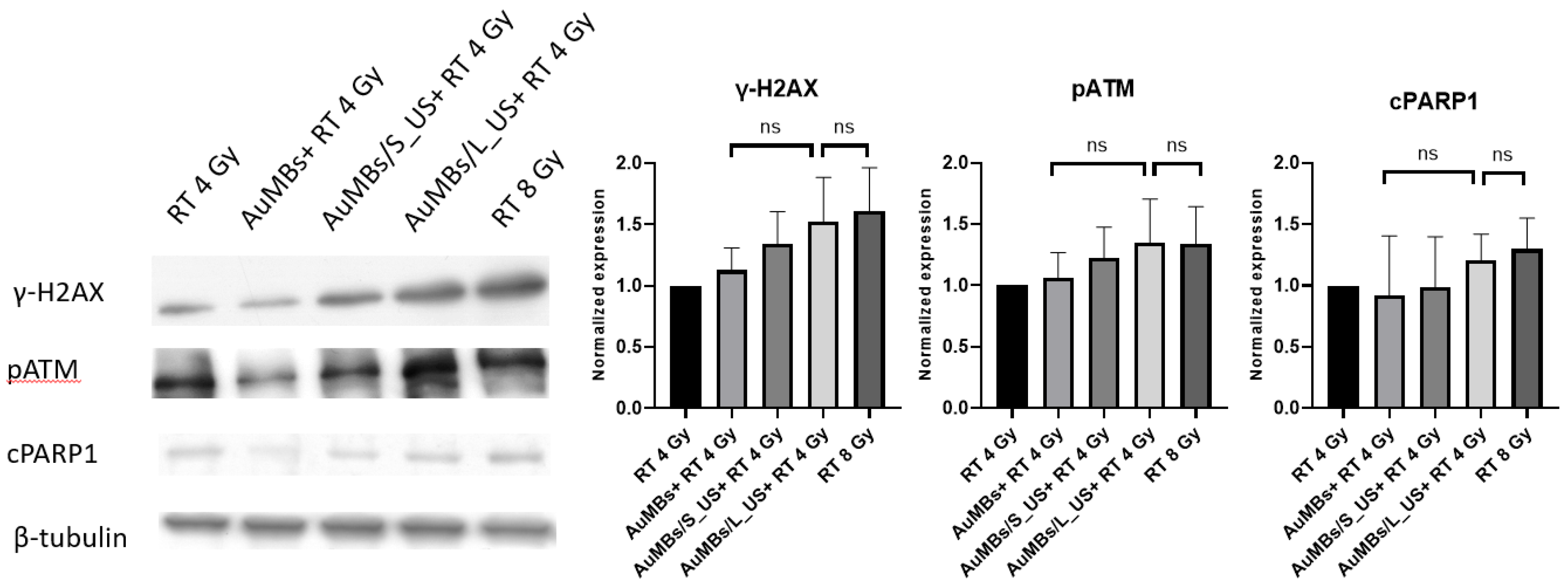
3.5. Western Blotting
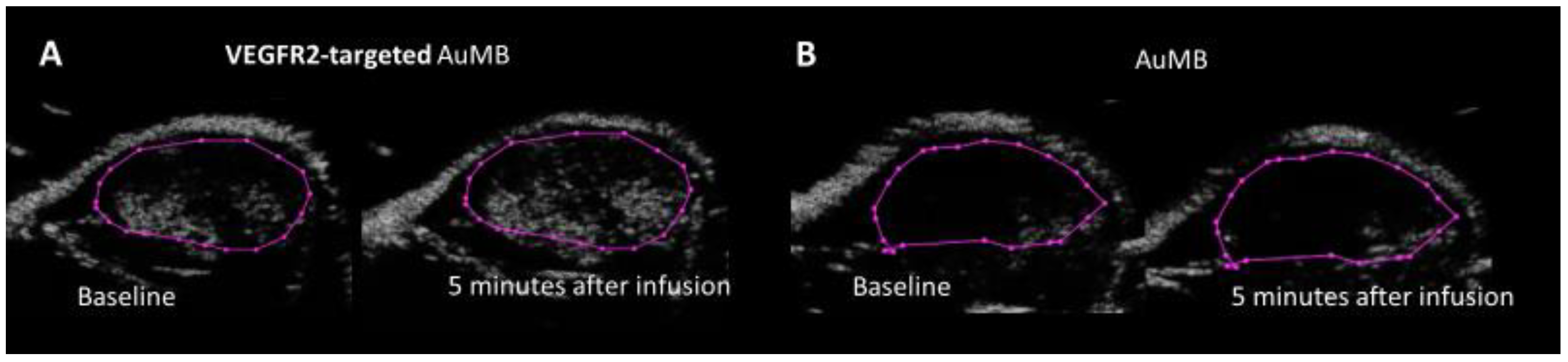
3.6. VEGFR2-Targed AuMBs to Enhance In Vivo Delivery
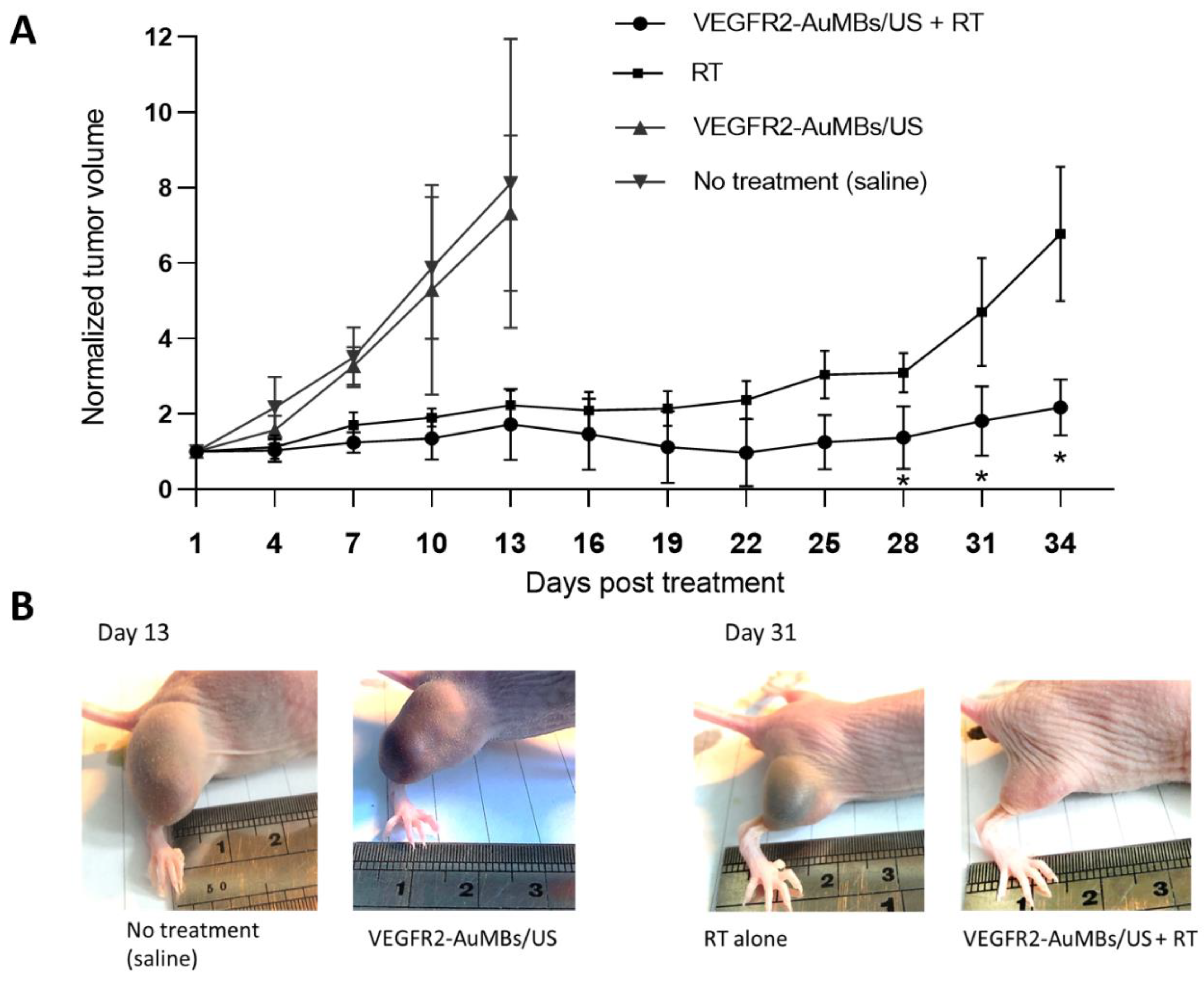
3.7. In Vivo Assessment of Xenograft Tumor-Growth Delay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harrington, K.J.; Billingham, L.J.; Brunner, T.B.; Burnet, N.G.; Chan, C.S.; Hoskin, P.; Mackay, R.I.; Maughan, T.S.; Macdougall, J.; McKenna, W.G.; et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer 2011, 105, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Her, S.; Borst, G.R.; Bristow, R.G.; Jaffray, D.A.; Allen, C. Radiosensitization by gold nanoparticles: Will they ever make it to the clinic? Radiother. Oncol. 2017, 124, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.; He, H.; Zhang, X.-D. Cancer radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; Volume 6. [Google Scholar]
- Pottier, A.; Borghi, E.; Levy, L. The future of nanosized radiation enhancers. Br. J. Radiol. 2015, 88, 20150171. [Google Scholar] [CrossRef] [PubMed]
- van Ballegooie, C.; Man, A.; Win, M.; Yapp, D.T. Spatially Specific Liposomal Cancer Therapy Triggered by Clinical External Sources of Energy. Pharmaceutics 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Jeremic, B.; Aguerri, A.R.; Filipovic, N. Radiosensitization by gold nanoparticles. Clin. Transl. Oncol. 2013, 15, 593–601. [Google Scholar] [CrossRef]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to clinical use of gold nanoparticles for radiation sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2020, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y.; Li, X.; Hu, L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer 2015, 22, 413–420. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Wang, Y.; Liu, Z.; Fu, L.; Hu, L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J. Nanoparticle Res. 2013, 15, 1642. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Cai, Z.; Kwon, Y.L.; Lechtman, E.; Pignol, J.-P.; Reilly, R.M. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res. Treat. 2013, 137, 81–91. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine 2015, 11, 1277–1283. [Google Scholar] [CrossRef]
