Functional Domain Order of an Anti-EGFR × Anti-CD16 Bispecific Diabody Involving NK Cell Activation
Abstract
1. Introduction
2. Results
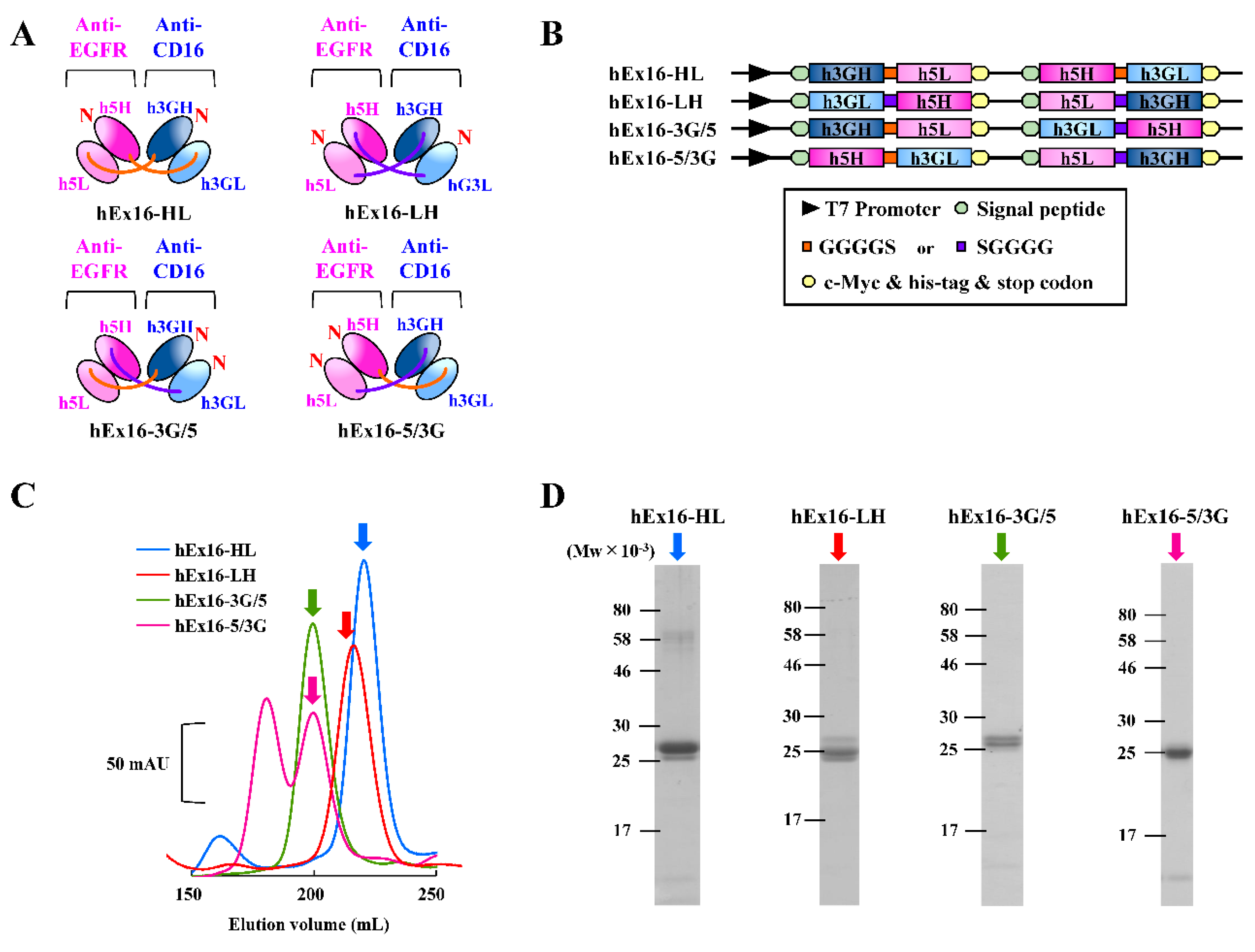
2.1. Preparation of hEx16-Dbs with Four Different Domain Orders
2.2. Effect of the Domain Order of hEx16-Dbs on Growth Inhibition
2.3. Preparation of hEx16-scDbs with Different Domain Orders
2.4. Effect of the Domain Order of hEx16-scDbs on Growth Inhibition
2.5. Binding Ability of Each hEx16-Db
2.6. Cytokine Release Induced by hEx16-Db Binding
3. Discussion
4. Materials and Methods
4.1. Construction of Expression Vectors for hEx16-Dbs and hEx16-scDbs
4.2. Preparation of hEx16-Dbs and hEx16-scDbs
4.3. In Vitro Growth Inhibition Assay
4.4. Surface Plasmon Resonance Spectroscopy
4.5. Confirmation of Cross-Linking Ability
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Cell Lines
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bsAbs | bispecific antibodies |
| bsDbs | bispecific diabodies |
| VH | variable heavy domain |
| VL | variable light domain |
| EGFR | epidermal growth factor receptor |
| TCR | T-cell receptor |
| NK | natural killer |
| Dbs | diabodies |
| scDbs | single-chain diabodies |
| taFvs | tandem single-chain variable fragments |
| CTLs | cytotoxic T lymphocytes |
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| PBMCs | peripheral blood mononuclear cells |
| IMAC | immobilized metal affinity chromatography |
| PBS | phosphate buffered saline |
| MTS | 3-(4,5-dimethylthiazole-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt |
| sEGFR | soluble EGFR |
| sCD16 | soluble CD16 |
| ELISA | enzyme-linked immunosorbent assays |
| TNF | tumor necrosis factor |
| IFN | interferon |
References
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to watch in 2020. MAbs 2020, 12, 1703531. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.M.; Trinh, H.; Le, J.; Siegel, S.; Shealy, D.; McDonough, M.; Scallon, B.; Moore, M.A.; Vilcek, J.; Daddona, P.; et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol. Immunol. 1993, 30, 1443–1453. [Google Scholar] [CrossRef]
- Nunez-Prado, N.; Compte, M.; Harwood, S.; Alvarez-Mendez, A.; Lykkemark, S.; Sanz, L.; Alvarez-Vallina, L. The coming of age of engineered multivalent antibodies. Drug Discov. Today 2015, 20, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Rathi, C.; Meibohm, B. Clinical Pharmacology of Bispecific Antibody Constructs. J. Clin. Pharmacol. 2015, 55, S21–S28. [Google Scholar] [CrossRef]
- Holliger, P.; Prospero, T.; Winter, G. Diabodies—Small Bivalent and Bispecific Antibody Fragments. Proc. Natl. Acad. Sci. USA 1993, 90, 6444–6448. [Google Scholar] [CrossRef]
