E2F1 Promotes Progression of Bladder Cancer by Modulating RAD54L Involved in Homologous Recombination Repair
Abstract
:1. Introduction
2. Results
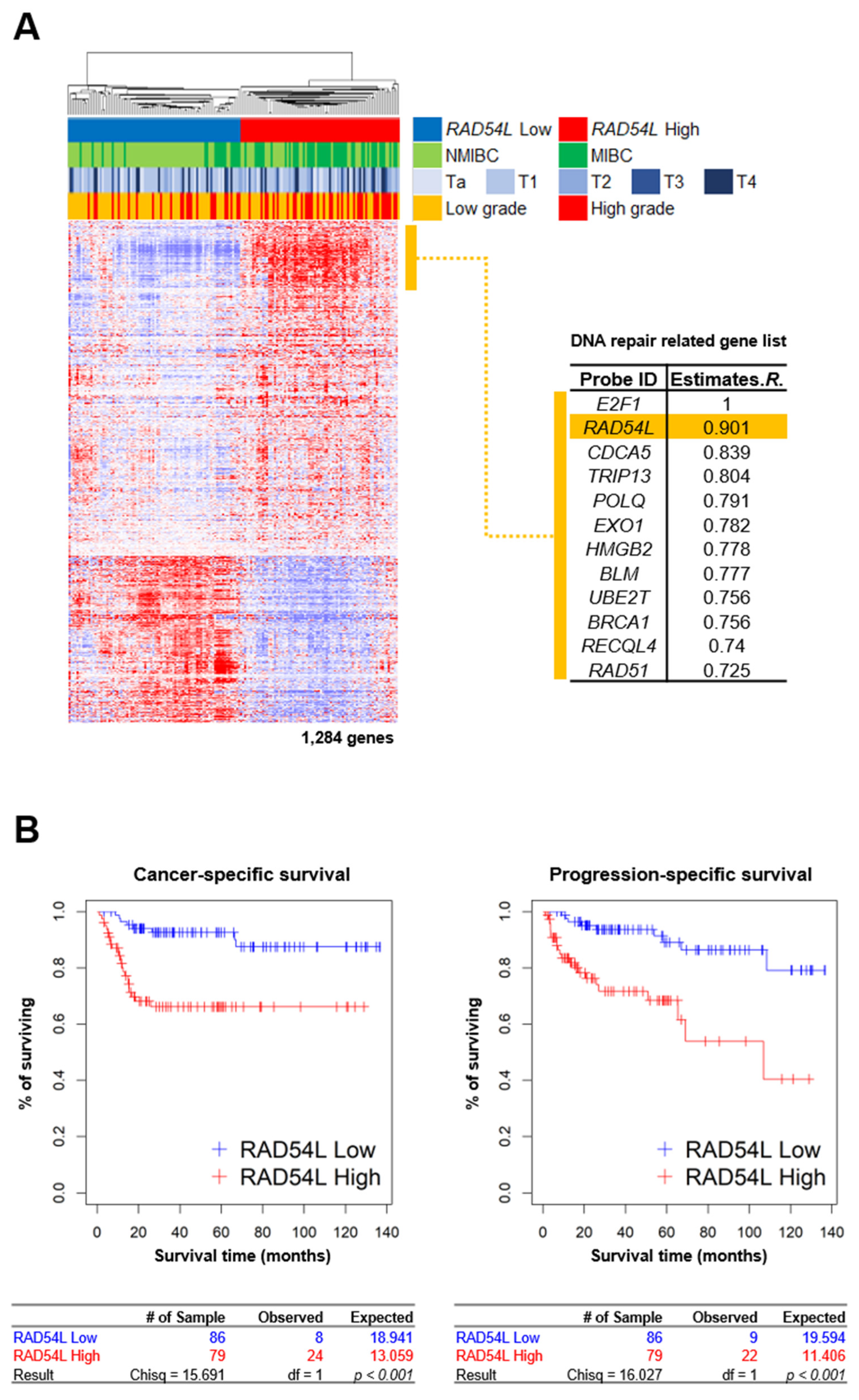
2.1. DNA Repair Gene RAD54L Is Strongly Correlated with E2F1 in Gene Expression Profiles of Bladder Cancer Patients
2.2. E2F1 Directly Regulates HRR-Related RAD54L Expression
2.3. E2F1 Is Involved in HRR Pathway via Regulation of RAD54L
2.4. High Expression Levels of E2F1 and RAD54L Are Correlated with Cancer Progression and Poor Prognosis in Patients with Bladder Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Plasmids and Reagents
4.3. Luciferase Assay
4.4. Quantitative Real Time-PCR (qRT-PCR)
4.5. Western Blot and Chromatin Immunoprecipitation (ChIP) Assay
4.6. Comet Assay and HR Repair Assay
4.7. Immunohistochemical (IHC) Analysis
4.8. Microarray Gene Expression Profiling and Clinical Information
4.9. Gene Set Networks and Ontology Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redondo-Gonzalez, E.; de Castro, L.N.; Moreno-Sierra, J.; de las Casas, M.L.M.; Vera-Gonzalez, V.; Ferrari, D.G.; Corchado, J.M. Bladder Carcinoma Data with Clinical Risk Factors and Molecular Markers: A Cluster Analysis. BioMed Res. Int. 2015, 2015, 168682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldousari, S.; Kassouf, W. Update on the management of non-muscle invasive bladder cancer. CUAJ Can. Urol. Assoc. J. 2010, 4, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvester, R.J.; van der Meijden, A.P.M.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Park, J.C.; Citrin, D.E.; Agarwal, P.K.; Apolo, A.B. Multimodal management of muscle-invasive bladder cancer. Curr. Probl. Cancer 2014, 38, 80–108. [Google Scholar] [CrossRef] [Green Version]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Raghavan, S.C. DNA Double-Strand Break Repair Inhibitors as Cancer Therapeutics. Chem. Biol. 2015, 22, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Lieber, M.R. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End-Joining Pathway. In Annual Review of Biochemistry; Kornberg, R.D., Raetz, C.R.H., Rothman, J.E., Thorner, J.W., Eds.; Annual Reviews: Palo Alto, CA, USA, 2010; Volume 79, pp. 181–211. [Google Scholar]
- Sishc, B.J.; Davis, A.J. The Role of the Core Non-Homologous End Joining Factors in Carcinogenesis and Cancer. Cancers 2017, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Heyer, W.D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Hannay, J.A.F.; Liu, J.H.; Zhu, Q.S.; Bolshakov, S.V.; Li, L.; Pisters, P.W.T.; Lazar, A.J.F.; Yu, D.H.; Pollock, R.E.; Lev, D. Rad51 overexpression contributes to chemoresistance in human soft tissue sarcoma cells: A role for p53/activator protein 2 transcriptional regulation. Mol. Cancer Ther. 2007, 6, 1650–1660. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.Y.; Bozzella, M.; Seluanov, A.; Gorbunova, V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle 2008, 7, 2902–2906. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Jameson, C.; Barbachano, Y.; Sanchez, L.; Kote-Jarai, Z.; Peock, S.; Sodha, N.; Bancroft, E.; Fletcher, A.; Cooper, C.; et al. Overexpression of RAD51 occurs in aggressive prostatic cancer. Histopathology 2009, 55, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Marignac, V.L.; Rodrigue, A.; Davidson, D.; Couillard, M.; Al-Moustafa, A.E.; Abramovitz, M.; Foulkes, W.D.; Masson, J.Y.; Aloyz, R. The Effect of a DNA Repair Gene on Cellular Invasiveness: Xrcc3 Over-Expression in Breast Cancer Cells. PLoS ONE 2011, 6, e16394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stornetta, A.; Zimmermann, M.; Cimino, G.D.; Henderson, P.T.; Sturla, S.J. DNA Adducts from Anticancer Drugs as Candidate Predictive Markers for Precision Medicine. Chem. Res. Toxicol. 2017, 30, 388–409. [Google Scholar] [CrossRef]
- Birkelbach, M.; Ferraiolo, N.; Gheorghiu, L.; Pfaffle, H.N.; Daly, B.; Ebright, M.I.; Spencer, C.; O’Hara, C.; Whetstine, J.R.; Benes, C.H.; et al. Detection of Impaired Homologous Recombination Repair in NSCLC Cells and Tissues. J. Thorac. Oncol. 2013, 8, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Leem, S.H.; Lee, S.Y.; Kim, S.C.; Park, E.S.; Kim, S.B.; Kim, S.K.; Kim, Y.J.; Kim, W.J.; Chu, I.S. Expression Signature of E2F1 and Its Associated Genes Predict Superficial to Invasive Progression of Bladder Tumors. J. Clin. Oncol. 2010, 28, 2660–2667. [Google Scholar] [CrossRef]
- Muller, H.; Bracken, A.P.; Vernell, R.; Moroni, M.C.; Christians, F.; Grassilli, E.; Prosperini, E.; Vigo, E.; Oliner, J.D.; Helin, K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001, 15, 267–285. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.K.; Johnson, D.G. Transcriptional and Nontranscriptional Functions of E2F1 in Response to DNA Damage. Cancer Res. 2012, 72, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Berton, T.R.; Mitchell, D.L.; Guo, R.F.; Johnson, D.G. Regulation of epidermal apoptosis and DNA repair by E2F1 in response to ultraviolet B radiation. Oncogene 2005, 24, 2449–2460. [Google Scholar] [CrossRef] [Green Version]
- Dupuy, A.; Sarasin, A. DNA damage and gene therapy of xeroderma pigmentosum, a human DNA repair-deficient disease. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2015, 776, 2–8. [Google Scholar] [CrossRef]
- Kiwerska, K.; Szyfter, K. DNA repair in cancer initiation, progression, and therapy-a double-edged sword. J. Appl. Genet. 2019, 60, 329–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietlein, F.; Thelen, L.; Reinhardt, H.C. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet. 2014, 30, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Tsai, S.Y.; Leone, G. Emerging roles of E2Fs in cancer: An exit from cell cycle control. Nat. Rev. Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothkamm, K.; Kruger, I.; Thompson, L.H.; Lobrich, M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 2003, 23, 5706–5715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.G.; Cooper, J.P. Two modes of DNA double-strand break repair are reciprocally regulated through the fission yeast cell cycle. Genes Dev. 2004, 18, 2249–2254. [Google Scholar] [CrossRef] [Green Version]