- Lai, C.Y.; Wu, C.H.; Chen, C.C.; Li, P.C. Quantitative relations of acoustic inertial cavitation with sonoporation and cell viability. Ultrasound Med. Biol. 2006, 32, 1931–1941. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Schlicher, R.K.; Hicks, H.K.; Prausnitz, M.R. Saving cells from ultrasound-induced apoptosis: Quantification of cell death and uptake following sonication and effects of targeted calcium chelation. Ultrasound Med. Biol. 2010, 36, 1008–1021. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, S.P.; Liao, A.H.; Yang, Y.C.; Lee, C.R.; Wu, C.H.; Wu, P.C.; Liu, T.M.; Wang, C.R.; Li, P.C. Synergistic delivery of gold nanorods using multifunctional microbubbles for enhanced plasmonic photothermal therapy. Sci. Rep. 2014, 4, 5685. [Google Scholar] [CrossRef]
- Liu, W.W.; Liu, S.W.; Liou, Y.R.; Wu, Y.H.; Yang, Y.C.; Wang, C.R.; Li, P.C. Nanodroplet-Vaporization-Assisted Sonoporation for Highly Effective Delivery of Photothermal Treatment. Sci. Rep. 2016, 6, 24753. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liao, A.H.; Chen, J.H.; Wang, C.R.; Li, P.C. Photoacoustic/ultrasound dual-modality contrast agent and its application to thermotherapy. J. Biomed. Opt. 2012, 17, 045001. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.R.; Francisco Neto, M.J.; Garcia, R.G.; Rahal Junior, A.; Salvalaggio, P.; Funari, M.B. High correlation between microbubble contrast-enhanced ultrasound, magnetic resonance and histopathology in the evaluation of hepatocellular carcinoma. Einstein 2013, 11, 500–506. [Google Scholar] [CrossRef]
- Rossi, S.; Ghittoni, G.; Ravetta, V.; Torello Viera, F.; Rosa, L.; Serassi, M.; Scabini, M.; Vercelli, A.; Tinelli, C.; Dal Bello, B.; et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur. Radiol. 2008, 18, 1749–1756. [Google Scholar] [CrossRef]
- Takahashi, M.; Maruyama, H.; Ishibashi, H.; Yoshikawa, M.; Yokosuka, O. Contrast-enhanced ultrasound with perflubutane microbubble agent: Evaluation of differentiation of hepatocellular carcinoma. Am. J. Roentgenol. 2011, 196, W123–W131. [Google Scholar] [CrossRef]
