Leptin Modulates the Metastasis of Canine Inflammatory Mammary Adenocarcinoma Cells through Downregulation of Lysosomal Protective Protein Cathepsin A (CTSA)
Abstract
:1. Introduction
2. Results
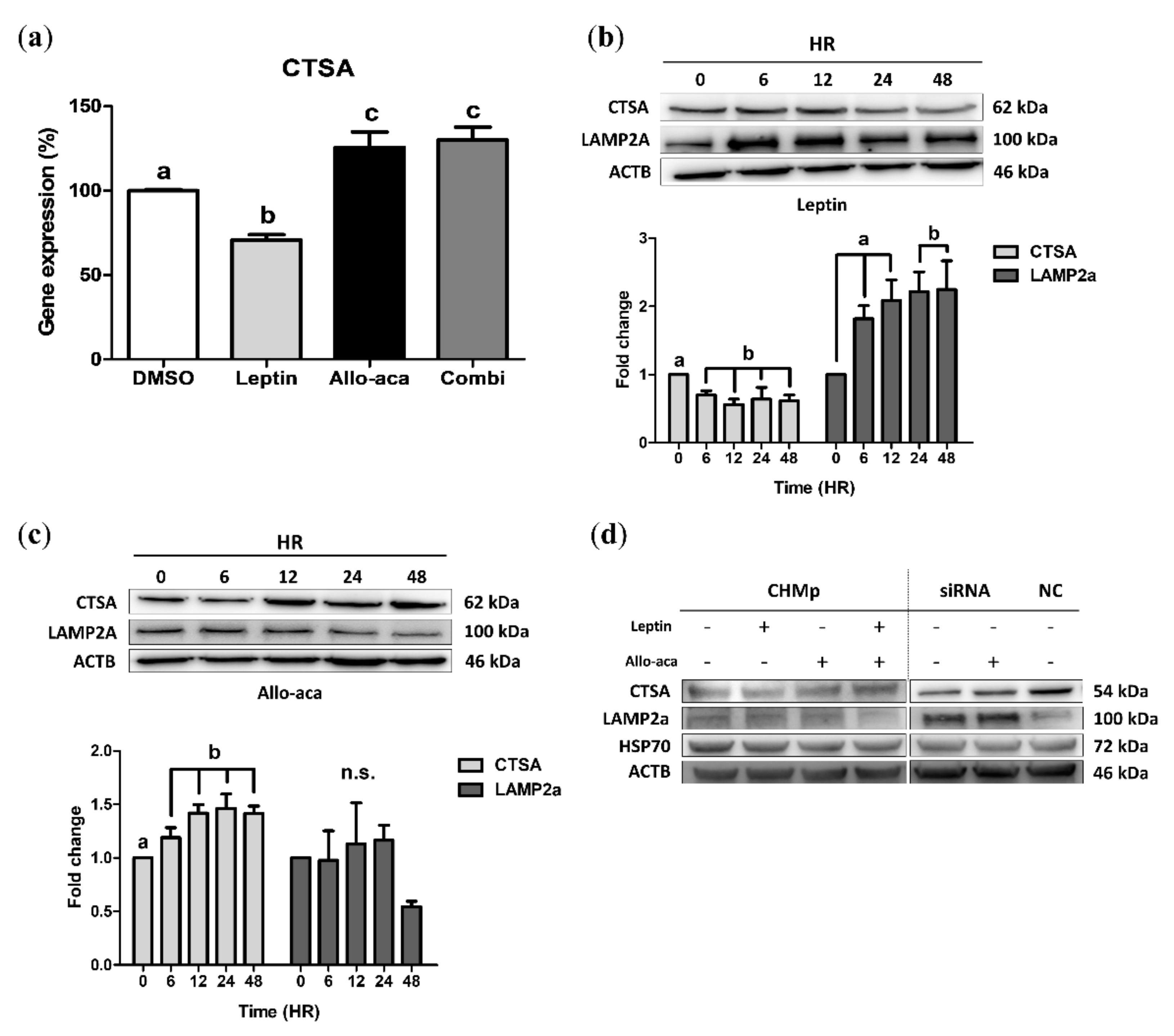
2.1. Leptin Downregulated CTSA and Upregulated LAMP2a in CHMp Cells
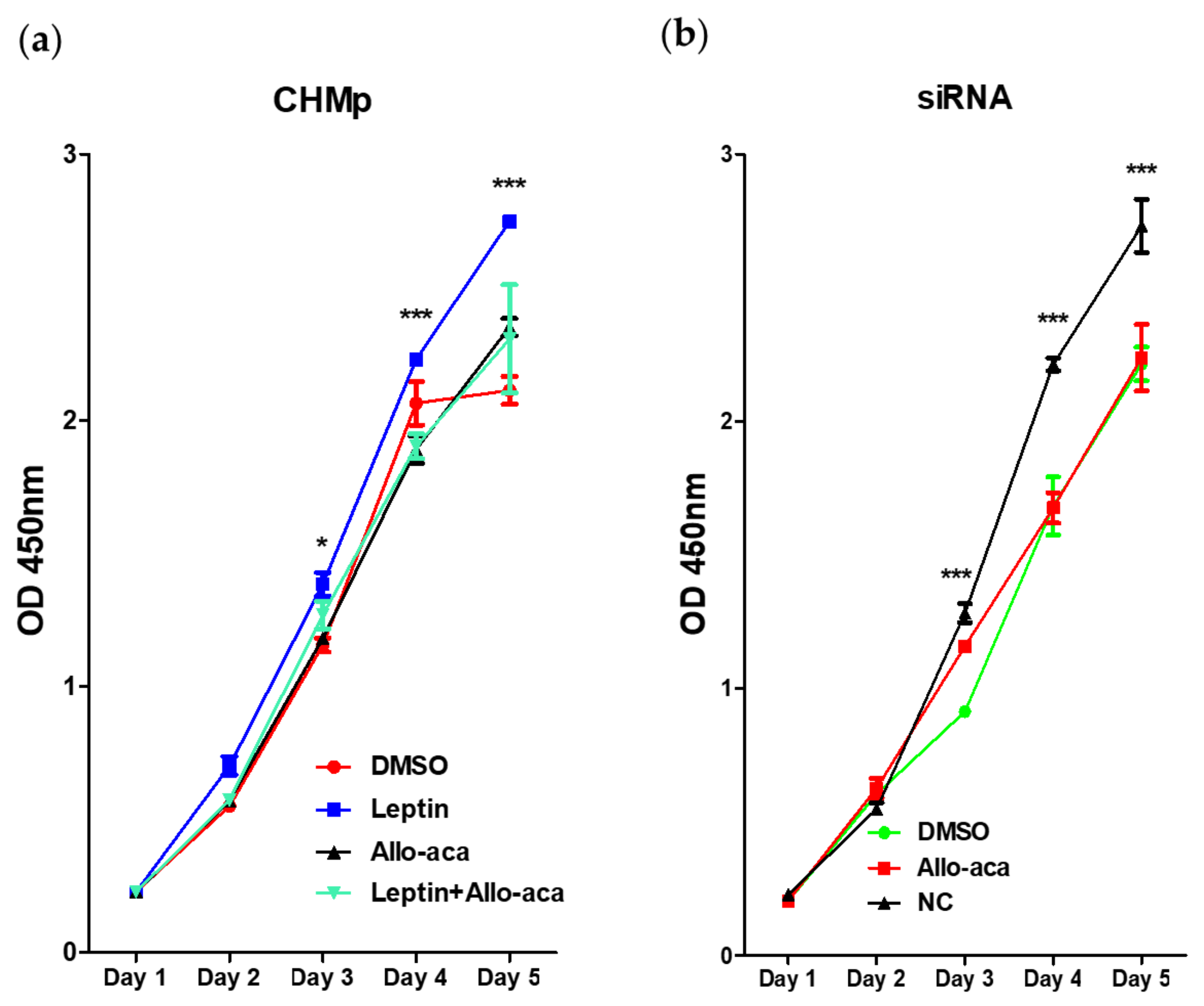
2.2. Leptin Promoted Cell Proliferation in CHMp Cells
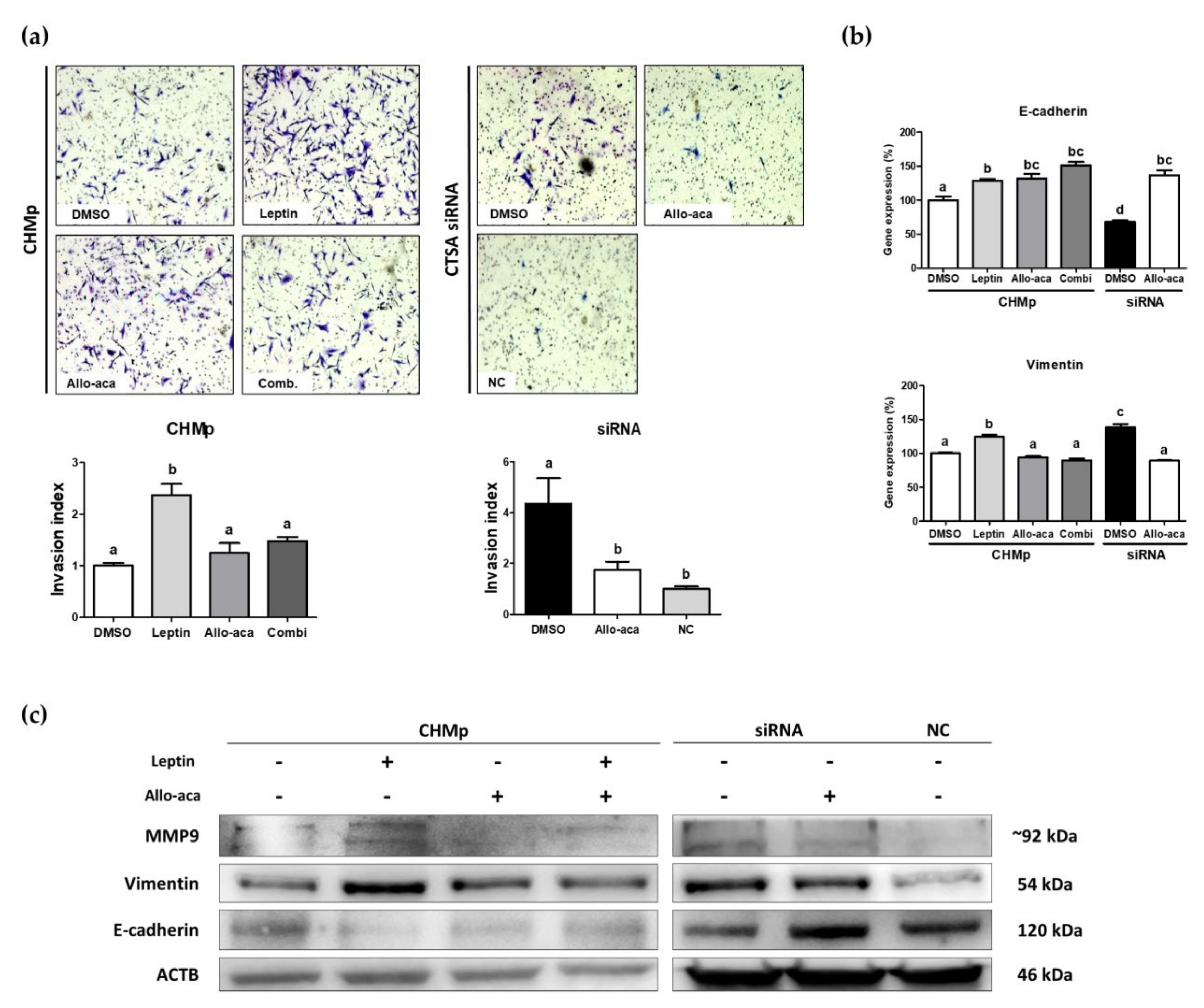
2.3. Leptin Stimulated EMT in CHMp Cells
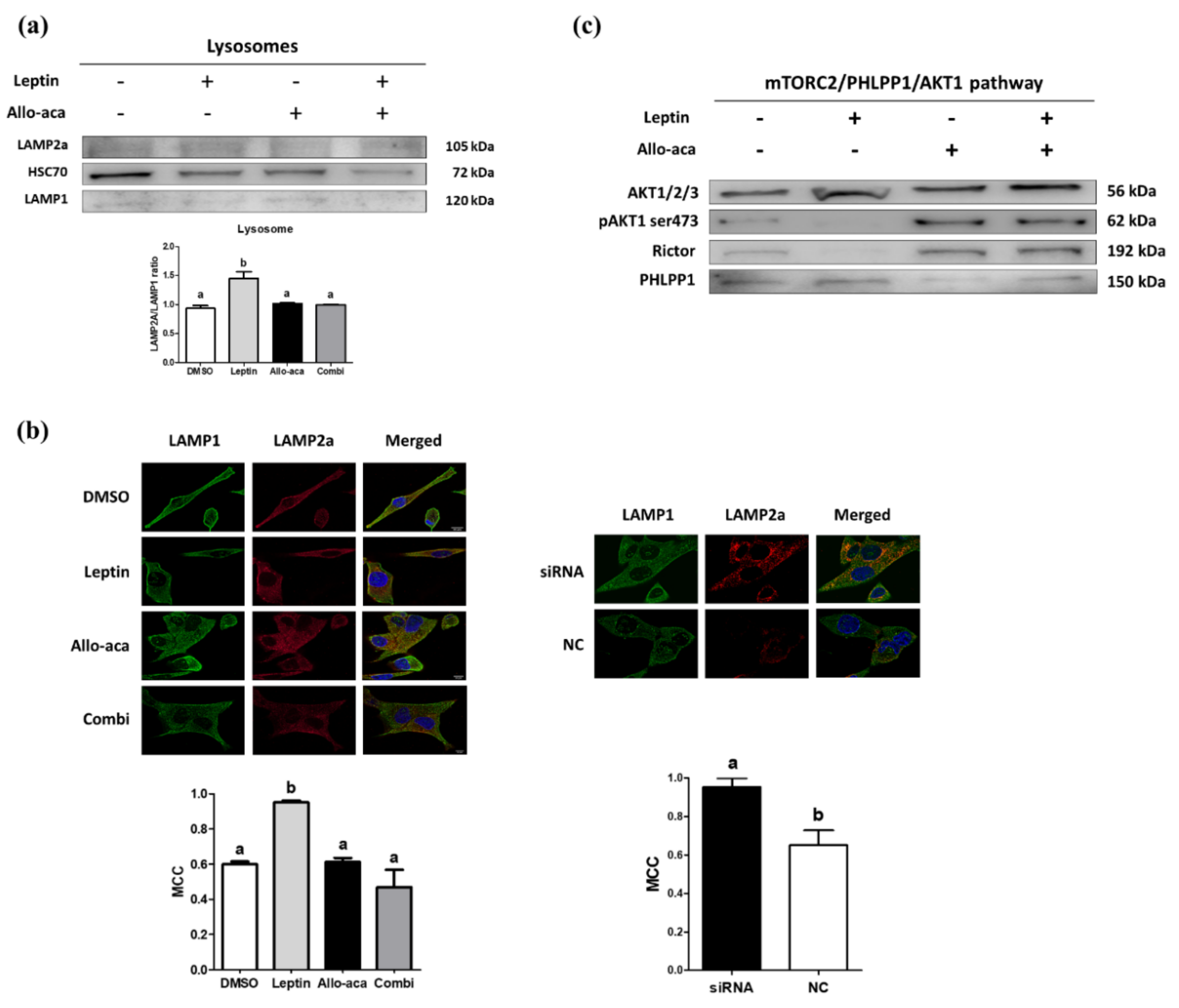
2.4. Leptin Delayed the Degradation of LAMP2a Through Downregulation of CTSA
2.5. Leptin May Promote Lysosomal LAMP2a Multimerization Through the mTORC2/PHLPP1/AKT1 Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture Methods
4.2. Chemicals and Antibodies
4.3. Reverse siRNA Transfection
4.4. Cell Proliferation Assay
4.5. Cell Invasion Assay
4.6. RNA Extraction and Real-time Quantitative PCR Analysis (RT-qPCR)
4.7. Immunofluorescence Analysis
4.8. Lysosome Isolation and Immunoblot
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CMT | Canine mammary gland tumor |
| cIMC | Canine inflammatory mammary carcinoma |
| CTSA | Lysosomal protective protein cathepsin A (PPCA) |
| LAMP1 | Lysosomal-associated membrane protein 1 |
| LAMP2a | Lysosomal-associated membrane protein 2a |
| CMA | Chaperone-mediated autophagy |
| OBR | Leptin receptor |
| PHLPP1 | PH domain and leucine rich repeat protein phosphatase 1 |
| ACTB | Beta-actin |
| VIM | Vimentin |
| CDH1 | E-cadherin |
| siRNA | Small interfering ribonucleic acid |
| cDNA | Complementary deoxyribonucleic acid |
| mRNA | Messenger ribonucleic acid |
| RNAi | RNA interference |
