A Cell for the Ages: Human γδ T Cells across the Lifespan
Abstract
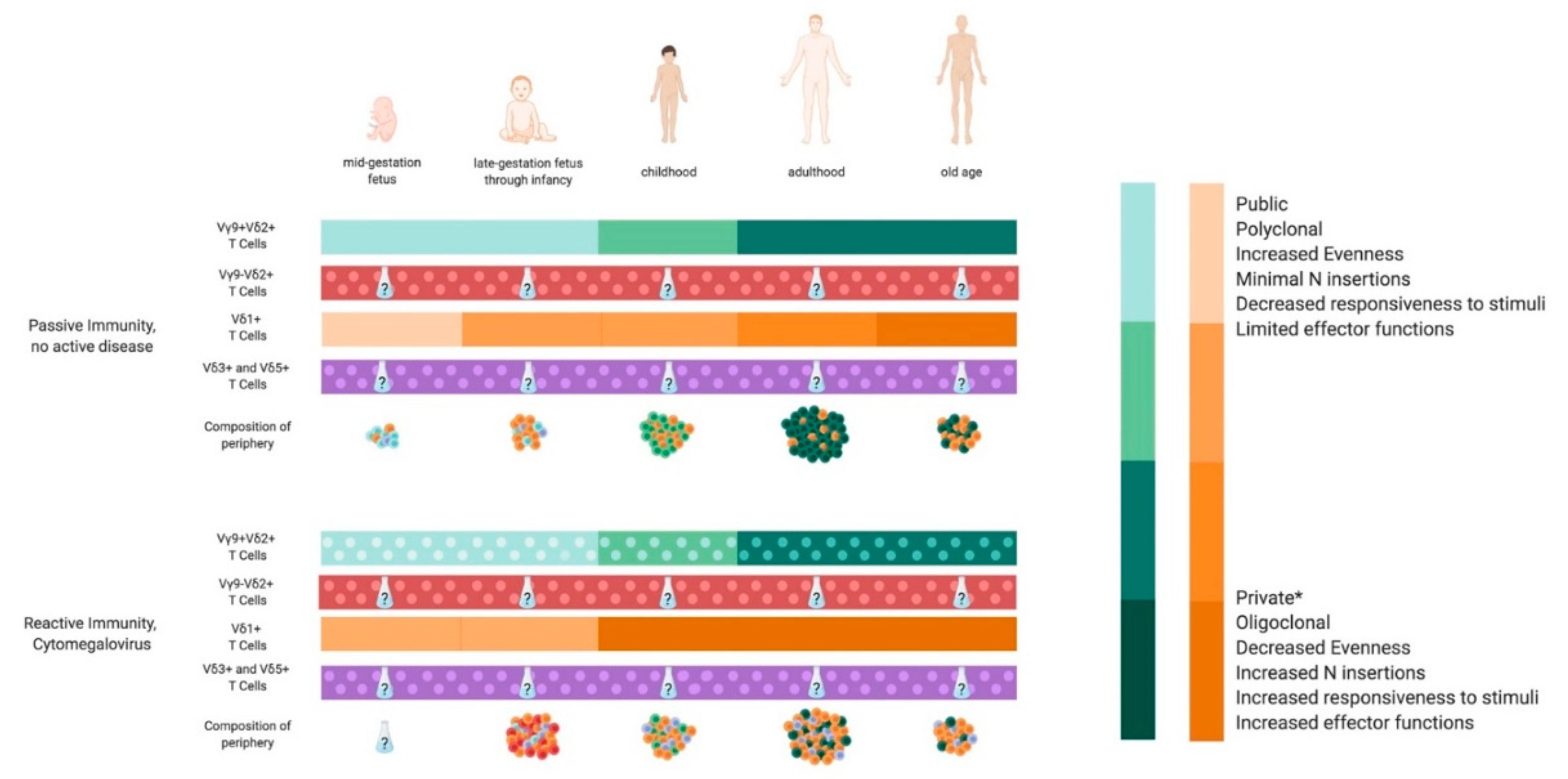
1. Introduction
2. Passive γδ T Cell Immunity
3. Reactive γδ T Cell Immunity
3.1. Cancer
3.2. Transplant
3.3. Infectious Disease
3.3.1. Cytomegalovirus
3.3.2. Influenza
3.3.3. Tuberculosis
3.3.4. Miscellaneous Microbes
3.3.5. Miscellaneous Chronic Infections
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| pAg | phosphoantigen |
| HMB-PP | (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate |
| IPP | isopentenyl pyrophosphate |
| HSCT | hematopoietic stem cell transplant |
| GVHD | graft-vs-host disease |
| HCMV | human cytomegalovirus |
| EPCR | endothelial protein C receptor |
References
- Gaimann, M.U.; Nguyen, M.; Desponds, J.; Mayer, A. Early life imprints the hierarchy of T cell clone sizes. arXiv 2020, arXiv:2007.11113. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Fülöp, T.; Pawelec, G. Immunosenescence in vertebrates and invertebrates. Immun. Ageing 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Rane, S.; Das, R.; Faulkner, M.; Gund, R.; Kandpal, U.; Lewis, V.; Mattoo, H.; Prabhu, S.; Ranganathan, V.; et al. T cell ageing: Effects of age on development, survival & function. Indian J. Med. Res. 2013, 138, 595–608. [Google Scholar] [PubMed]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Malavolta, M.; Pawelec, G. Age and immunity. Immun. Ageing 2006, 3, 2. [Google Scholar] [CrossRef]
- Woodland, D.L.; Blackman, M.A. Immunity and age: Living in the past? Trends Immunol. 2006, 27, 303–307. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity 2012, 37, 771–783. [Google Scholar] [CrossRef]
- Hill, D.L.; Carr, E.J.; Rutishauser, T.; Moncunill, G.; Campo, J.J.; Innocentin, S.; Mpina, M.; Nhabomba, A.; Tumbo, A.; Jairoce, C.; et al. Immune system development varies according to age, location, and anemia in African children. Sci. Transl. Med. 2020, 12, eaaw9522. [Google Scholar] [CrossRef] [PubMed]
- Teran, R.; Mitre, E.; Vaca, M.; Erazo, S.; Oviedo, G.; Hübner, M.P.; Chico, M.E.; Mattapallil, J.J.; Bickle, Q.; Rodrigues, L.C.; et al. Immune system development during early childhood in tropical Latin America: Evidence for the age-dependent down regulation of the innate immune response. Clin. Immunol. 2011, 138, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Graham, J.E.; Christian, L.M.; Kiecolt-Glaser, J.K. Stress, Age, and Immune Function: Toward a Lifespan Approach. J. Behav. Med. 2006, 29, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef]
- Kalyan, S.; Kabelitz, D. Defining the nature of human γδ T cells: A biographical sketch of the highly empathetic. Cell. Mol. Immunol. 2013, 10, 21–29. [Google Scholar] [CrossRef]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef]
- Xu, W.; Lau, Z.W.X.; Fulop, T.; Larbi, A. The Aging of γδ T Cells. Cells 2020, 9, 1181. [Google Scholar] [CrossRef]
- Schatz, D.G.; Swanson, P.C. V(D)J Recombination: Mechanisms of Initiation. Annu. Rev. Genet. 2011, 45, 167–202. [Google Scholar] [CrossRef]
- Bradley, P.; Thomas, P.G. Using T Cell Receptor Repertoires to Understand the Principles of Adaptive Immune Recognition. Annu. Rev. Immunol. 2019, 37, 547–570. [Google Scholar] [CrossRef]
- Brenner, M.B.; McLean, J.; Dialynas, D.P.; Strominger, J.L.; Smith, J.A.; Owen, F.L.; Seidman, J.G.; Ip, S.; Rosen, F.; Krangel, M.S. Identification of a putative second T-cell receptor. Nature 1986, 322, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Havran, W.L.; Allison, J.P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 1988, 335, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M.; Fowlkes, B.J.; Bluestone, J.A.; Kruisbeek, A.; Maloy, W.L.; Coligan, J.E.; Schwartz, R.H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature 1987, 326, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Meyer, C.; Bonneville, M. γδ T Cells: First Line of Defense and Beyond. Annu. Rev. Immunol. 2014, 32, 121–155. [Google Scholar] [CrossRef]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Baker, A.T.; Hunter, S.; Willcox, B.E. Recasting Human Vδ1 Lymphocytes in an Adaptive Role. Trends Immunol. 2018, 39, 446–459. [Google Scholar] [CrossRef]
- Khairallah, C.; Chu, T.H.; Sheridan, B.S. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front. Immunol. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Lefranc, M.-P.; Giudicelli, V.; Ginestoux, C.; Bodmer, J.; Müller, W.; Bontrop, R.; Lemaitre, M.; Malik, A.; Barbié, V.; Chaume, D. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999, 27, 209–212. [Google Scholar] [CrossRef]