- Liu, W.L.; Gao, M.; Tzen, K.Y.; Tsai, C.L.; Hsu, F.M.; Cheng, A.L.; Cheng, J.C. Targeting Phosphatidylinositide3-Kinase/Akt pathway by BKM120 for radiosensitization in hepatocellular carcinoma. Oncotarget 2014, 5, 3662–3672. [Google Scholar] [CrossRef]
- Smith, N.R.; Baker, D.; James, N.H.; Ratcliffe, K.; Jenkins, M.; Ashton, S.E.; Sproat, G.; Swann, R.; Gray, N.; Ryan, A.; et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 2010, 16, 3548–3561. [Google Scholar] [CrossRef]
- Bagi, C.M.; Gebhard, D.F.; Andresen, C.J. Antitumor effect of vascular endothelial growth factor inhibitor sunitinib in preclinical models of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2012, 24, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Yardeni, T.; Eckhaus, M.; Morris, H.D.; Huizing, M.; Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab. Anim. 2011, 40, 155–160. [Google Scholar] [CrossRef]
- Brenner, D.J. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin. Radiat. Oncol. 2008, 18, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Morozov, K.V.; Kolyvanova, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dement’eva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by Gold Nanoparticles: Impact of the Size, Dose Rate, and Photon Energy. Nanomater 2020, 10, 952. [Google Scholar] [CrossRef]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The role of ultrasound-driven microbubble dynamics in drug delivery: From microbubble fundamentals to clinical translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Fan, Z.; Kumon, R.E.; Deng, C.X. Mechanisms of microbubble-facilitated sonoporation for drug and gene delivery. Ther. Deliv. 2014, 5, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; E Wilson, K.; Machtaler, S.; K Willmann, J. Ultrasound and microbubble guided drug delivery: Mechanistic understanding and clinical implications. Curr. Pharm. Biotechnol. 2013, 14, 743–752. [Google Scholar] [CrossRef]
- Karshafian, R.; Bevan, P.D.; Williams, R.; Samac, S.; Burns, P.N. Sonoporation by ultrasound-activated microbubble contrast agents: Effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med. Biol. 2009, 35, 847–860. [Google Scholar] [CrossRef]
