Intracerebroventricular Neuropeptide FF Diminishes the Number of Apneas and Cardiovascular Effects Produced by Opioid Receptors’ Activation
Abstract
1. Introduction
2. Results
2.1. The Effects of Iv NPFF on Cardiovascular and Respiratory Pattern
2.2. The Effects of Iv NPFF on Apnea and Cardiovascular Effects Induced by EM-1
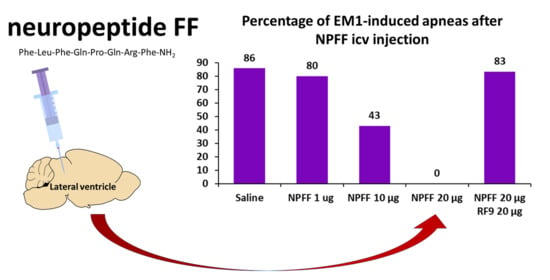
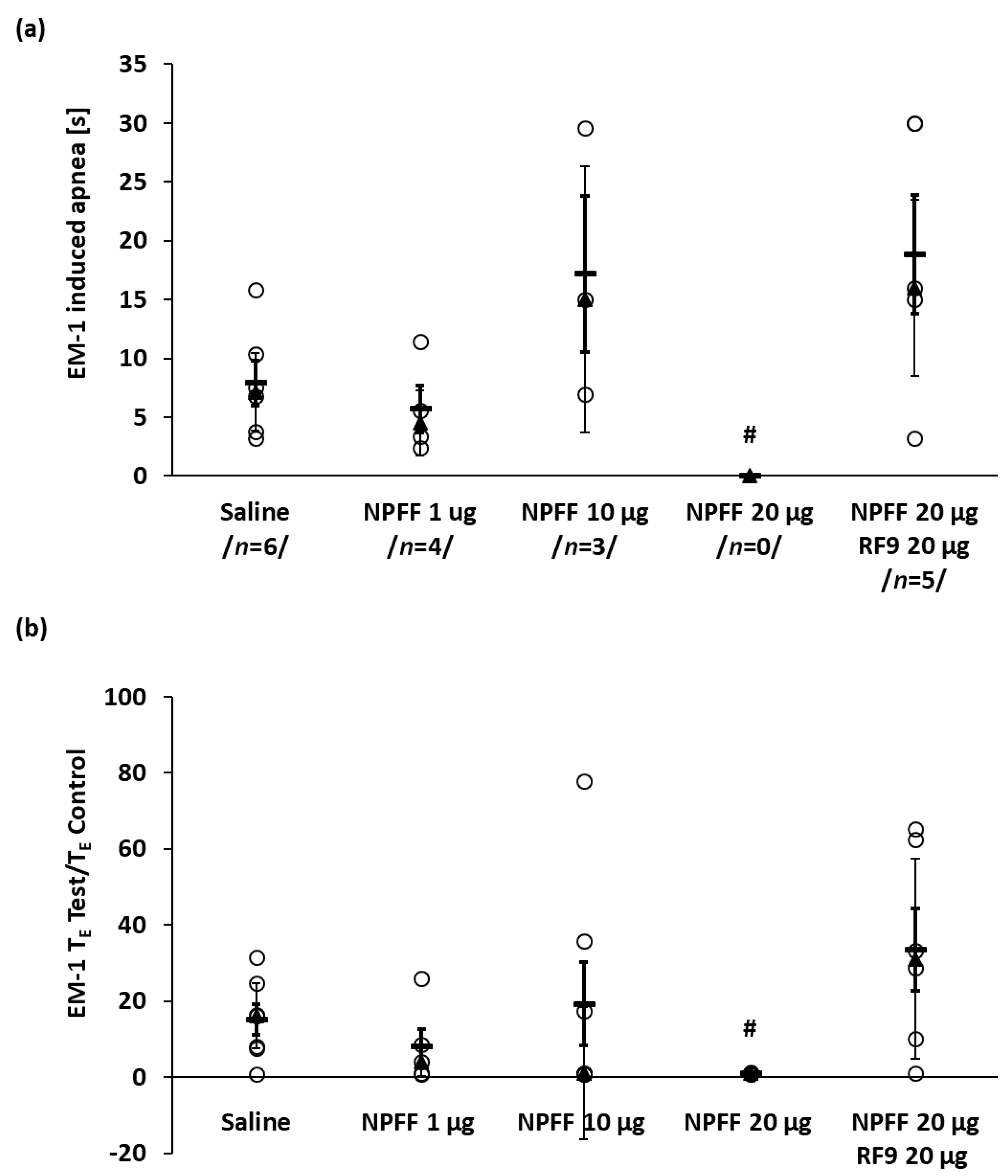
2.3. The Effects of Icv NPFF on Apnea and Cardiovascular Effects Induced by EM-1
2.4. The Effect of Blockade with RF9
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedures
4.3. Registration and Counting of Parameters
4.4. Drugs
- First, control group of animals received an intravenous (iv) injection of EM-1 in a dose of 50 μg/kg;
- Second group was divided on rats that received iv injection of NPFF in doses of 0.1, 0.3, 0.6 and 1.2 mg/kg 2 min prior to the EM-1 challenge;
- Third group of animals was divided on rats that were administered icv (right cerebral ventricle) with vehicle (saline) and NPFF in doses of 1, 10 and 20 μg per rat before iv EM-1 injection;
- Fourth group was injected icv with the mixture (per rat) of 20 μg of neuropeptide FF and 20 μg of RF9—neuropeptide FF receptor antagonist, before the intravenous EM-1 challenge.
4.5. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Han, J.-S. Chapter 210–Antiopioid peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1543–1549. ISBN 978-0-12-385095-9. [Google Scholar]
- Yang, H.Y.; Fratta, W.; Majane, E.A.; Costa, E. Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acad. Sci. USA 1985, 82, 7757–7761. [Google Scholar] [CrossRef]
- Allard, M.; Zajac, J.-M.; Simonnet, G. Autoradiographic distribution of receptors to FLFQPQRFamide, a morphine-modulating peptide, in rat central nervous system. Neuroscience 1992, 49, 101–116. [Google Scholar] [CrossRef]
- Majane, E.A.; Panula, P.; Yang, H.-Y.T. Rat brain regional distribution and spinal cord neuronal pathway of FLFQPQRF-NH2, a mammalian FMRF-NH2-like peptide. Brain Res. 1989, 494, 1–12. [Google Scholar] [CrossRef]
- Panula, P.; Aarnisalo, A.A.; Wasowicz, K. Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog. Neurobiol. 1996, 48, 461–487. [Google Scholar] [CrossRef]
- Kalliomäki, M.-L.; Panula, P. Neuropeptide ff, but not prolactin-releasing peptide, mRNA is differentially regulated in the hypothalamic and medullary neurons after salt loading. Neuroscience 2004, 124, 81–87. [Google Scholar] [CrossRef]
- Sunter, D.; Hewson, A.K.; Lynam, S.; Dickson, S.L. Intracerebroventricular injection of neuropeptide FF, an opioid modulating neuropeptide, acutely reduces food intake and stimulates water intake in the rat. Neurosci. Lett. 2001, 313, 145–148. [Google Scholar] [CrossRef]
- Dockray, G.J. The expanding family of-RFamide peptides and their effects on feeding behaviour. Exp. Physiol. 2004, 89, 229–235. [Google Scholar] [CrossRef]