| DMSO | Dimethyl sulfoxide |
References
- Moschos, S.; Chan, J.L.; Mantzoros, C.S. Leptin and reproduction: A review. Fertil. Steril. 2002, 77, 433–444. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Surmacz, E. Leptin and cancer. J. Cell Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Kim, M.J.; Hong, J.T.; Kim, S.H.; Sohn, D.H.; Lee, S.H.; Song, K.; Choi, D.Y.; Lee, E.S.; Park, P.H. Autophagy induction by leptin contributes to suppression of apoptosis in cancer cells and xenograft model: Involvement of p53/FoxO3A axis. Oncotarget 2015, 6, 7166–7181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.; Avtanski, D.; Saxena, N.K.; Sharma, D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires beta-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J. Biol. Chem. 2012, 287, 8598–8612. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Li, K.; Pang, X.; Guo, B.; Su, M.; Huang, Y.; Wang, N.; Ji, F.; Zhong, C.; Yang, J.; et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J. Exp. Clin. Cancer Res. 2016, 35, 166. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Liu, Q.; Zhang, N.; Zheng, L.; Sang, M.; Feng, J.; Zhang, J.; Wu, X.; Shan, B. Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol. Res. 2013, 21, 165–171. [Google Scholar] [CrossRef]
- Xu, M.; Cao, F.L.; Li, N.; Gao, X.; Su, X.; Jiang, X. Leptin induces epithelial-to-mesenchymal transition via activation of the ERK signaling pathway in lung cancer cells. Oncol. Lett. 2018, 16, 4782–4788. [Google Scholar] [CrossRef] [Green Version]
