Design, Synthesis and Evaluation of New Bioactive Oxadiazole Derivatives as Anticancer Agents Targeting Bcl-2
Abstract
1. Introduction
2. Results
2.1. Synthesis of Target Compounds
2.2. In Vitro Antiproliferative Activity
2.3. Evaluation of Bcl-2 Binding (ELISA Assay)
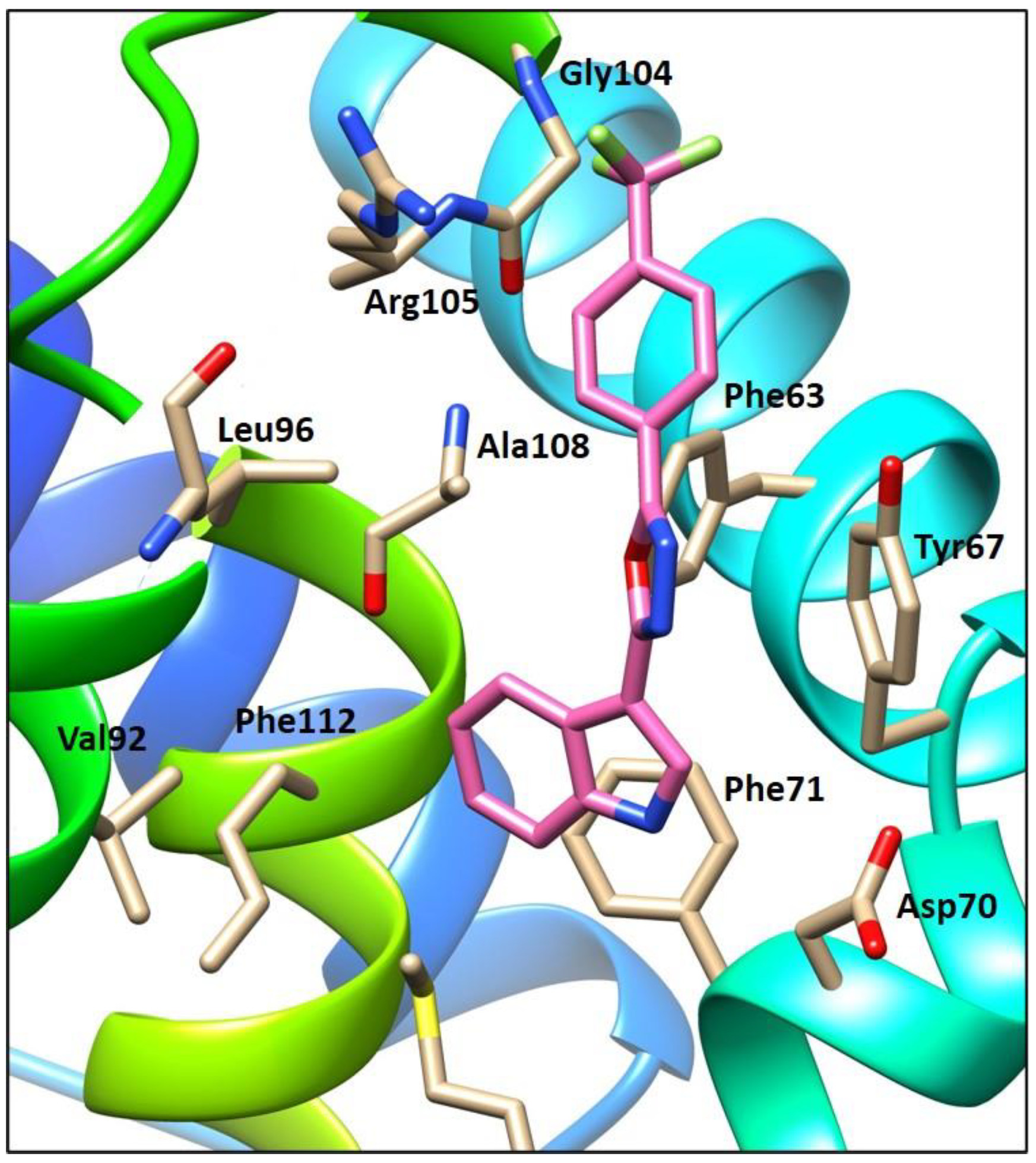
2.4. Molecular Modeling of Compound 4j within the Bcl-2 Binding Pocket
2.5. Physicochemical Properties and Assessment of Hit Optimization Potential
3. Discussion
4. Materials and Methods
4.1. General Experimental Details—Chemistry
4.2. Chemical Synthesis
4.2.1. Synthesis of 3-Indolylcarboxlic Acid Hydrazide (2)
4.2.2. General Method for Synthesis of 2-(1H-Indol-3-Yl)-5-Substituted-1,3,4-Oxadiazoles (4a–m)
4.3. Cell Viability—MTT Assay (MDA-MB-231 and HeLa Cells)
4.4. Cell Viability—CellTiter-Blue® Assay (KG1a and Jurkat Cells)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Molecular Modeling and Docking
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashkenazi, A.; Fairbrother, A.A.W.J.; Leverson, J.D.; Souers, J.D.L.A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Valentin, R.; Grabow, S.; Davids, M.S. The rise of apoptosis: Targeting apoptosis in hematologic malignancies. Blood 2018, 132, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Westwell, A.D.; Brahemi, G. Protein–protein interactions as targets for small-molecule therapeutics in cancer. Expert Rev. Mol. Med. 2008, 10, e8. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, A.D.; O’Brien, S.; Kantarjian, H.; Brandwein, J.; Cheson, B.D.; Minden, M.D.; Yee, K.; Ravandi, F.; Giles, F.J.; Schuh, A.; et al. A Phase I Study of the Pan Bcl-2 Family Inhibitor Obatoclax Mesylate in Patients with Advanced Hematologic Malignancies. Clin. Cancer Res. 2008, 14, 8295–8301. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Ziedan, N.I.; Ali, S.; Bordoni, C.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 5-(1H-indol-3-yl)-N-aryl-1,3,4-oxadiazol-2-amines as Bcl-2 inhibitory anticancer agents. Bioorganic Med. Chem. Lett. 2017, 27, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Ziedan, N.I.; Hamdy, R.; Cavaliere, A.; Kourti, M.; Prencipe, F.; Brancale, A.; Jones, A.T.; Westwell, A.D. Virtual screening, SAR, and discovery of 5-(indole-3-yl)-2-[(2-nitrophenyl)amino] [1,3,4]-oxadiazole as a novel Bcl-2 inhibitor. Chem. Biol. Drug Des. 2017, 90, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.I.; Ziedan, N.; Ali, S.