- Maletínská, L.; Tichá, A.; Nagelová, V.; Spolcová, A.; Blechová, M.; Elbert, T.; Zelezná, B. Neuropeptide FF analog RF9 is not an antagonist of NPFF receptor and decreases food intake in mice after its central and peripheral administration. Brain Res. 2013, 1498, 33–40. [Google Scholar] [CrossRef]
- Labrouche, S.; Laulin, J.-P.; Le Moal, M.; Tramu, G.; Simonnet, G. Neuropeptide FF in the rat adrenal gland: Presence, distribution and pharmacological effects. J. Neuroendocrinol. 1998, 10, 559–565. [Google Scholar] [CrossRef]
- Saito, T.H.; Nakane, R.; Akazome, Y.; Abe, H.; Oka, Y. Electrophysiological analysis of the inhibitory effects of FMRFamide-Like peptides on the pacemaker activity of gonadotropin-releasing hormone neurons. J. Neurophysiol. 2010, 104, 3518–3529. [Google Scholar] [CrossRef]
- Decker, B.; Vadokas, B.; Kutschenreuter, U.; Golenhofen, K.; Voigt, K.; Mcgregor, G.P.; Mandrek, K. Action of FMRFamide-like peptides on porcine gastrointestinal motility in vitro. Peptides 1997, 18, 1531–1537. [Google Scholar] [CrossRef]
- Fang, Q.; Guo, J.; Chang, M.; Chen, L.; Chen, Q.; Wang, R. Neuropeptide FF receptors exert contractile activity via inhibition of nitric oxide release in the mouse distal colon. Peptides 2005, 26, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Desprat, C.; Zajac, J.-M. Hypothermic effects of neuropeptide FF analogues in mice. Pharmacol. Biochem. Behav. 1997, 58, 559–563. [Google Scholar] [CrossRef]
- Fang, Q.; Li, N.; Jiang, T.; Liu, Q.; Li, Y.; Wang, R. Pressor and tachycardic responses to intrathecal administration of neuropeptide FF in anesthetized rats. Peptides 2010, 31, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.D.; Buijs, R.M.; Jhamandas, J.H.; Swaab, D.F. Vasopressin (VP) and neuropeptide FF (NPFF) systems in the normal and hypertensive human brainstem. J. Comp. Neurol. 2011, 519, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.-K.; Li, J.-Y.; Tan, P.P.-C.; Wong, C.-H.; Chen, J.-C. The cardiovascular effects of PFRFamide and PFR(Tic)amide, a possible agonist and antagonist of neuropeptide FF (NPFF). Peptides 2000, 21, 205–210. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Goncharuk, V. Role of neuropeptide FF in central cardiovascular and neuroendocrine regulation. Front. Endocrinol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Roth, B.L.; Disimone, J.; Majane, E.A.; Yang, H.-Y.T. Elevation of arterial pressure in rats by two new vertebrate peptides FLF QPQRF-NH2 and AGE GLSSPFWSLAAPQRF-NH2 which are immunoreactive to FMRF-NH2 antiserum. Neuropeptides 1987, 10, 37–42. [Google Scholar] [CrossRef]
- Allard, M.; Labrouche, S.; Nosjean, A.; Laguzzi, R. Mechanisms underlying the cardiovascular responses to peripheral administration of NPFF in the rat. J. Pharmacol. Exp. Ther. 1995, 274, 577–583. [Google Scholar]