- Candelaria, P.V.; Rampoldi, A.; Harbuzariu, A.; Gonzalez-Perez, R.R. Leptin signaling and cancer chemoresistance: Perspectives. World J. Clin. Oncol. 2017, 8, 106–119. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Kovalszky, I.; Riolfi, M.; Ferla, R.; Olah, J.; Sztodola, A.; Nama, K.; Molino, A.; Piubello, Q.; Wade, J.D.; et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur. J. Cancer 2011, 47, 1578–1584. [Google Scholar] [CrossRef]
- Alenza, M.D.P.; Pena, L.; del Castillo, N.; Nieto, A.I. Factors influencing the incidence and prognosis of canine mammary tumours. J. Small Anim. Pract. 2000, 41, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Strollo, F.; More, M.; Caprio, M.; Aversa, A.; Moretti, C.; Frajese, G.; Riondino, G.; Fabbri, A. Leptin and aging: Correlation with endocrine changes in male and female healthy adult populations of different body weights. J. Clin. Endocr Metab. 2000, 85, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Im, K.S.; Kim, N.H.; Kim, H.W.; Shin, J.I.; Yhee, J.Y.; Sur, J.H. Effects of Obesity and Obesity-Related Molecules on Canine Mammary Gland Tumors. Vet. Pathol. 2015, 52, 1045–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishioka, K.; Hosoya, K.; Kitagawa, H.; Shibata, H.; Honjoh, T.; Kimura, K.; Saito, M. Plasma leptin concentration in dogs: Effects of body condition score, age, gender and breeds. Res. Vet. Sci. 2007, 82, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishioka, K.; Soliman, M.M.; Sagawa, M.; Nakadomo, F.; Shibata, H.; Honjoh, T.; Hashimoto, A.; Kitamura, H.; Kimura, K.; Saito, M. Experimental and clinical studies on plasma leptin in obese dogs. J. Vet. Med. Sci. 2002, 64, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, M.; Meehan, J.; Martinez-Perez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Perez Alenza, M.D.; Tabanera, E.; Pena, L. Inflammatory mammary carcinoma in dogs: 33 cases (1995–1999). J. Am. Vet. Med. Assoc. 