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J. Control. Release 2005, 104, 213–222. [Google Scholar] [CrossRef]
- Czarnota, G.J.; Karshafian, R.; Burns, P.N.; Wong, S.; Al Mahrouki, A.; Lee, J.W.; Caissie, A.; Tran, W.; Kim, C.; Furukawa, M.; et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc. Natl. Acad. Sci. USA 2012, 109, E2033–E2041. [Google Scholar] [CrossRef]
- El Kaffas, A.; Gangeh, M.J.; Farhat, G.; Tran, W.T.; Hashim, A.; Giles, A.; Czarnota, G.J. Tumour vascular shutdown and cell death following ultrasound-microbubble enhanced radiation therapy. Theranostics 2018, 8, 314. [Google Scholar] [CrossRef]
- Tran, W.; Iradji, S.; Sofroni, E.; Giles, A.; Eddy, D.; Czarnota, G. Microbubble and ultrasound radioenhancement of bladder cancer. Br. J. Cancer 2012, 107, 469. [Google Scholar] [CrossRef]
- Moding, E.J.; Castle, K.D.; Perez, B.A.; Oh, P.; Min, H.D.; Norris, H.; Ma, Y.; Cardona, D.M.; Lee, C.-L.; Kirsch, D.G. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci. Transl. Med. 2015, 7, 278ra34. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.D.; Bhatia, S. The radiobiological targets of SBRT: Tumor cells or endothelial cells? Ann. Transl. Med. 2015, 3, 290. [Google Scholar]
- Tran, D.M.; Harrang, J.; Song, S.; Chen, J.; Smith, B.M.; Miao, C.H. Prolonging pulse duration in ultrasound-mediated gene delivery lowers acoustic pressure threshold for efficient gene transfer to cells and small animals. J. Control. Release 2018, 279, 345–354. [Google Scholar] [CrossRef]
- Snipstad, S.; Berg, S.; Mørch, Ý.; Bjørkøy, A.; Sulheim, E.; Hansen, R.; Grimstad, I.; van Wamel, A.; Maaland, A.F.; Torp, S.H. Ultrasound improves the delivery and therapeutic effect of nanoparticle-stabilized microbubbles in breast cancer xenografts. Ultrasound Med. Biol. 2017, 43, 2651–2669. [Google Scholar] [CrossRef]