- Kiianitsa, K.; Solinger, J.A.; Heyer, W.D. Terminal association of Rad54 protein with the Rad51-dsDNA filament. Proc. Natl. Acad. Sci. USA 2006, 103, 9767–9772. [Google Scholar] [CrossRef] [Green Version]
- Heyer, W.D.; Li, X.; Rolfsmeier, M.; Zhang, X.P. Rad54: The Swiss Army knife of homologous recombination? Nucleic Acids Res. 2006, 34, 4115–4125. [Google Scholar] [CrossRef]
- Roh, Y.G.; Mun, J.Y.; Kim, S.K.; Park, W.Y.; Jeong, M.S.; Kim, T.N.; Kim, W.T.; Choi, Y.H.; Chu, I.S.; Leem, S.H. Fanconi Anemia Pathway Activation by FOXM1 is Critical to Bladder Cancer Recurrence and Anticancer Drug Resistance. Cancers 2020, 12, 1417. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Pierce, A.J.; Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 2001, 7, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Lin, C.C.J.; Mo, W.; Dai, H.; Park, Y.Y.; Kim, S.M.; Peng, Y.; Mo, Q.X.; Siwko, S.; Hu, R.Z.; et al. Genome-wide transcriptome profiling of homologous recombination DNA repair. Nat. Commun. 2014, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; Yim, E.K.; Dai, H.; Jackson, A.P.; van der Burgt, I.; Pan, M.R.; Hu, R.Z.; Li, K.Y.; Lin, S.Y. BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nat. Cell Biol. 2009, 11, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgovnick, A.; Schumacher, B. DNA repair mechanisms in cancer development and therapy. Front. Genet. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herr, H.W. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J. Urol. 2000, 163, 60–61. [Google Scholar] [CrossRef]
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [Green Version]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-Damaging Agents in Cancer Chemotherapy: Serendipity and Chemical Biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhu, F.; Weaks, R.L.; Biswas, A.K.; Guo, R.F.; Li, Y.J.; Johnson, D.G. E2F1 promotes the recruitment of DNA repair factors to sites of DNA double-strand breaks. Cell Cycle 2011, 10, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.H.; Kim, K.P. E2F1 facilitates DNA break repair by localizing to break sites and enhancing the expression of homologous recombination factors. Exp. Mol. Med. 2019, 51, 12. [Google Scholar] [CrossRef]
- Ceballos, S.J.; Heyer, W.D. Functions of the Snf2/Swi2 family Rad54 motor protein in homologous recombination. Biochim. Biophys. Acta Gene Regul. Mech. 2011, 1809, 509–523. [Google Scholar] [CrossRef] [Green Version]