- Stork, R.; Campigna, E.; Robert, B.; Muller, D.; Kontermann, R.E. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J. Biol. Chem. 2009, 284, 25612–25619. [Google Scholar] [CrossRef]
- Bargou, R.; Leo, E.; Zugmaier, G.; Klinger, M.; Goebeler, M.; Knop, S.; Noppeney, R.; Viardot, A.; Hess, G.; Schuler, M.; et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008, 321, 974–977. [Google Scholar] [CrossRef]
- Shahied, L.S.; Tang, Y.; Alpaugh, R.K.; Somer, R.; Greenspon, D.; Weiner, L.M. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J. Biol. Chem. 2004, 279, 53907–53914. [Google Scholar] [CrossRef]
- Portner, L.M.; Schonberg, K.; Hejazi, M.; Brunnert, D.; Neumann, F.; Galonska, L.; Reusch, U.; Little, M.; Haas, R.; Uhrberg, M. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 x CD3 or CD19 x CD16. Cancer Immunol. Immun. 2012, 61, 1869–1875. [Google Scholar] [CrossRef]
- Robinson, M.K.; Doss, M.; Shaller, C.; Narayanan, D.; Marks, J.D.; Adler, L.P.; Gonzalez Trotter, D.E.; Adams, G.P. Quantitative immuno-positron emission tomography imaging of HER2-positive tumor xenografts with an iodine-124 labeled anti-HER2 diabody. Cancer Res. 2005, 65, 1471–1478. [Google Scholar] [CrossRef]
- Sundaresan, G.; Yazaki, P.J.; Shively, J.E.; Finn, R.D.; Larson, S.M.; Raubitschek, A.A.; Williams, L.E.; Chatziioannou, A.F.; Gambhir, S.S.; Wu, A.M. 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J. Nucl. Med. 2003, 44, 1962–1969. [Google Scholar] [PubMed]
- Kipriyanov, S.M.; Cochlovius, B.; Schafer, H.J.; Moldenhauer, G.; Bahre, A.; Le Gall, F.; Knackmuss, S.; Little, M. Synergistic antitumor effect of bispecific CD19 x CD3 and CD19 x CD16 diabodies in a preclinical model of non-Hodgkin’s lymphoma. J. Immunol. 2002, 169, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, G.; Chu, Y.; Cairo, M.S. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front. Oncol. 2019, 9, 51. [Google Scholar] [CrossRef]
- Mandelboim, O.; Malik, P.; Davis, D.M.; Jo, C.H.; Boyson, J.E.; Strominger, J.L. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc. Natl. Acad. Sci. USA 1999, 96, 5640–5644. [Google Scholar] [CrossRef]
- Ellwanger, K.; Reusch, U.; Fucek, I.; Wingert, S.; Ross, T.; Muller, T.; Schniegler-Mattox, U.; Haneke, T.; Rajkovic, E.; Koch, J.; et al. Redirected optimized cell killing (ROCK(R)): A highly versatile multispecific fit-for-purpose antibody platform for engaging innate immunity. MAbs 2019, 11, 899–918. [Google Scholar] [CrossRef]