- Pouliopoulos, A.N.; Li, C.; Tinguely, M.; Garbin, V.; Tang, M.-X.; Choi, J.J. Rapid short-pulse sequences enhance the spatiotemporal uniformity of acoustically driven microbubble activity during flow conditions. J. Acoust. Soc. Am. 2016, 140, 2469–2480. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Choe, J.W.; Pu, K.; Devulapally, R.; Bachawal, S.; Machtaler, S.; Chowdhury, S.M.; Luong, R.; Tian, L.; Khuri-Yakub, B. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. J. Control. Release 2015, 203, 99–108. [Google Scholar] [CrossRef]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]
- Shanei, A.; Akbari-Zadeh, H. Investigating the Sonodynamic-Radiosensitivity Effect of Gold Nanoparticles on HeLa Cervical Cancer Cells. J. Korean Med. Sci. 2019, 34, e243. [Google Scholar] [CrossRef]
- Anderson Christopher, R.; Rychak Joshua, J.; Backer Marina, B.J.; Ley Klaus, K.A.L. scVEGF microbubble ultrasound contrast agents: A novel probe for ultrasound molecular imaging of tumor angiogenesis. Investig. Radiol. 2010, 45, 579. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Zhang, H.; Lutz, A.M.; Tian, L.; Hristov, D.; Willmann, J.K. VEGFR2-targeted three-dimensional ultrasound imaging can predict responses to antiangiogenic therapy in preclinical models of colon cancer. Cancer Res. 2016, 76, 4081–4089. [Google Scholar] [CrossRef] [PubMed]
- Toaldo, M.B.; Salvatore, V.; Marinelli, S.; Palamà, C.; Milazzo, M.; Croci, L.; Venerandi, L.; Cipone, M.; Bolondi, L.; Piscaglia, F. Use of VEGFR-2 targeted ultrasound contrast agent for the early evaluation of response to sorafenib in a mouse model of hepatocellular carcinoma. Mol. Imaging Biol. 2015, 17, 29–37. [Google Scholar] [CrossRef]
- Chan, E.S.; Patel, A.R.; Larchian, W.A.; Heston, W.D. In vivo targeted contrast enhanced micro-ultrasound to measure intratumor perfusion and vascular endothelial growth factor receptor 2 expression in a mouse orthotopic bladder cancer model. J. Urol. 2011, 185, 2359–2365. [Google Scholar] [CrossRef]
- Lyshchik, A.; Fleischer, A.C.; Huamani, J.; Hallahan, D.E.; Brissova, M.; Gore, J.C. Molecular imaging of vascular endothelial growth factor receptor 2 expression using targeted contrast-enhanced high-frequency ultrasonography. J. Ultrasound Med. 2007, 26, 1575–1586. [Google Scholar] [CrossRef]
- Rychak, J.J.; Graba, J.; Cheung, A.M.; Mystry, B.S.; Lindner, J.R.; Kerbel, R.S.; Foster, F.S. Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol. Imaging 2007, 6, 289–296. [Google Scholar] [CrossRef]