- Nijsen, M.J.; de Ruiter, G.J.; Kasbergen, C.M.; Hoogerhout, P.; de Wildt, D.J. Relevance of the C-terminal Arg-Phe sequence in gamma(2)-melanocyte-stimulating hormone (gamma(2)-MSH) for inducing cardiovascular effects in conscious rats. Br. J. Pharmacol. 2000, 131, 1468–1474. [Google Scholar] [CrossRef]
- Prokai, L.; Zharikova, A.D.; Juhasz, A.; Prokai-Tatrai, K. Cardiovascular effects of neuropeptide FF antagonists. Peptides 2006, 27, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Laguzzi, R.; Nosjean, A.; Mazarguil, H.; Allard, M. Cardiovascular effects induced by the stimulation of neuropeptide FF receptors in the dorsal vagal complex: An autoradiographic and pharmacological study in the rat. Brain Res. 1996, 711, 193–202. [Google Scholar] [CrossRef]
- Elhabazi, K.; Trigo, J.; Mollereau, C.; Moulédous, L.; Zajac, J.-M.; Bihel, F.; Schmitt, M.; Bourguignon, J.; Meziane, H.; Petit-demoulière, B.; et al. Involvement of neuropeptide FF receptors in neuroadaptive responses to acute and chronic opiate treatments: NPFF receptors in neuroadaptive responses to opiates. Br. J. Pharmacol. 2012, 165, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Lameh, J.; Bertozzi, F.; Kelly, N.; Jacobi, P.M.; Nguyen, D.; Bajpai, A.; Gaubert, G.; Olsson, R.; Gardell, L.R. Neuropeptide FF receptors have opposing modulatory effects on nociception. J. Pharmacol. Exp. Ther. 2010, 334, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Kalso, E.; Nieminen, M.; Kontinen, V.K.; Brandt, A.; Pertovaara, A. Neuropeptide FF and modulation of pain. Brain Res. 1999, 848, 191–196. [Google Scholar] [CrossRef]
- Roumy, M.; Zajac, J.-M. Neuropeptide FF, pain and analgesia. Eur. J. Pharmacol. 1998, 345, 1–11. [Google Scholar] [CrossRef]
- Yang, H.-Y.T.; Tao, T.; Iadarola, M.J. Modulatory role of neuropeptide FF system in nociception and opiate analgesia. Neuropeptides 2008, 42, 1–18. [Google Scholar] [CrossRef]
- Gouardères, C.; Puget, A.; Zajac, J.-M. Detailed distribution of neuropeptide FF receptors (NPFF1 and NPFF2) in the rat, mouse, octodon, rabbit, guinea pig, and marmoset monkey brains: A comparative autoradiographic study. Synapse 2004, 51, 249–269. [Google Scholar] [CrossRef]
- Mansour, A.; Fox, C.A.; Akil, H.; Watson, S.J. Opioid-receptor mRNA expression in the rat CNS: Anatomical and functional implications. Trends Neurosci. 1995, 18, 22–29. [Google Scholar] [CrossRef]
- Mansour, A.; Fox, C.A.; Burke, S.; Akil, H.; Watson, S.J. Immunohistochemical localization of the cloned μ opioid receptor in the rat CNS. J. Chem. Neuroanat. 1995, 8, 283–305. [Google Scholar] [CrossRef]
- Mansour, A.; Khachaturian, H.; Lewis, M.E.; Akil, H.; Watson, S.J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988, 11, 308–314. [Google Scholar] [CrossRef]