- Asano, R.; Kumagai, T.; Nagai, K.; Taki, S.; Shimomura, I.; Arai, K.; Ogata, H.; Okada, M.; Hayasaka, F.; Sanada, H.; et al. Domain order of a bispecific diabody dramatically enhances its antitumor activity beyond structural format conversion: The case of the hEx3 diabody. Protein Eng. Des. Sel. 2013, 26, 359–367. [Google Scholar] [CrossRef]
- Asano, R.; Nagai, K.; Makabe, K.; Takahashi, K.; Kumagai, T.; Kawaguchi, H.; Ogata, H.; Arai, K.; Umetsu, M.; Kumagai, I. Structural considerations for functional anti-EGFR x anti-CD3 bispecific diabodies in light of domain order and binding affinity. Oncotarget 2018, 9, 13884–13893. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Umetsu, M.; Nakazawa, H.; Niide, T.; Onodera, T.; Hosokawa, K.; Hattori, S.; Asano, R.; Kumagai, I. A semi high-throughput method for screening small bispecific antibodies with high cytotoxicity. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Wucherpfennig, K.W.; Gagnon, E.; Call, M.J.; Huseby, E.S.; Call, M.E. Structural Biology of the T-cell Receptor: Insights into Receptor Assembly, Ligand Recognition, and Initiation of Signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a005140. [Google Scholar] [CrossRef]
- Dong, D.; Zheng, L.; Lin, J.; Zhang, B.; Zhu, Y.; Li, N.; Xie, S.; Wang, Y.; Gao, N.; Huang, Z. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature 2019, 573, 546–552. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Kadowaki, K.; Udaka, S. Screening and Characterization of Protein-Hyperproducing Bacteria without Detectable Exoprotease Activity. Agric. Biol. Chem. 1989, 53, 691–699. [Google Scholar]
- Zou, C.; Duan, X.G.; Wu, J. Efficient extracellular expression of Bacillus deramificans pullulanase in Brevibacillus choshinensis. J. Ind. Microbiol. Biotechnol. 2016, 43, 495–504. [Google Scholar] [CrossRef]
- Ilk, N.; Schumi, C.T.; Bohle, B.; Egelseer, E.M.; Sleytr, U.B. Expression of an endotoxin-free S-layer/allergen fusion protein in gram-positive Bacillus subtilis 1012 for the potential application as vaccines for immunotherapy of atopic allergy. Microb. Cell Factories 2011, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Kuroki, Y.; Honma, S.; Akabane, M.; Watanabe, S.; Mayuzumi, S.; Hiyamuta, S.; Kumagai, I.; Sode, K. Comprehensive study of domain rearrangements of single-chain bispecific antibodies to determine the best combination of configurations and microbial host cells. Mabs 2018, 10, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, P.; Huber, R.; Oosthuizen, V.; Jacob, U. The 3.2-angstrom crystal structure of the human IgG1 Fc fragment-Fc gamma RIII complex. Nature 2000, 406, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Nakayama, M.; Kawaguchi, H.; Kubota, T.; Nakanishi, T.; Umetsu, M.; Hayashi, H.; Katayose, Y.; Unno, M.; Kudo, T.; et al. Construction and humanization of a functional bispecific EGFR CD16 diabody using a refolding system. FEBS J. 2012, 279, 223–233. [Google Scholar] [CrossRef]