- Lum, A.F.; Borden, M.A.; Dayton, P.A.; Kruse, D.E.; Simon, S.I.; Ferrara, K.W. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J. Control. Release 2006, 111, 128–134. [Google Scholar] [CrossRef]
- Thomas, E.; Menon, J.U.; Owen, J.; Skaripa-Koukelli, I.; Wallington, S.; Gray, M.; Mannaris, C.; Kersemans, V.; Allen, D.; Kinchesh, P.; et al. Ultrasound-mediated cavitation enhances the delivery of an EGFR-targeting liposomal formulation designed for chemo-radionuclide therapy. Theranostics 2019, 9, 5595–5609. [Google Scholar] [CrossRef]
- Fan, C.H.; Ting, C.Y.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials 2013, 34, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Shiau, A.L.; Chen, Y.H.; Chang, C.J.; Chen, H.H.; Wu, C.L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar] [CrossRef]
- Sen, T.; Tufekcioglu, O.; Koza, Y. Mechanical index. Anatol. J. Cardiol. 2015, 15, 334–336. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-L.; Liu, W.-W.; Cheng, J.C.-H.; Lin, L.-C.; Wang, C.-R.C.; Li, P.-C. Enhanced Radiosensitization for Cancer Treatment with Gold Nanoparticles through Sonoporation. Int. J. Mol. Sci. 2020, 21, 8370. https://doi.org/10.3390/ijms21218370
Lu S-L, Liu W-W, Cheng JC-H, Lin L-C, Wang C-RC, Li P-C. Enhanced Radiosensitization for Cancer Treatment with Gold Nanoparticles through Sonoporation. International Journal of Molecular Sciences. 2020; 21(21):8370. https://doi.org/10.3390/ijms21218370
Chicago/Turabian StyleLu, Shao-Lun, Wei-Wen Liu, Jason Chia-Hsien Cheng, Lien-Chieh Lin, Churng-Ren Chris Wang, and Pai-Chi Li. 2020. "Enhanced Radiosensitization for Cancer Treatment with Gold Nanoparticles through Sonoporation" International Journal of Molecular Sciences 21, no. 21: 8370. https://doi.org/10.3390/ijms21218370
APA StyleLu, S.-L., Liu, W.-W., Cheng, J. C.-H., Lin, L.-C., Wang, C.-R. C., & Li, P.-C. (2020). Enhanced Radiosensitization for Cancer Treatment with Gold Nanoparticles through Sonoporation. International Journal of Molecular Sciences, 21(21), 8370. https://doi.org/10.3390/ijms21218370