- Gouardères, C.; Tafani, J.A.; Zajac, J.M. Affinity of neuropeptide FF analogs to opioid receptors in the rat spinal cord. Peptides 1998, 19, 727–730. [Google Scholar] [CrossRef]
- Fang, Q.; Jiang, T.; Li, N.; Han, Z.-I.; Wang, R. Central Administration of Neuropeptide FF and Related Peptides Attenuate Systemic Morphine Analgesia in Mice. Protein Pept. Lett. 2011, 18, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, V.; Zajac, J.M. Effects of neuropeptide FF analogs on morphine analgesia in the nucleus raphe dorsalis. Regul. Pept. 1995, 59, 349–356. [Google Scholar] [CrossRef]
- Gelot, A.; Francés, B.; Roussin, A.; Latapie, J.-P.; Zajac, J.-M. Anti-opioid efficacy of Neuropeptide FF in morphine-tolerant mice. Brain Res. 1998, 808, 166–173. [Google Scholar] [CrossRef]
- Malin, D.H.; Lake, J.R.; Fowler, D.E.; Hammond, M.V.; Brown, S.L.; Leyva, J.E.; Prasco, P.E.; Dougherty, T.M. FMRF-NH2-like mammalian peptide precipitates opiate-withdrawal syndrome in the rat. Peptides 1990, 11, 277–280. [Google Scholar] [CrossRef]
- Malin, D.H.; Lake, J.R.; Hammond, M.V.; Fowler, D.E.; Rogillio, R.B.; Brown, S.L.; Sims, J.L.; Leecraft, B.M.; Yang, H.-Y.T. FMRF-NH2-like mammalian octapeptide: Possible role in opiate dependence and abstinence. Peptides 1990, 11, 969–972. [Google Scholar] [CrossRef]
- Kontinen, V.K.; Kalso, E.A. Differential modulation of alpha 2-adrenergic and mu-opioid spinal antinociception by neuropeptide FF. Peptides 1995, 16, 973–977. [Google Scholar] [CrossRef]
- Gouardères, C.; Sutak, M.; Zajac, J.-M.; Jhamandas, K. Antinociceptive effects of intrathecally administered F8Famide and FMRFamide in the rat. Eur. J. Pharmacol. 1993, 237, 73–81. [Google Scholar] [CrossRef]
- Wojciechowski, P.; Kleczkowska, P.; Mollica, A.; Stefanucci, A.; Kaczyńska, K. Vagal apnea and hypotension evoked by systemic injection of an antinociceptive analogue of endomorphin-2. Eur. J. Pharmacol. 2020, 885, 173514. [Google Scholar] [CrossRef]
- Arima, H.; Murase, T.; Kondo, K.; Iwasaki, Y.; Oiso, Y. Centrally administered neuropeptide FF inhibits arginine vasopressin release in conscious rats. Endocrinology 1996, 137, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, Q.; Han, Z.; Pan, J.; Li, X.; Li, N.; Tang, H.; Wang, P.; Zheng, T.; Chang, X.; et al. Opposite effects of neuropeptide FF on central antinociception induced by endomorphin-1 and endomorphin-2 in mice. PLoS ONE 2014, 9, e103773. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.; Pachuta, A.; Dylag, T.; Silberring, J. Neuropeptide FF (NPFF) reduces the expression of morphine-but not of ethanol-induced conditioned place preference in rats. Peptides 2007, 28, 2235–2242. [Google Scholar] [CrossRef]
- Travagli, R.A. Nucleus tractus solitarii. In Encyclopedia of Neuroscience; Binder, M.D., Hirokawa, N., Windhorst, U., Eds.; Springer: Berlin, Germany, 2009; pp. 2908–2911. ISBN 978-3-540-29678-2. [Google Scholar]