- Carding, S.R.; Egan, P.J. γδ T cells: Functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002, 2, 336–345. [Google Scholar] [CrossRef]
- Fichtner, A.S.; Ravens, S.; Prinz, I. Human γδ TCR Repertoires in Health and Disease. Cells 2020, 9, 800. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C. The Role of Human γδ T Cells in Anti-Tumor Immunity and Their Potential for Cancer Immunotherapy. Cells 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, S.; Kumar, A.; Wan, G.S.; Ramanjaneyulu, G.S.; Cavallari, M.; El Daker, S.; Beddoe, T.; Theodossis, A.; Williams, N.K.; Gostick, E.; et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 2013, 14, 908–916. [Google Scholar] [CrossRef]
- Willcox, C.R.; Vantourout, P.; Salim, M.; Zlatareva, I.; Melandri, D.; Zanardo, L.; George, R.; Kjaer, S.; Jeeves, M.; Mohammed, F.; et al. Butyrophilin-like 3 Directly Binds a Human Vγ4+ T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity 2019, 51, 813–825.e4. [Google Scholar] [CrossRef]
- Hviid, L.; Akanmori, B.D.; Loizon, S.; Kurtzhals, J.A.L.; Ricke, C.H.; Lim, A.; Koram, K.A.; Nkrumah, F.K.; Mercereau-Puijalon, O.; Behr, C. High frequency of circulating γδ T cells with dominance of the Vδ1 subset in a healthy population. Int. Immunol. 2000, 12, 797–805. [Google Scholar] [CrossRef]
- Hunter, S.; Willcox, C.R.; Davey, M.S.; Kasatskaya, S.A.; Jeffery, H.C.; Chudakov, D.M.; Oo, Y.H.; Willcox, B.E. Human liver infiltrating γδ T cells are composed of clonally expanded circulating and tissue-resident populations. J. Hepatol. 2018, 69, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.T.; Parker, C.M.; Brenner, M.B.; Band, H. TCR usage and functional capabilities of human gamma delta T cells at birth. J. Immunol. 1994, 153, 3979–3988. [Google Scholar] [PubMed]
- Lalor, S.J.; McLoughlin, R.M. Memory γδ T Cells–Newly Appreciated Protagonists in Infection and Immunity. Trends Immunol. 2016, 37, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Pitard, V.; Roumanes, D.; Lafarge, X.; Couzi, L.; Garrigue, I.; Lafon, M.-E.; Merville, P.; Moreau, J.-F.; Déchanet-Merville, J. Long-term expansion of effector/memory Vδ2−γδ T cells is a specific blood signature of CMV infection. Blood 2008, 112, 1317–1324. [Google Scholar] [CrossRef]
- Willcox, B.E.; Willcox, C.R. γδ TCR ligands: The quest to solve a 500-million-year-old mystery. Nat. Immunol. 2019, 20, 121–128. [Google Scholar] [CrossRef]
- De Rosa, S.C.; Andrus, J.P.; Perfetto, S.P.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A.; Roederer, M. Ontogeny of gamma delta T cells in humans. J. Immunol. 2004, 172, 1637–1645. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Yang, L.; Li, B.; Chan, J.Y.-H.; Cai, D. TRGV and TRDV repertoire distribution and clonality of T cells from umbilical cord blood. Transpl. Immunol. 2009, 20, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.; Dimova, T.; Shey, M.; Briel, L.; Veldtsman, H.; Khomba, N.; Africa, H.; Steyn, M.; Hanekom, W.A.; Scriba, T.J.; et al. Fetal public Vγ9Vδ2 T cells expand and gain potent cytotoxic functions early after birth. Proc. Natl. Acad. Sci. USA 2020, 117, 18638–18648. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, A.; Ying, C.T.T.; Ayyadhury, S.; Puan, K.J.; Andiappan, A.K.; Nyunt, M.S.Z.; Shadan, N.B.; Mustafa, S.; Low, I.; Rotzschke, O.; et al. γ/δ T cell subsets in human aging using the classical α/β T cell model. J. Leukoc. Biol. 2014, 96, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Dimova, T.; Brouwer, M.; Gosselin, F.; Tassignon, J.; Leo, O.; Donner, C.; Marchant, A.; Vermijlen, D. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc. Natl. Acad. Sci. USA 2015, 112, E556–E565. [Google Scholar] [CrossRef] [PubMed]
- Cairo, C.; Armstrong, C.L.; Cummings, J.S.; Deetz, C.O.; Tan, M.; Lu, C.; Davis, C.E.; Pauza, C.D. Impact of age, gender, and race on circulating γδ T cells. Hum. Immunol. 2010, 71, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Jenkins, M.R.; Dash, P.; Watson, K.A.; Wang, Z.; Pizzolla, A.; Koutsakos, M.; Nguyen, T.H.; Lappas, M.; Crowe, J.; et al. Human γδ T-cell receptor repertoire is shaped by influenza viruses, age and tissue compartmentalisation. Clin. Transl. Immunol. 2019, 8, e1079. [Google Scholar] [CrossRef]
- Silva-Santos, B.; Strid, J. γδ T cells get adaptive. Nat. Immunol. 2017, 18, 370–372. [Google Scholar] [CrossRef]