- Makabe, K.; Nakanishi, T.; Tsumoto, K.; Tanaka, Y.; Kondo, H.; Umetsu, M.; Sone, Y.; Asano, R.; Kumagai, I. Thermodynamic consequences of mutations in vernier zone residues of a humanized anti-human epidermal growth factor receptor murine antibody, 528. J. Biol. Chem. 2008, 283, 1156–1166. [Google Scholar] [CrossRef]
- Nakanishi, T.; Maru, T.; Tahara, K.; Sanada, H.; Umetsu, M.; Asano, R.; Kumagai, I. Development of an affinity-matured humanized anti-epidermal growth factor receptor antibody for cancer immunotherapy. Protein Eng. Des. Sel. 2013, 26, 113–122. [Google Scholar] [CrossRef]
- Sanada, H.; Kobayashi, K.; Oyama, K.; Maru, T.; Nakanishi, T.; Umetsu, M.; Asano, R.; Kumagai, I. Affinity maturation of humanized anti-epidermal growth factor receptor antibody using a modified phage-based open sandwich selection method. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Asano, R.; Hosokawa, K.; Taki, S.; Konno, S.; Shimomura, I.; Ogata, H.; Okada, M.; Arai, K.; Onitsuka, M.; Omasa, T.; et al. Build-up functionalization of anti-EGFR x anti-CD3 bispecific diabodies by integrating high-affinity mutants and functional molecular formats. Sci. Rep. 2020, 10, 4913. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Uggla, C.K.; Geisberg, M.; Jondal, M.; Knowles, R.W. Agonistic effects of anti-CD2 and anti-CD16 antibodies on human natural killer killing. Scand. J. Immunol. 1989, 29, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Wuellner, U.; Klupsch, K.; Buller, F.; Attinger-Toller, I.; Santimaria, R.; Zbinden, I.; Henne, P.; Grabulovski, D.; Bertschinger, J.; Brack, S. Bispecific CD3/HER2 Targeting FynomAb Induces Redirected T Cell-Mediated Cytolysis with High Potency and Enhanced Tumor Selectivity. Antibodies 2015, 4, 426–440. [Google Scholar] [CrossRef]
- Asano, R.; Sone, Y.; Makabe, K.; Tsumoto, K.; Hayashi, H.; Katayose, Y.; Unno, M.; Kudo, T.; Kumagai, I. Humanization of the bispecific epidermal growth factor receptor x CD3 diabody and its efficacy as a potential clinical reagent. Clin. Cancer Res. 2006, 12, 4036–4042. [Google Scholar] [CrossRef]
- Saijyo, S.; Kudo, T.; Suzuki, M.; Katayose, Y.; Shinoda, M.; Muto, T.; Fukuhara, K.; Suzuki, T.; Matsuno, S. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J. Exp. Med. 1995, 177, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Asano, R.; Arai, K.; Shimomura, I.; Ogata, H.; Kawaguchi, H.; Hayashi, H.; Ohtsuka, H.; Yoshida, H.; Katayose, Y.; et al. In vitro and in vivo antitumor effects of recombinant bispecific antibodies based on humanized anti-EGFR antibody. Oncol. Rep. 2011, 26, 949–955. [Google Scholar]