- Dean, J.B.; Putnam, R.W. The caudal solitary complex is a site of central CO2 chemoreception and integration of multiple systems that regulate expired CO2. Respir. Physiol. Neurobiol. 2010, 173, 274–287. [Google Scholar] [CrossRef]
- Fu, C.; Shi, L.; Wei, Z.; Yu, H.; Hao, Y.; Tian, Y.; Liu, Y.; Zhang, Y.; Zhang, X.; Yuan, F.; et al. Activation of Phox2b-Expressing neurons in the nucleus tractus solitarii drives breathing in mice. J. Neurosci. 2019, 39, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Alheid, G.F.; Jiao, W.; McCrimmon, D.R. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience 2011, 190, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, L.; Aarnisalo, A.; Panula, P. Neuropeptide FF is colocalized with catecholamine-synthesizing enzymes in neurons of the nucleus of the solitary tract. Neurosci. Lett. 1992, 143, 190–194. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Mactavish, D. Central administration of neuropeptide FF (NPFF) causes increased neuronal activation and up-regulation of NPFF gene expression in the rat brainstem. J. Comp. Neurol. 2002, 447, 300–307. [Google Scholar] [CrossRef]
- Szereda-Przestaszewska, M.; Kaczyńska, K. Pharmacologically evoked apnoeas. Receptors and nervous pathways involved. Life Sci. 2019, 217, 237–242. [Google Scholar] [CrossRef]
- Zhuang, J.; Gao, X.; Gao, F.; Xu, F. Mu-opioid receptors in the caudomedial NTS are critical for respiratory responses to stimulation of bronchopulmonary C-fibers and carotid body in conscious rats. Respir. Physiol. Neurobiol. 2017, 235, 71–78. [Google Scholar] [CrossRef]
- Xia, Y.; Haddad, G.G. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. 1991, 549, 181–193. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Kaneko, T.; Nomura, S.; Mizuno, N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J. Comp. Neurol. 1996, 367, 375–402. [Google Scholar] [CrossRef]
- Goodman, C.B.; Heyliger, S.; Emilien, B.; Partilla, J.S.; Yang, H.-Y.T.; Lee, C.-H.; Cadet, J.L.; Rothman, R.B. Regulation of μ binding sites after chronic administration of antibodies directed against specific anti-opiate peptides. Peptides 1998, 19, 1703–1709. [Google Scholar] [CrossRef]
- Simonin, F.; Schmitt, M.; Laulin, J.-P.; Laboureyras, E.; Jhamandas, J.H.; MacTavish, D.; Matifas, A.; Mollereau, C.; Laurent, P.; Parmentier, M.; et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. USA 2006, 103, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Journigan, V.B.; Mésangeau, C.; Vyas, N.; Eans, S.O.; Cutler, S.J.; McLaughlin, J.P.; Mollereau, C.; McCurdy, C.R. Nonpeptide small molecule agonist and antagonist original leads for neuropeptide FF1 and FF2 receptors. J. Med. Chem. 2014, 57, 8903–8927. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, Y.