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 3-(benzylthio)-5-(1H-indol-3-yl)-1,2,4-triazol-4-amines as Bcl-2 inhibitory anticancer agents. Bioorganic Med. Chem. Lett. 2013, 23, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; Jones, A.T.; Westwell, A.D. New Quinoline-Based Heterocycles as Anticancer Agents Targeting Bcl-2. Molecules 2019, 24, 1274. [Google Scholar] [CrossRef] [PubMed]
- Arisan, E.D.; Kutuk, O.; Tezil, T.; Bodur, C.; Telci, D.; Basaga, S.H. Small inhibitor of Bcl-2, HA14-1, selectively enhanced the apoptotic effect of cisplatin by modulating Bcl-2 family members in MDA-MB-231 breast cancer cells. Breast Cancer Res. Treat. 2010, 119, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.J.; Cusick, C.L.; Soltaninassab, S.; Sekhar, K.S.; Lu, S.; Freeman, M.L. Expression of Bcl-2 Increases Intracellular Glutathione by Inhibiting Methionine-Dependent GSH Efflux. Biochem. Biophys. Res. Commun. 1998, 248, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Sayers, E.J.; Allender, C.J.; Barrow, D.; Fegan, C.; Brennan, P.; Jones, A.T. Co-operative Membrane Disruption Between Cell-penetrating Peptide and Cargo: Implications for the Therapeutic Use of the Bcl-2 Converter Peptide D-NuBCP-9-r8. Mol. Ther. 2011, 19, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Xu, D.; Zheng, F.; Long, Z.-J.; Huang, S.-S.; Wu, X.; Zhou, W.-H.; Huang, R.-W.; Liu, Q. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J. Transl. Med. 2011, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tang, W.; Dai, Y.; Wu, X.; Liu, M.; Ji, Q.; Ji, M.; Pienta, K.; Lawrence, T.; Xu, L. Natural BH3 mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol. Cancer Ther. 2008, 7, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Research Collaboration for Structural Bioinformatics (RCSB) Protein Data Bank. Available online: www.rcsb.org/pdb (accessed on 13 August 2020).
- Molinspiration® Software. Available online: www.molinspiration.com (accessed on 12 October 2020).