- Parker, C.M.; Groh, V.; Band, H.; Porcelli, S.A.; Morita, C.; Fabbi, M.; Glass, D.; Strominger, J.L.; Brenner, M.B. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J. Exp. Med. 1990, 171, 1597–1612. [Google Scholar] [CrossRef]
- Michishita, Y.; Hirokawa, M.; Guo, Y.-M.; Abe, Y.; Liu, J.; Ubukawa, K.; Fujishima, N.; Fujishima, M.; Yoshioka, T.; Kameoka, Y.; et al. Age-associated alteration of γδ T-cell repertoire and different profiles of activation-induced death of Vδ1 and Vδ2 T cells. Int. J. Hematol. 2011, 94, 230–240. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Haehnel, K.; Janssen, N.; Demuth, I.; Pawelec, G. Peripheral blood T-cell signatures from high-resolution immune phenotyping of γδ and αβ T-cells in younger and older subjects in the Berlin Aging Study II. Immun. Ageing 2015, 12, 25. [Google Scholar] [CrossRef]
- Cairo, C.; Propp, N.; Auricchio, G.; Armstrong, C.L.; Abimiku, A.; Mancino, G.; Colizzi, V.; Blattner, W.; Pauza, C.D. Altered cord blood γδ T cell repertoire in Nigeria: Possible impacts of environmental factors on neonatal immunity. Mol. Immunol. 2008, 45, 3190–3197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vδ2 + T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9-subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.C.; Mitra, D.K.; Watanabe, N.; Herzenberg, L.A.; Herzenberg, L.A.; Roederer, M. Vdelta1 and Vdelta2 gammadelta T cells express distinct surface markers and might be developmentally distinct lineages. J. Leukoc. Biol. 2001, 70, 518–526. [Google Scholar] [PubMed]
- Colonna-Romano, G.; Potestio, M.; Aquino, A.; Candore, G.; Lio, D.; Caruso, C. Gamma/delta T lymphocytes are affected in the elderly. Exp. Gerontol. 2002, 37, 205–211. [Google Scholar] [CrossRef]
- van der Heiden, M.; Björkander, S.; Rahman Qazi, K.; Bittmann, J.; Hell, L.; Jenmalm, M.C.; Marchini, G.; Vermijlen, D.; Abrahamsson, T.; Nilsson, C.; et al. Characterization of the γδ T-cell compartment during infancy reveals clear differences between the early neonatal period and 2 years of age. Immunol. Cell Biol. 2020, 98, 79–87. [Google Scholar] [CrossRef]
- Caccamo, N.; La Mendola, C.; Orlando, V.; Meraviglia, S.; Todaro, M.; Stassi, G.; Sireci, G.; Fournié, J.J.; Dieli, F. Differentiation, phenotype, and function of interleukin-17–producing human Vγ9Vδ2 T cells. Blood 2011, 118, 129–138. [Google Scholar] [CrossRef]
- Caccamo, N.; Battistini, L.; Bonneville, M.; Poccia, F.; Fournié, J.J.; Meraviglia, S.; Borsellino, G.; Kroczek, R.A.; Mendola, C.L.; Scotet, E.; et al. CXCR5 Identifies a Subset of Vγ9Vδ2 T Cells which Secrete IL-4 and IL-10 and Help B Cells for Antibody Production. J. Immunol. 2006, 177, 5290–5295. [Google Scholar] [CrossRef]
- Patil, R.S.; Bhat, S.A.; Dar, A.A.; Chiplunkar, S.V. The Jekyll and Hyde story of IL17 producing γδT (Tγδ17) cells. Front. Immunol. 2015, 6, 37. [Google Scholar] [CrossRef]
- Mao, Y.; Yin, S.; Zhang, J.; Hu, Y.; Huang, B.; Cui, L.; Kang, N.; He, W. A new effect of IL-4 on human γδ T cells: Promoting regulatory Vδ1 T cells via IL-10 production and inhibiting function of Vδ2 T cells. Cell. Mol. Immunol. 2016, 13, 217–228. [Google Scholar] [CrossRef]
- Agrati, C.; Alonzi, T.; De Santis, R.; Castilletti, C.; Abbate, I.; Capobianchi, M.R.; D’Offizi, G.; Siepi, F.; Fimia, G.M.; Tripodi, M.; et al. Activation of Vγ9Vδ2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int. Immunol. 2006, 18, 11–18. [Google Scholar] [CrossRef]
- Legut, M.; Cole, D.K.; Sewell, A.K. The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell. Mol. Immunol. 2015, 12, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.-X.; Duan, J.; Li, M.-Q.; Xu, B.; Li, D.-J.; Jin, L.-P. The decidual gamma-delta T cells up-regulate the biological functions of trophoblasts via IL-10 secretion in early human pregnancy. Clin. Immunol. 2011, 141, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Bank, I. The Role of Gamma Delta T Cells in Autoimmune Rheumatic Diseases. Cells 2020, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, Y.; Lau, Y.-L.; Tu, W. γδ-T cells: An unpolished sword in human anti-infection immunity. Cell. Mol. Immunol. 2013, 10, 50–57. [Google Scholar] [CrossRef]
- Qin, G.; Liu, Y.; Zheng, J.; Ng, I.H.Y.; Xiang, Z.; Lam, K.-T.; Mao, H.; Li, H.; Peiris, J.S.M.; Lau, Y.-L.; et al. Type 1 Responses of Human Vγ9Vδ2 T Cells to Influenza A Viruses. J. Virol. 2011, 85, 10109–10116. [Google Scholar] [CrossRef]