-Q.; He, F.; Guo, J.; Guo, J.; Chen, Q.; Wang, R. Inhibition of neuropeptide FF (NPFF)-induced hypothermia and anti-morphine analgesia by RF9, a new selective NPFF receptors antagonist. Regul. Pept. 2008, 147, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.M.; Greenberg, M.J.; Tse, S.Y. Cardiovascular effects of intraventricular injection of FMRFamide, Met-enkephalin and their common analogues in the rat. Comp. Biochem. Physiol. C 1985, 81, 175–179. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition, 6th ed.; Academic Press: London, UK, 2006; ISBN 978-0-08-047515-8. [Google Scholar]



| Saline | NPFF 100 μg | NPFF 300 μg | NPFF 600 μg | ||
|---|---|---|---|---|---|
| n | 7 | 6 | 5 | 6 | |
| HR 1/min | Baseline | 473 ± 37 | 454 ± 19 | 491 ± 0 | 505 ± 38 |
| MIN 0–30 s | 396 ± 18 * | 397 ± 22 * | 450 ± 9 *# | 450 ± 20 | |
| Recovery (1 min) | 454 ± 38 | 438 ± 19 | 483 ± 2 | 492 ± 39 | |
| MAP mmHg | Baseline | 80 ± 6 | 83 ± 6 | 75 ± 1 | 84 ± 3 |
| MIN 0–30 s | 29 ± 11 * | 66 ± 13 *# | 47 ± 5 * | 59 ± 9 * | |
| Recovery (1 min) | 81 ± 4 | 84 ± 12 | 75 ± 3 | 79 ± 17 |
| Saline | NPFF 1 μg | NPFF 10 μg | NPFF 20 μg | NPFF 20 μg RF9 20 μg | ||
|---|---|---|---|---|---|---|
| n | 7 | 5 | 7 | 5 | 6 | |
| HR 1/min | Baseline | 428 ± 39 | 410 ± 32 | 430 ± 31 | 459 ± 15 | 413 ± 56 |
| MIN 0–30 s | 387 ± 11 * | 394 ± 34 * | 398 ± 33 | 400 ± 5 # | 354 ± 33 * | |
| Recovery (1 min) | 412 ± 18 | 426 ± 12 | 455 ± 20 * | 456 ± 23 | 432 ± 31 | |
| MAP mmHg | Baseline | 81 ± 4 | 75 ± 15 | 77 ± 6 | 76 ± 7 | 73 ± 7 |
| MIN 0–30 s | 51 ± 2 * | 43 ± 3 *# | 68 ± 21 | 66 ± 5 | 52 ± 16 * | |
| Recovery (1 min) | 81 ± 7 | 67 ± 15 | 89 ± 10 | 85 ± 7 * | 73 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowski, P.; Andrzejewski, K.; Kaczyńska, K. Intracerebroventricular Neuropeptide FF Diminishes the Number of Apneas and Cardiovascular Effects Produced by Opioid Receptors’ Activation. Int. J. Mol. Sci. 2020, 21, 8931. https://doi.org/10.3390/ijms21238931
Wojciechowski P, Andrzejewski K, Kaczyńska K. Intracerebroventricular Neuropeptide FF Diminishes the Number of Apneas and Cardiovascular Effects Produced by Opioid Receptors’ Activation. International Journal of Molecular Sciences. 2020; 21(23):8931. https://doi.org/10.3390/ijms21238931
Chicago/Turabian StyleWojciechowski, Piotr, Kryspin Andrzejewski, and Katarzyna Kaczyńska. 2020. "Intracerebroventricular Neuropeptide FF Diminishes the Number of Apneas and Cardiovascular Effects Produced by Opioid Receptors’ Activation" International Journal of Molecular Sciences 21, no. 23: 8931. https://doi.org/10.3390/ijms21238931
APA StyleWojciechowski, P., Andrzejewski, K., & Kaczyńska, K. (2020). Intracerebroventricular Neuropeptide FF Diminishes the Number of Apneas and Cardiovascular Effects Produced by Opioid Receptors’ Activation. International Journal of Molecular Sciences, 21(23), 8931. https://doi.org/10.3390/ijms21238931