- Nakadate, Y.; Kodera, Y.; Kitamura, Y.; Shirasawa, S.; Tachibana, T.; Tamura, T.; Koizumi, F. KRAS mutation confers resistance to antibody-dependent cellular cytotoxicity of cetuximab against human colorectal cancer cells. Int. J. Cancer 2014, 134, 2146–2155. [Google Scholar] [CrossRef]
- Sondermann, P.; Jacob, U. Human Fcgamma receptor IIb expressed in Escherichia coli reveals IgG binding capability. Biol. Chem. 1999, 380, 717–721. [Google Scholar] [CrossRef]
- Osaki, T.; Fujisawa, S.; Kitaguchi, M.; Kitamura, M.; Nakanishi, T. Development of a bispecific antibody tetramerized through hetero-associating peptides. FEBS J. 2015, 282, 4389–4401. [Google Scholar] [CrossRef]





| sEGFR | sCD16 | |||||
|---|---|---|---|---|---|---|
| kon (×104 M−1s−1) | koff (×10−2 s−1) | KD (×10−8 M) | kon (×104 M−1s−1) | koff (×10−2 s−1) | KD (×10−8 M) | |
| hEx16-HL | 7.44 | 0.637 | 8.57 | 2.45 | 1.45 | 59.1 |
| hEx16-LH | 6.66 | 0.877 | 13.2 | 5.07 | 2.69 | 53.2 |
| hEx16-3G/5 | 0.000742 | 0.00106 | 143 | 4.33 | 2.29 | 52.8 |
| hEx16-5/3G | 3.76 | 0.801 | 21.3 | 0.0073 | 2.24 | 30,600 |
| sEGFR | sCD16 | |||||
|---|---|---|---|---|---|---|
| kon (×104 M−1s−1) | koff (×10−2 s−1) | KD (×10−8 M) | kon (×104 M−1s−1) | koff (×10−2 s−1) | KD (×10−8 M) | |
| hEx16-scDb-HL | 18.0 | 0.470 | 2.61 | 10.5 | 2.59 | 24.7 |
| hEx16-scDb-LH | 10.6 | 0.170 | 1.60 | 2.77 | 0.579 | 20.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwahara, A.; Nagai, K.; Nakanishi, T.; Kumagai, I.; Asano, R. Functional Domain Order of an Anti-EGFR × Anti-CD16 Bispecific Diabody Involving NK Cell Activation. Int. J. Mol. Sci. 2020, 21, 8914. https://doi.org/10.3390/ijms21238914
Kuwahara A, Nagai K, Nakanishi T, Kumagai I, Asano R. Functional Domain Order of an Anti-EGFR × Anti-CD16 Bispecific Diabody Involving NK Cell Activation. International Journal of Molecular Sciences. 2020; 21(23):8914. https://doi.org/10.3390/ijms21238914
Chicago/Turabian StyleKuwahara, Atsushi, Keisuke Nagai, Takeshi Nakanishi, Izumi Kumagai, and Ryutaro Asano. 2020. "Functional Domain Order of an Anti-EGFR × Anti-CD16 Bispecific Diabody Involving NK Cell Activation" International Journal of Molecular Sciences 21, no. 23: 8914. https://doi.org/10.3390/ijms21238914
APA StyleKuwahara, A., Nagai, K., Nakanishi, T., Kumagai, I., & Asano, R. (2020). Functional Domain Order of an Anti-EGFR × Anti-CD16 Bispecific Diabody Involving NK Cell Activation. International Journal of Molecular Sciences, 21(23), 8914. https://doi.org/10.3390/ijms21238914