- Dong, P.; Ju, X.; Yan, Y.; Zhang, S.; Cai, M.; Wang, H.; Chen, H.; Hu, Y.; Cui, L.; Zhang, J.; et al. γδ T Cells Provide Protective Function in Highly Pathogenic Avian H5N1 Influenza A Virus Infection. Front. Immunol. 2018, 9, 2812. [Google Scholar] [CrossRef]
- Kroca, M.; Johansson, A.; Sjöstedt, A.; Tärnvik, A. Vγ9Vδ2 T Cells in Human Legionellosis. Clin. Diagn. Lab. Immunol. 2001, 8, 949–954. [Google Scholar] [CrossRef]
- Poccia, F.; Gioia, C.; Martini, F.; Sacchi, A.; Piacentini, P.; Tempestilli, M.; Agrati, C.; Amendola, A.; Abdeddaim, A.; Vlassi, C.; et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vγ9Vδ2 T cells. AIDS 2009, 23, 555–565. [Google Scholar] [CrossRef]
- Laggner, U.; Meglio, P.D.; Perera, G.K.; Hundhausen, C.; Lacy, K.E.; Ali, N.; Smith, C.H.; Hayday, A.C.; Nickoloff, B.J.; Nestle, F.O. Identification of a Novel Proinflammatory Human Skin-Homing Vγ9Vδ2 T Cell Subset with a Potential Role in Psoriasis. J. Immunol. 2011, 187, 2783–2793. [Google Scholar] [CrossRef]
- Corvaisier, M.; Moreau-Aubry, A.; Diez, E.; Bennouna, J.; Mosnier, J.-F.; Scotet, E.; Bonneville, M.; Jotereau, F. Vγ9Vδ2 T Cell Response to Colon Carcinoma Cells. J. Immunol. 2005, 175, 5481–5488. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, D.; Zhen, W.; Shi, Q.; Liu, Y.; Ling, N.; Peng, M.; Tang, K.; Hu, P.; Hu, H.; et al. Characteristics of Circulating T Cell Receptor γδ T Cells from Individuals Chronically Infected with Hepatitis B Virus (HBV): An Association between Vδ2 Subtype and Chronic HBV Infection. J. Infect. Dis. 2008, 198, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, J.-Y.; Huang, A.; Li, Y.-Y.; Zhang, S.; Wei, J.; Xia, S.; Wan, Y.; Chen, W.; Zhang, Z.; et al. Decreased Vδ2 γδ T Cells Associated with Liver Damage by Regulation of Th17 Response in Patients with Chronic Hepatitis B. J. Infect. Dis. 2013, 208, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Sengeløv, H. The Role of Gamma Delta T Cells in Haematopoietic Stem Cell Transplantation. Scand. J. Immunol. 2015, 81, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Alejenef, A.; Pachnio, A.; Halawi, M.; Christmas, S.E.; Moss, P.A.H.; Khan, N. Cytomegalovirus drives Vδ2neg γδ T cell inflation in many healthy virus carriers with increasing age. Clin. Exp. Immunol. 2014, 176, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Szereday, L.; Baliko, Z.; Szekeres-Bartho, J. The role of Vδ2+T-cells in patients with active Mycobacterium tuberculosis infection and tuberculin anergy. Int. J. Tuberc. Lung Dis. 2008, 12, 262–268. [Google Scholar]
- Xi, X.; Han, X.; Li, L.; Zhao, Z. γδ T cells response to Mycobacterium tuberculosis in pulmonary tuberculosis patients using preponderant complementary determinant region 3 sequence. Indian J. Med. Res. 2011, 134, 356–361. [Google Scholar]
- Cordova, A.; Toia, F.; Mendola, C.L.; Orlando, V.; Meraviglia, S.; Rinaldi, G.; Todaro, M.; Cicero, G.; Zichichi, L.; Donni, P.L.; et al. Characterization of Human γδ T Lymphocytes Infiltrating Primary Malignant Melanomas. PLoS ONE 2012, 7, e49878. [Google Scholar] [CrossRef]
- Ramstead, A.G.; Jutila, M.A. Complex Role of γδ T-Cell-Derived Cytokines and Growth Factors in Cancer. J. Interferon Cytokine Res. 2012, 32, 563–569. [Google Scholar] [CrossRef]
- Tseng, C.-T.K.; Miskovsky, E.; Houghton, M.; Klimpel, G.R. Characterization of liver T-cell receptor γδ+ T cells obtained from individuals chronically infected with hepatitis C virus (HCV): Evidence for these T cells playing a role in the liver pathology associated with HCV infections. Hepatology 2001, 33, 1312–1320. [Google Scholar] [CrossRef]
- Rong, L.; Li, K.; Li, R.; Liu, H.-M.; Sun, R.; Liu, X.-Y. Analysis of tumor-infiltrating gamma delta T cells in rectal cancer. World J. Gastroenterol. 2016, 22, 3573–3580. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Wevers, E.; Schwartz, T.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; et al. Tumor-Infiltrating γδ T Lymphocytes Predict Clinical Outcome in Human Breast Cancer. J. Immunol. 2012, 189, 5029–5036. [Google Scholar] [CrossRef] [PubMed]
- Fisch, P.; Malkovsky, M.; Kovats, S.; Sturm, E.; Braakman, E.; Klein, B.S.; Voss, S.D.; Morrissey, L.W.; DeMars, R.; Welch, W.J.; et al. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science 1990, 250, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Bompas, E.; Neidhardt, E.M.; Rolland, F.; Philip, I.; Galéa, C.; Salot, S.; Saiagh, S.; Audrain, M.; Rimbert, M.; et al. Phase-I study of Innacell γδTM, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2008, 57, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; Scotet, E. Human Vγ9Vδ2 T cells: Promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 2006, 18, 539–546. [Google Scholar] [CrossRef]