2001, 219, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.N. A comparative study of canine and human breast cancer. Investig. Cell Pathol. 1979, 2, 257–275. [Google Scholar]
- Mohammed, S.I.; Utturkar, S.; Lee, M.; Yang, H.H.; Cui, Z.; Lanman, N.A.; Zhang, G.; Cardona, X.E.R.; Mittal, S.K.; Miller, M.A. Ductal Carcinoma In Situ Progression in Dog Model of Breast Cancer. Cancers 2020, 12, 418. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, N.; Haim, Y.; Mattar, P.; Hadadi-Bechor, S.; Maixner, N.; Kovacs, P.; Bluher, M.; Rudich, A. Leptin stimulates autophagy/lysosome-related degradation of long-lived proteins in adipocytes. Adipocyte 2019, 8, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Dice, J.F. Chaperone-mediated autophagy. Autophagy 2007, 3, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kon, M.; Kiffin, R.; Koga, H.; Chapochnick, J.; Macian, F.; Varticovski, L.; Cuervo, A.M. Chaperone-Mediated Autophagy Is Required for Tumor Growth. Sci. Transl. Med. 2011, 3, 109ra117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, T. LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy 2012, 8, 1643–1656. [Google Scholar] [CrossRef] [Green Version]
- Arias, E.; Koga, H.; Diaz, A.; Mocholi, E.; Patel, B.; Cuervo, A.M. Lysosomal mTORC2/PHLPP1/Akt Regulate Chaperone-Mediated Autophagy. Mol. Cell 2015, 59, 270–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuervo, A.M.; Mann, L.; Bonten, E.J.; d’Azzo, A.; Dice, J.F. Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 2003, 22, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, K.; Alroy, J.; Castagnaro, M.; Raghavan, S.S.; Jakowski, R.M.; Freden, G.O. Adult-onset lysosomal storage disease in a Schipperke dog: Clinical, morphological and biochemical studies. Acta Neuropathol. 1993, 86, 306–312. [Google Scholar] [CrossRef]