- Mazin, A.V.; Alexeev, A.A.; Kowalczykowski, S.C. A novel function of Rad54 protein—Stabilization of the Rad51 nucleoprotein filament. J. Biol. Chem. 2003, 278, 14029–14036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; van Cappellen, W.A.; Guenole, A.; Eppink, B.; Linsen, S.E.V.; Meijering, E.; Houtsmuller, A.; Kanaar, R.; Essers, J. ATP-dependent and independent functions of Rad54 in genome maintenance. J. Cell Biol. 2011, 192, 735–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbaum, J.C.; Bonilla, B.; Hengel, S.R.; Mertz, T.M.; Herken, B.W.; Kazemier, H.G.; Pressimone, C.A.; Ratterman, T.C.; MacNary, E.; De Magis, A.; et al. The Rad51 paralogs facilitate a novel DNA strand specific damage tolerance pathway. Nat. Commun. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; Miyagawa, K.; Takahashi, M.; Fukuda, T.; Kataoka, T.; Asahara, T.; Inui, H.; Watatani, M.; Yasutomi, M.; Kamada, N.; et al. Mutations in the RAD54 recombination gene in primary cancers. Oncogene 1999, 18, 3427–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.R.; Roh, Y.G.; Kim, S.K.; Lee, J.S.; Seol, S.Y.; Lee, H.H.; Kim, W.T.; Kim, W.J.; Heo, J.; Cha, H.J.; et al. Activation of EZH2 and SUZ12 Regulated by E2F1 Predicts the Disease Progression and Aggressive Characteristics of Bladder Cancer. Clin. Cancer Res. 2015, 21, 5391–5403. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Roh, Y.G.; Lee, H.H.; Lee, S.Y.; Kim, S.I.; Lee, B.J.; Leem, S.H. The E2F1 Oncogene Transcriptionally Regulates NELL2 in Cancer Cells. DNA Cell Biol. 2013, 32, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Roh, Y.G.; Mun, M.H.; Jeong, M.S.; Kim, W.T.; Lee, S.R.; Chung, J.W.; Kim, S.I.; Kim, T.N.; Nam, J.K.; Leem, S.H. Drug resistance of bladder cancer cells through activation of ABCG2 by FOXM1. BMB Rep. 2018, 51, 98–103. [Google Scholar] [CrossRef]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. Open Comet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, J.-Y.; Baek, S.-W.; Park, W.Y.; Kim, W.-T.; Kim, S.-K.; Roh, Y.-G.; Jeong, M.-S.; Yang, G.-E.; Lee, J.-H.; Chung, J.W.; et al. E2F1 Promotes Progression of Bladder Cancer by Modulating RAD54L Involved in Homologous Recombination Repair. Int. J. Mol. Sci. 2020, 21, 9025. https://doi.org/10.3390/ijms21239025
Mun J-Y, Baek S-W, Park WY, Kim W-T, Kim S-K, Roh Y-G, Jeong M-S, Yang G-E, Lee J-H, Chung JW, et al. E2F1 Promotes Progression of Bladder Cancer by Modulating RAD54L Involved in Homologous Recombination Repair. International Journal of Molecular Sciences. 2020; 21(23):9025. https://doi.org/10.3390/ijms21239025
Chicago/Turabian StyleMun, Jeong-Yeon, Seung-Woo Baek, Won Young Park, Won-Tae Kim, Seon-Kyu Kim, Yun-Gil Roh, Mi-So Jeong, Gi-Eun Yang, Jong-Ho Lee, Jin Woong Chung, and et al. 2020. "E2F1 Promotes Progression of Bladder Cancer by Modulating RAD54L Involved in Homologous Recombination Repair" International Journal of Molecular Sciences 21, no. 23: 9025. https://doi.org/10.3390/ijms21239025
APA StyleMun, J.-Y., Baek, S.-W., Park, W. Y., Kim, W.-T., Kim, S.-K., Roh, Y.-G., Jeong, M.-S., Yang, G.-E., Lee, J.-H., Chung, J. W., Choi, Y. H., Chu, I.-S., & Leem, S.-H. (2020). E2F1 Promotes Progression of Bladder Cancer by Modulating RAD54L Involved in Homologous Recombination Repair. International Journal of Molecular Sciences, 21(23), 9025. https://doi.org/10.3390/ijms21239025