- Kabelitz, D.; Wesch, D.; Pitters, E.; Zöller, M. Characterization of Tumor Reactivity of Human Vγ9Vδ2 γδ T Cells In Vitro and in SCID Mice In Vivo. J. Immunol. 2004, 173, 6767–6776. [Google Scholar] [CrossRef]
- Lamb, L.S.; Lopez, R.D. γδ T cells: A new frontier for immunotherapy? Biol. Blood Marrow Transplant. 2005, 11, 161–168. [Google Scholar] [CrossRef]
- Meraviglia, S.; Eberl, M.; Vermijlen, D.; Todaro, M.; Buccheri, S.; Cicero, G.; Mendola, C.L.; Guggino, G.; D’Asaro, M.; Orlando, V.; et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 2010, 161, 290–297. [Google Scholar] [CrossRef]
- Rei, M.; Pennington, D.J.; Silva-Santos, B. The Emerging Protumor Role of γδ T Lymphocytes: Implications for Cancer Immunotherapy. Cancer Res. 2015, 75, 798–802. [Google Scholar] [CrossRef]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.-F. Tumor-Infiltrating γδ T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef]
- Sullivan, L.C.; Shaw, E.M.; Stankovic, S.; I Snell, G.; Brooks, A.G.; Westall, G.P. The complex existence of γδ T cells following transplantation: The good, the bad and the simply confusing. Clin. Transl. Immunol. 2019, 8, e1078. [Google Scholar] [CrossRef]
- Arruda, L.C.M.; Gaballa, A.; Uhlin, M. Impact of γδ T cells on clinical outcome of hematopoietic stem cell transplantation: Systematic review and meta-analysis. Blood Adv. 2019, 3, 3436–3448. [Google Scholar] [CrossRef] [PubMed]
- Perko, R.; Kang, G.; Sunkara, A.; Leung, W.; Thomas, P.G.; Dallas, M.H. Gamma Delta T Cell Reconstitution Is Associated with Fewer Infections and Improved Event-Free Survival after Hematopoietic Stem Cell Transplantation for Pediatric Leukemia. Biol. Blood Marrow Transplant. 2015, 21, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ravens, S.; Schultze-Florey, C.; Raha, S.; Sandrock, I.; Drenker, M.; Oberdörfer, L.; Reinhardt, A.; Ravens, I.; Beck, M.; Geffers, R.; et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2017, 18, 393–401. [Google Scholar] [CrossRef] [PubMed]
- De Witte, M.A.; Sarhan, D.; Davis, Z.; Felices, M.; Vallera, D.A.; Hinderlie, P.; Curtsinger, J.; Cooley, S.; Wagner, J.; Kuball, J.; et al. Early Reconstitution of NK and γδ T Cells and Its Implication for the Design of Post-Transplant Immunotherapy. Biol. Blood Marrow Transplant. 2018, 24, 1152–1162. [Google Scholar] [CrossRef]
- Handgretinger, R.; Schilbach, K. The potential role of γδ T cells after allogeneic HCT for leukemia. Blood 2018, 131, 1063–1072. [Google Scholar] [CrossRef]
- Fujishima, N.; Hirokawa, M.; Fujishima, M.; Yamashita, J.; Saitoh, H.; Ichikawa, Y.; Horiuchi, T.; Kawabata, Y.; Sawada, K.-I. Skewed T cell receptor repertoire of Vδ1+ γδ T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein–Barr virus-infected B cells in clonal restriction. Clin. Exp. Immunol. 2007, 149, 70–79. [Google Scholar] [CrossRef]
- Lawand, M.; Déchanet-Merville, J.; Dieu-Nosjean, M.-C. Key Features of Gamma-Delta T-Cell Subsets in Human Diseases and Their Immunotherapeutic Implications. Front. Immunol. 2017, 8, 761. [Google Scholar] [CrossRef]
- Sciammas, R.; Bluestone, J.A. TCRγδ cells and viruses. Microbes Infect. 1999, 1, 203–212. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. Available online: https://www.hindawi.com/journals/jir/2018/5081634/ (accessed on 18 June 2020).
- Jackson, S.E.; Redeker, A.; Arens, R.; van Baarle, D.; van den Berg, S.P.H.; Benedict, C.A.; Čičin-Šain, L.; Hill, A.B.; Wills, M.R. CMV immune evasion and manipulation of the immune system with aging. GeroScience 2017, 39, 273–291. [Google Scholar] [CrossRef]
- Picarda, G.; Benedict, C.A. Cytomegalovirus: Shape-Shifting the Immune System. J. Immunol. 2018, 200, 3881–3889. [Google Scholar] [CrossRef]
- Souquette, A.; Frere, J.; Smithey, M.; Sauce, D.; Thomas, P.G. A constant companion: Immune recognition and response to cytomegalovirus with aging and implications for immune fitness. GeroScience 2017, 39, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, C.; Déchanet-Merville, J.; Capone, M. γδ T Cell-Mediated Immunity to Cytomegalovirus Infection. Front. Immunol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- About Cytomegalovirus and Congenital CMV Infection. Available online: https://www.cdc.gov/cmv/overview.html (accessed on 23 November 2020).