- Caciotti, A.; Catarzi, S.; Tonin, R.; Lugli, L.; Perez, C.R.; Michelakakis, H.; Mavridou, I.; Donati, M.A.; Guerrini, R.; d’Azzo, A.; et al. Galactosialidosis: Review and analysis of CTSA gene mutations. Orphanet. J. Rare Dis. 2013, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naour, N.; Rouault, C.; Fellahi, S.; Lavoie, M.E.; Poitou, C.; Keophiphath, M.; Eberle, D.; Shoelson, S.; Rizkalla, S.; Bastard, J.P.; et al. Cathepsins in human obesity: Changes in energy balance predominantly affect cathepsin s in adipose tissue and in circulation. J. Clin. Endocrinol. Metab. 2010, 95, 1861–1868. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.; Assis, D.M.; Paschoalin, T.; Miranda, A.; Ribeiro, E.B.; Juliano, M.A.; Bromme, D.; Christoffolete, M.A.; Barros, N.M.; Carmona, A.K. Cysteine cathepsin S processes leptin, inactivating its biological activity. J. Endocrinol. 2012, 214, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlix, A.; Lamy, P.J.; Lopez-Crapez, E.; Braccini, A.L.; Firmin, N.; Romieu, G.; Thezenas, S.; Jacot, W. Serum NSE, MMP-9 and HER2 extracellular domain are associated with brain metastases in metastatic breast cancer patients: Predictive biomarkers for brain metastases? Int. J. Cancer 2016, 139, 2299–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama, A.; Paredes, J.; Gartner, F.; Alves, A.; Schmitt, F. Expression of E-cadherin, P-cadherin and beta-catenin in canine malignant mammary