| Compound | X | R | IC50 (μM) 1 | |||
|---|---|---|---|---|---|---|
| MDA-MB-2312 | HeLa 2 | KG1a 3 | Jurkat 3 | |||
| 4a | OCH2 | 4-F-C6H4 | 36.11 ± 3.4 | 12.86 ± 1.33 | >100 | >100 |
| 4b | OCH2 | 4-CH3-C6H4 | 31.53 ± 4.8 | 13.31 ± 0.32 | >100 | >100 |
| 4c | OCH2 | 4-tBu-C6H4 | 3.50 ± 0.16 | 0.9 ± 0.03 | 3.45 ± 0.8 | >100 |
| 4d | OCH2 | C6H5 | 1.63 ± 0.08 | 0.57 ± 0.04 | 2.56 ± 0.07 | >100 |
| 4e | CH2 | 3-OMe-C6H4 | 6.28 ± 0.20 | 3.14 ± 0.14 | 3.66 ± 0.15 | >100 |
| 4f | CH2 | C6H5 | >100 | >100 | >100 | >100 |
| 4g | - | 4-NO2-C6H4 | 6.95 ± 0.5 | 1.60 ± 0.12 | >100 | >100 |
| 4h | - | 4-OMe-C6H4 | 6.02 ± 0.12 | 0.92 ± 0.08 | 2.21 ± 0.1 | 60.59 ± 0.12 |
| 4i | - | 3,4,5-triOMe- C6H2 | 1.19 ± 0.1 | 1.22 ± 0.9 | 0.975 ± 0.02 | 12.25 ± 1.13 |
| 4j | - | 4-CF3-C6H4 | 0.52 ± 0.15 | 0.88 ± 0.16 | 0.73 ± 0.09 | >100 |
| 4k | - | 4-CH3-C6H4 | 0.92 ± 0.19 | 1.28 ± 0.35 | 1.47 ± 0.13 | >100 |
| 4l | - | 6(1-piperidinyl)-pyridin-3-yl | 2.97 ± 0.06 | 1.52 ± 0.03 | 4.5 ± 0.31 | 61.4 ± 1.4 |
| 4m | - | 5-OMe-indol-3-yl | 1.79 ± 0.07 | 1.19 ± 0.4 | 1.87 ± 0.14 | 57.45 ± 0.58 |
| Compound | ELISA IC50 (µM) 1 |
|---|---|
| 4b | >100 |
| 4c | 1.17 ± 0.13 |
| 4d | 1.41 ± 0.01 |
| 4e | 2.72 ± 0.2 |
| 4i | 21.05 ± 1.09 |
| 4j | 0.33 ± 0.05 |
| 4k | 0.43 ± 0.05 |
| 4m | 0.84 ± 0.05 |
| Gossypol | 0.60 ± 0.09 |
| Compound | X | R | MW | clogP | TPSA/Å | nON | nOHNH |
|---|---|---|---|---|---|---|---|
| 4d | OCH2 | C6H5 | 291.31 | 3.16 | 63.95 | 5 | 1 |
| 4e | CH2 | 3-OMe-C6H4 | 305.34 | 3.50 | 63.95 | 5 | 1 |
| 4j | - | 4-CF3-C6H4 | 329.28 | 4.77 | 54.72 | 4 | 1 |
| Obatoclax | - | - | 317.39 | 4.08 | 53.71 | 4 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdy, R.; Elseginy, S.A.; Ziedan, N.I.; El-Sadek, M.; Lashin, E.; Jones, A.T.; Westwell, A.D. Design, Synthesis and Evaluation of New Bioactive Oxadiazole Derivatives as Anticancer Agents Targeting Bcl-2. Int. J. Mol. Sci. 2020, 21, 8980. https://doi.org/10.3390/ijms21238980
Hamdy R, Elseginy SA, Ziedan NI, El-Sadek M, Lashin E, Jones AT, Westwell AD. Design, Synthesis and Evaluation of New Bioactive Oxadiazole Derivatives as Anticancer Agents Targeting Bcl-2. International Journal of Molecular Sciences. 2020; 21(23):8980. https://doi.org/10.3390/ijms21238980
Chicago/Turabian StyleHamdy, Rania, Samia A. Elseginy, Noha I. Ziedan, Mohamed El-Sadek, Elsaid Lashin, Arwyn T. Jones, and Andrew D. Westwell. 2020. "Design, Synthesis and Evaluation of New Bioactive Oxadiazole Derivatives as Anticancer Agents Targeting Bcl-2" International Journal of Molecular Sciences 21, no. 23: 8980. https://doi.org/10.3390/ijms21238980
APA StyleHamdy, R., Elseginy, S. A., Ziedan, N. I., El-Sadek, M., Lashin, E., Jones, A. T., & Westwell, A. D. (2020). Design, Synthesis and Evaluation of New Bioactive Oxadiazole Derivatives as Anticancer Agents Targeting Bcl-2. International Journal of Molecular Sciences, 21(23), 8980. https://doi.org/10.3390/ijms21238980