- Bayard, C.; Lepetitcorps, H.; Roux, A.; Larsen, M.; Fastenackels, S.; Salle, V.; Vieillard, V.; Marchant, A.; Stern, M.; Boddaert, J.; et al. Coordinated expansion of both memory T cells and NK cells in response to CMV infection in humans. Eur. J. Immunol. 2016, 46, 1168–1179. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, S.P.H.; Pardieck, I.N.; Lanfermeijer, J.; Sauce, D.; Klenerman, P.; van Baarle, D.; Arens, R. The hallmarks of CMV-specific CD8 T-cell differentiation. Med. Microbiol. Immunol. 2019, 208, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Liu, L.L.; Malmberg, J.-A.; Ivarsson, M.A.; Sohlberg, E.; Björklund, A.T.; Retière, C.; Sverremark-Ekström, E.; Traherne, J.; Ljungman, P.; et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 2013, 121, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Faist, B.; Fleischer, B.; Jacobsen, M. Cytomegalovirus Infection- and Age-Dependent Changes in Human CD8+ T-Cell Cytokine Expression Patterns. Clin. Vaccine Immunol. 2010, 17, 986–992. [Google Scholar] [CrossRef][Green Version]
- Goodier, M.R.; Jonjić, S.; Riley, E.M.; Lisnić, V.J. CMV and natural killer cells: Shaping the response to vaccination. Eur. J. Immunol. 2018, 48, 50–65. [Google Scholar] [CrossRef]
- Wallace, D.L.; Masters, J.E.; de Lara, C.M.; Henson, S.M.; Worth, A.; Zhang, Y.; Kumar, S.R.; Beverley, P.C.; Akbar, A.N.; Macallan, D.C. Human cytomegalovirus-specific CD8+ T-cell expansions contain long-lived cells that retain functional capacity in both young and elderly subjects. Immunology 2011, 132, 27–38. [Google Scholar] [CrossRef]
- Déchanet, J.; Merville, P.; Lim, A.; Retière, C.; Pitard, V.; Lafarge, X.; Michelson, S.; Méric, C.; Hallet, M.-M.; Kourilsky, P.; et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J. Clin. Investig. 1999, 103, 1437–1449. [Google Scholar] [CrossRef]
- Kallemeijn, M.J.; Boots, A.M.H.; van der Klift, M.Y.; Brouwer, E.; Abdulahad, W.H.; Verhaar, J.A.N.; van Dongen, J.J.M.; Langerak, A.W. Ageing and latent CMV infection impact on maturation, differentiation and exhaustion profiles of T-cell receptor gammadelta T-cells. Sci. Rep. 2017, 7, 5509. [Google Scholar] [CrossRef]
- Déchanet, J.; Merville, P.; Bergé, F.; Bone-Mane, G.; Taupin, J.-L.; Michel, P.; Joly, P.; Bonneville, M.; Potaux, L.; Moreau, J.-F. Major Expansion of γδ T Lymphocytes following Cytomegalovirus Infection in Kidney Allograft Recipients. J. Infect. Dis. 1999, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Madrigal, A.J.; Grace, S.; Sivakumaran, J.; Kottaridis, P.; Mackinnon, S.; Travers, P.J.; Lowdell, M.W. The role of Vδ2-negative γδ T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood 2010, 116, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Mourin, G.; Larsen, M.; Fastenackels, S.; Urrutia, A.; Gorochov, G.; Autran, B.; Donner, C.; Sidi, D.; Sibony-Prat, J.; et al. Differential Impact of Age and Cytomegalovirus Infection on the γδ T Cell Compartment. J. Immunol. 2013, 191, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Wistuba-Hamprecht, K.; Frasca, D.; Blomberg, B.; Pawelec, G.; Derhovanessian, E. Age-associated alterations in γδ T-cells are present predominantly in individuals infected with Cytomegalovirus. Immun. Ageing 2013, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Vermijlen, D.; Brouwer, M.; Donner, C.; Liesnard, C.; Tackoen, M.; Van Rysselberge, M.; Twité, N.; Goldman, M.; Marchant, A.; Willems, F. Human cytomegalovirus elicits fetal γδ T cell responses in utero. J. Exp. Med. 2010, 207, 807–821. [Google Scholar] [CrossRef]
- Halary, F.; Pitard, V.; Dlubek, D.; Krzysiek, R.; de la Salle, H.; Merville, P.; Dromer, C.; Emilie, D.; Moreau, J.-F.; Déchanet-Merville, J. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 2005, 201, 1567–1578. [Google Scholar] [CrossRef]
- Willcox, C.R.; Pitard, V.; Netzer, S.; Couzi, L.; Salim, M.; Silberzahn, T.; Moreau, J.-F.; Hayday, A.C.; Willcox, B.E.; Déchanet-Merville, J. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 2012, 13, 872–879. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Aging, cytomegalovirus (CMV) and influenza vaccine responses. Hum. Vaccines Immunother. 2016, 12, 682–690. [Google Scholar] [CrossRef]
- Merani, S.; Pawelec, G.; Kuchel, G.A.; McElhaney, J.E. Impact of Aging and Cytomegalovirus on Immunological Response to Influenza Vaccination and Infection. Front. Immunol. 2017, 8, 784. [Google Scholar] [CrossRef]
- Oshansky, C.M.; Gartland, A.J.; Wong, S.-S.; Jeevan, T.; Wang, D.; Roddam, P.L.; Caniza, M.A.; Hertz, T.; DeVincenzo, J.P.; Webby, R.J.; et al. Mucosal Immune Responses Predict Clinical Outcomes during Influenza Infection Independently of Age and Viral Load. Am. J. Respir. Crit. Care Med. 2013, 189, 449–462. [Google Scholar] [CrossRef]