tumours in relation to clinicopathological parameters, proliferation and survival. Vet. J. 2008, 177, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yin, S.; Zhang, L.; Liu, W.; Chen, B. Prognostic value of reduced E-cadherin expression in breast cancer: A meta-analysis. Oncotarget 2017, 8, 16445–16455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Liu, S.; Wong, H.L. Nanotoxicity: A key obstacle to clinical translation of siRNA-based nanomedicine. Nanomedicine 2014, 9, 295–312. [Google Scholar] [CrossRef] [Green Version]
- Uyama, R.; Nakagawa, T.; Hong, S.H.; Mochizuki, M.; Nishimura, R.; Sasaki, N. Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression. Vet. Comp. Oncol. 2006, 4, 104–113. [Google Scholar] [CrossRef]
- Otvos, L.; Kovalszky, I.; Scolaro, L.; Sztodola, A.; Olah, J.; Cassone, M.; Knappe, D.; Hoffmann, R.; Lovas, S.; Hatfield, M.P.D.; et al. Peptide-Based leptin Receptor Antagonists for Cancer Treatment and Appetite Regulation. Biopolymers 2011, 96, 117–125. [Google Scholar] [CrossRef]

| Target | Catalog Number | Host | Clone | Dilution | Manufacturer |
|---|---|---|---|---|---|
| LAMP2a | ab18528 | Rabbit | Polyclonal | 1:1000 | Abcam |
| LAMP1 | ab24170 | Rabbit | Polyclonal | 1:1000 | Abcam |
| HSP70 | ab53496 | Mouse | Monoclonal | 1:1000 | Abcam |
| VIM | ab1316 | Mouse | Monoclonal | 1:400 | Abcam |
| MMP9 | ab38898 | Rabbit | Polyclonal | 1:1000 | Abcam |
| pAKT1(ser473) | ab81283 | Rabbit | Monoclonal | 1:5000 | Abcam |
| ACTB | ab8227 | Mouse | Monoclonal | 1:5000 | Abcam |
| CDH1 | 14-3249-82 | Rat | Monoclonal | 1:250 | Invitrogen |
| AKT1 | sc-135829 | Mouse | Monoclonal | 1:1000 | SCBT |