- Castle, S.C. Clinical Relevance of Age-Related Immune Dysfunction. Clin. Infect. Dis. 2000, 31, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Jojic, V.; Sharma, S.; Shen-Orr, S.S.; Angel, C.J.L.; Onengut-Gumuscu, S.; Kidd, B.A.; Maecker, H.T.; Concannon, P.; Dekker, C.L.; et al. Cytomegalovirus infection enhances the immune response to influenza. Sci. Transl. Med. 2015, 7, 281ra43. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Mao, H.; Zheng, J.; Sia, S.F.; Liu, Y.; Chan, P.-L.; Lam, K.-T.; Peiris, J.S.M.; Lau, Y.-L.; Tu, W. Phosphoantigen-Expanded Human γδ T Cells Display Potent Cytotoxicity against Monocyte-Derived Macrophages Infected with Human and Avian Influenza Viruses. J. Infect. Dis. 2009, 200, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Liu, Y.; Zheng, J.; Xiang, Z.; Ng, I.H.Y.; Malik Peiris, J.S.; Lau, Y.-L.; Tu, W. Phenotypic and Functional Characterization of Human γδ T-Cell Subsets in Response to Influenza A Viruses. J. Infect. Dis. 2012, 205, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiang, Z.; Feng, T.; Li, J.; Liu, Y.; Fan, Y.; Lu, Q.; Yin, Z.; Yu, M.; Shen, C.; et al. Human Vγ9Vδ2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cell. Mol. Immunol. 2013, 10, 159–164. [Google Scholar] [CrossRef]
- Tu, W.; Zheng, J.; Liu, Y.; Sia, S.F.; Liu, M.; Qin, G.; Ng, I.H.Y.; Xiang, Z.; Lam, K.-T.; Peiris, J.S.M.; et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice. J. Exp. Med. 2011, 208, 1511–1522. [Google Scholar] [CrossRef]
- Stervbo, U.; Pohlmann, D.; Baron, U.; Bozzetti, C.; Jürchott, K.; Mälzer, J.N.; Nienen, M.; Olek, S.; Roch, T.; Schulz, A.R.; et al. Age dependent differences in the kinetics of γδ T cells after influenza vaccination. PLoS ONE 2017, 12, e0181161. [Google Scholar] [CrossRef]
- McArdle, A.J.; Turkova, A.; Cunnington, A.J. When do co-infections matter? Curr. Opin. Infect. Dis. 2018, 31, 209–215. [Google Scholar] [CrossRef]
- Souquette, A.; Thomas, P.G. Past Life and Future Effects—How Heterologous Infections Alter Immunity to Influenza Viruses. Front. Immunol. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Balbi, B.; Valle, M.T.; Oddera, S.; Giunti, O.; Manca, F.; Rossi, G.A.; Allegra, L. T-Lymphocytes with γδ + Vδ2+ Antigen Receptors Are Present in Increased Proportions in a Fraction of Patients with Tuberculosis or with Sarcoidosis. Am. Rev. Respir. Dis. 1993, 148, 1685–1690. [Google Scholar] [CrossRef]
- Janis, E.M.; Kaufmann, S.H.; Schwartz, R.H.; Pardoll, D.M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science 1989, 244, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Meraviglia, S.; El Daker, S.; Dieli, F.; Martini, F.; Martino, A. γδ T Cells Cross-Link Innate and Adaptive Immunity in Mycobacterium Tuberculosis Infection. Available online: https://www.hindawi.com/journals/jir/2011/587315/ (accessed on 14 August 2020).
- Ding, Y.; Ma, F.; Wang, Z.; Li, B. Characteristics of the Vδ2 CDR3 Sequence of Peripheral γδ T Cells in Patients with Pulmonary Tuberculosis and Identification of a New Tuberculosis-Related Antigen Peptide. Clin. Vaccine Immunol. 2015, 22, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, B.; Gao, L.; Liu, J.; Chen, X.; Huang, H.; Zhao, Z. Next generation sequencing reveals changes of the γδ T cell receptor repertoires in patients with pulmonary tuberculosis. Sci. Rep. 2018, 8, 3956. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Han, X.; Li, L.; Zhao, Z. Identification of a New Tuberculosis Antigen Recognized by γδ T Cell Receptor. Clin. Vaccine Immunol. 2013, 20, 530–539. [Google Scholar] [CrossRef]
- Constant, P.; Poquet, Y.; Peyrat, M.A.; Davodeau, F.; Bonneville, M.; Fournié, J.J. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human gamma delta T cells. Infect. Immun. 1995, 63, 4628–4633. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, D.; Qiu, L.; Lai, X.; Simon, M.; Shen, L.; Kou, Z.; Wang, Q.; Jiang, L.; Estep, J.; et al. Adaptive Immune Response of Vγ2Vδ2+ T Cells During Mycobacterial Infections. Science 2002, 295, 2255–2258. [Google Scholar] [CrossRef]
- Cairo, C.; Mancino, G.; Cappelli, G.; Pauza, C.D.; Galli, E.; Brunetti, E.; Colizzi, V. Vδ2 T-lymphocyte responses in cord blood samples from Italy and Côte d’Ivoire. Immunology 2008, 124, 380–387. [Google Scholar] [CrossRef]
- Panchamoorthy, G.; McLean, J.; Modlin, R.L.; Morita, C.T.; Ishikawa, S.; Brenner, M.B.; Band, H. A predominance of the T cell receptor V gamma 2/V delta 2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J. Immunol. 1991, 147, 3360–3369. [Google Scholar]
- Vesosky, B.; Turner, J. The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol. Rev. 2005, 205, 229–243. [Google Scholar] [CrossRef]
- Vesosky, B.; Flaherty, D.K.; Rottinghaus, E.K.; Beamer, G.L.; Turner, J. Age dependent increase in early resistance of mice to Mycobacterium tuberculosis is associated with an increase in CD8 T cells that are capable of antigen independent IFN-γ production. Exp. Gerontol. 2006, 41, 1185–1194. [Google Scholar] [CrossRef]
- Piergallini, T.J.; Turner, J. Tuberculosis in the elderly: Why inflammation matters. Exp. Gerontol. 2018, 105, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Eberl, M.; Roberts, G.W.; Meuter, S.; Williams, J.D.; Topley, N.; Moser, B. A Rapid Crosstalk of Human γδ T Cells and Monocytes Drives the Acute Inflammation in Bacterial Infections. PLoS Pathog. 2009, 5, e1000308. [Google Scholar] [CrossRef] [PubMed]
- Puan, K.-J.; Jin, C.; Wang, H.; Sarikonda, G.; Raker, A.M.; Lee, H.K.; Samuelson, M.I.; Märker-Hermann, E.; Pasa-Tolic, L.; Nieves, E.; et al. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int. Immunol. 2007, 19, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Ding, Y.-P.; Tanaka, Y.; Shen, L.-W.; Wei, C.-H.; Minato, N.; Zhang, W. γδ T Cells and Their Potential for Immunotherapy. Int. J. Biol. Sci. 2014, 10, 119–135. [Google Scholar] [CrossRef]