| PHLPP1 | sc-390129 | Mouse | Monoclonal | 1:500 | SCBT |
| mTOR | sc-517464 | Mouse | Monoclonal | 1:200 | SCBT |
| CTSA | 139645 | Rabbit | Polyclonal | 1:1000 | US Biological |
| Gene | GenBank Accession No. | Primer Sequences | Product Size (bp) |
|---|---|---|---|
| CTSA | NM_001109915.1 | F: 5′-CAG ACC CAC TGC TGT TCT CA-3′ | 56 |
| R: 5′-CTG CAG ATT TGT CAC GCA TT-3′ | |||
| R: 5′-GAG TAC TTC AGG GCC GTC AG-3′ | |||
| VIM | NM_001287023.1 | F: 5′-CCG ACA GGA TGT TGA CAA TG-3′ | 116 |
| R: 5′-GCT CCT GGA TTT CCT CAT CA-3′ | |||
| CDH11 | NM_001287125.1 | F: 5′-AAT GAC CCA GCT CGT GAA TC-3′ | 108 |
| R: 5′-CAC CTG GTC CTT GTT CTG GT-3′ | |||
| ACTB | NM_001195845.2 | F: 5′-GCG CAA GTA CTC TGT GTG GA-3′ | 65 |
| R: 5′-ACA TTT GCT GGA AGG TGG AC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Mahiddine, F.Y.; Kim, G.A. Leptin Modulates the Metastasis of Canine Inflammatory Mammary Adenocarcinoma Cells through Downregulation of Lysosomal Protective Protein Cathepsin A (CTSA). Int. J. Mol. Sci. 2020, 21, 8963. https://doi.org/10.3390/ijms21238963
Kim J-W, Mahiddine FY, Kim GA. Leptin Modulates the Metastasis of Canine Inflammatory Mammary Adenocarcinoma Cells through Downregulation of Lysosomal Protective Protein Cathepsin A (CTSA). International Journal of Molecular Sciences. 2020; 21(23):8963. https://doi.org/10.3390/ijms21238963
Chicago/Turabian StyleKim, Jin-Wook, Feriel Yasmine Mahiddine, and Geon A Kim. 2020. "Leptin Modulates the Metastasis of Canine Inflammatory Mammary Adenocarcinoma Cells through Downregulation of Lysosomal Protective Protein Cathepsin A (CTSA)" International Journal of Molecular Sciences 21, no. 23: 8963. https://doi.org/10.3390/ijms21238963