- Pauza, C.D.; Poonia, B.; Li, H.; Cairo, C.; Chaudhry, S. γδ T Cells in HIV Disease: Past, Present, and Future. Front. Immunol. 2015, 5, 687. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Eriksson, E.M. γδ T-cell responses during HIV infection and antiretroviral therapy. Clin. Transl. Immunol. 2019, 8, e01069. [Google Scholar] [CrossRef]
- Belkina, A.C.; Starchenko, A.; Drake, K.A.; Proctor, E.A.; Pihl, R.M.F.; Olson, A.; Lauffenburger, D.A.; Lin, N.; Snyder-Cappione, J.E. Multivariate Computational Analysis of Gamma Delta T cell Inhibitory Receptor Signatures Reveals the Divergence of Healthy and ART-Suppressed HIV+ Aging. Front. Immunol. 2018, 9, 2783. [Google Scholar] [CrossRef]
- Fang, H.; Welte, T.; Zheng, X.; Chang, G.-J.J.; Holbrook, M.R.; Soong, L.; Wang, T. γδd T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol. Med. Microbiol. 2010, 59, 71–80. [Google Scholar] [CrossRef]
- Kallemeijn, M.J.; Kavelaars, F.G.; van der Klift, M.Y.; Wolvers-Tettero, I.L.M.; Valk, P.J.M.; van Dongen, J.J.M.; Langerak, A.W. Next-Generation Sequencing Analysis of the Human TCRγδ+ T-Cell Repertoire Reveals Shifts in Vγ- and Vδ-Usage in Memory Populations upon Aging. Front. Immunol. 2018, 9, 448. [Google Scholar] [CrossRef]
- Lei, L.; Qian, H.; Yang, X.; Zhang, X.; Zhang, D.; Dai, T.; Guo, R.; Shi, L.; Cheng, Y.; Zhang, B.; et al. The phenotypic changes of γδ T cells in COVID-19 patients. J. Cell. Mol. Med. 2020, 24, 11603–11606. [Google Scholar] [CrossRef]
- Deroost, K.; Langhorne, J. Gamma/Delta T Cells and Their Role in Protection Against Malaria. Front. Immunol. 2018, 9, 2973. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Freedman, M.S. CD16+ γδ T cells mediate antibody dependent cellular cytotoxicity: Potential mechanism in the pathogenesis of multiple sclerosis. Clin. Immunol. 2008, 128, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Usui, T.; Kobayashi, S.; Iguchi-Hashimoto, M.; Ito, H.; Yoshitomi, H.; Nakamura, T.; Shimizu, M.; Kawabata, D.; Yukawa, N.; et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009, 60, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Papotto, P.H.; Reinhardt, A.; Prinz, I.; Silva-Santos, B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 2018, 87, 26–37. [Google Scholar] [CrossRef] [PubMed]
| Subset(s) | Location | Age | Effector Molecules | Disease State * |
|---|---|---|---|---|
| Vδ2+ | Periphery | Cord Blood | IFNγ ** | Homeostasis [55] |
| Vδ2+ | Periphery | Neonates (14 d) | IFNγ | Homeostasis [55] |
| Vδ1+Vδ2+ | Periphery | Infant (4 m) | IFNγ, perforin | Homeostasis [53] |
| Vγ9+Vδ2+ | Periphery | Infant (1 y) | IFNγ | Homeostasis [53] |
| Vδ2+ | Periphery | Children (2–5 y) | IFNγ | Homeostasis [55] |
| Vγ9+Vδ2+ | Periphery, Cerebrospinal fluid | Children (3–14 y) | IL-17A ** | Bacterial meningitis [56] |
| Vγ9+Vδ2+ | Periphery, Tonsils | Children (9–14 y) | IL-2, IL-4, IL-10 | Tonsillectomy patients [57] (Caccamo 2006) |
| Vγ9+Vδ2+ | Periphery | Adult | IFNγ, TNFα **, IL-2, IL-4, IL-10, IL-17A | Homeostasis [26,53,56,57,58,59,60,61] |
| Vδ1+ | Periphery | Adult | Perforin, granzymes | Homeostasis [26] |
| Unspecified | Periphery | Adult | IFNγ, TNFα, IL-6, IL-17A, IL-10 | Homeostasis [59] |
| Unspecified | Mucous Membrane | Adult | IL-10, TGF-β **, TNFα, IFNγ, IL-4, IL-2 | Pregnancy [62] |
| Vγ9+Vδ2+ | Periphery | Adult | IFNγ, TNFα, IL-17A | Rheumatic disease [63] |
| Vγ9+Vδ2+ | Periphery | Adult | IFNγ, CCL3 **, CCL4, CCL5 | Influenza [64,65,66] |
| Vγ9+Vδ2+ | Periphery | Adult | IFNγ, TNFα | Legionellosis [67] |
| Vγ9+Vδ2+ | Periphery | Adult | IFNγ | Human immunodeficiency virus (HIV) [68] |
| Vγ9+Vδ2+ | Lesions | Adult | IL-17A, IL-8, IFNγ, TNFα | Psoriasis [69] |
| Vγ9+Vδ2+ | Tumor-infiltrating | Adult | IFNγ, TNFα | Colon carcinoma [70] |
| Vδ2+ | Periphery | Adult | IFNγ, IL-17A | Hepatitis B virus (HBV) [71,72] |
| Vδ2− | Periphery | Adult | IFNγ, TNFα | Human cytomegalovirus (HCMV) [64,73,74] |
| Vδ1+ | Periphery, Synovial fluid | Adult | IFNγ, IL-4 | Rheumatic disease [63] |
| Unspecified | Periphery | Adult | IFNγ, TNFα, IL-4, IL-10 | Mycobacterium tuberculosis [75,76] |
| Unspecified | Periphery | Adult | IL-17A, TNFα | Various bacterial infections, Plasmodium falciparum [64] |
| Unspecified | Tumor-infiltrating | Adult | IFNγ, TNFα, IL-17A, IL-4, TNFβ | Miscellaneous adult cancers [77,78] |
| Unspecified | Liver-derived celllines | Adult | IFNγ, TNFα, IL-8 | Viral hepatitis [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, B.L.; Thomas, P.G. A Cell for the Ages: Human γδ T Cells across the Lifespan. Int. J. Mol. Sci. 2020, 21, 8903. https://doi.org/10.3390/ijms21238903
Clark BL, Thomas PG. A Cell for the Ages: Human γδ T Cells across the Lifespan. International Journal of Molecular Sciences. 2020; 21(23):8903. https://doi.org/10.3390/ijms21238903
Chicago/Turabian StyleClark, Brandi L., and Paul G. Thomas. 2020. "A Cell for the Ages: Human γδ T Cells across the Lifespan" International Journal of Molecular Sciences 21, no. 23: 8903. https://doi.org/10.3390/ijms21238903
APA StyleClark, B. L., & Thomas, P. G. (2020). A Cell for the Ages: Human γδ T Cells across the Lifespan. International Journal of Molecular Sciences, 21(23), 8903. https://doi.org/10.3390/ijms21238903





