Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications
Abstract
1. Introduction
2. Importance of Conifers
3. Green Synthesis of Nanoparticles Mediated by Conifers Extract Synthesis Mechanism, Characterization
3.1. Mechanism
3.2. Characterization Techniques
4. Types of Nanoparticles
4.1. Metal Nanoparticles
4.1.1. Silver Nanoparticles
4.1.2. Gold
4.2. Nanoparticles of Metallic Oxides
5. Conifer-Derived Metallic Nanoparticles Potential Applications
5.1. Biomedical Applications
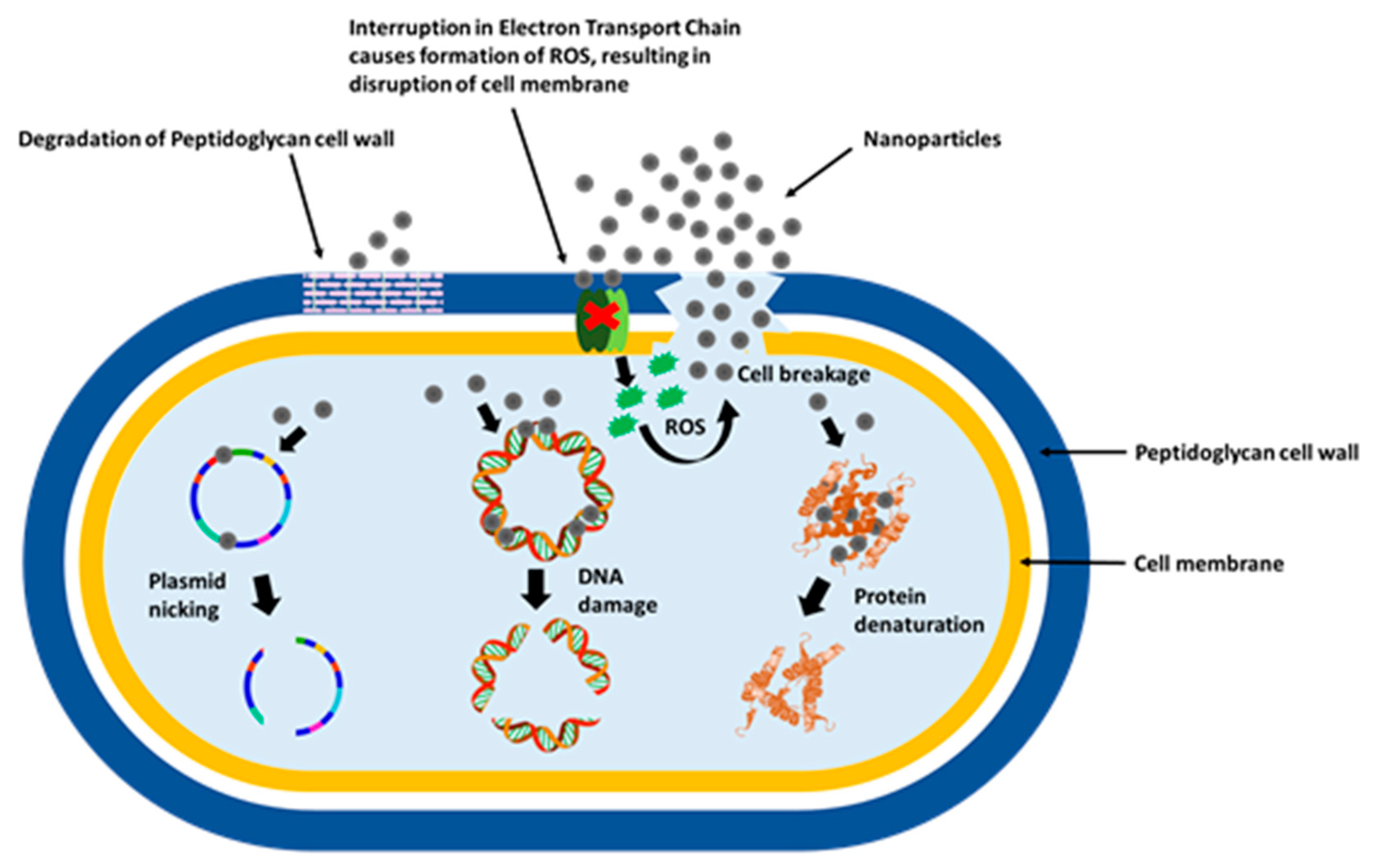
5.1.1. Antimicrobial Action
Mechanism of Action Towards Microbes
Direct Contact with the Cell Membrane
Destabilization of the Cell Membrane, Changes in Its Permeability and Metal Ions Release
Production of Reactive Oxygen Species (ROS) and Free Radicals
Signal Transduction Pathways Modulation
5.1.2. Anticancer
Mechanism behind anticancer activity
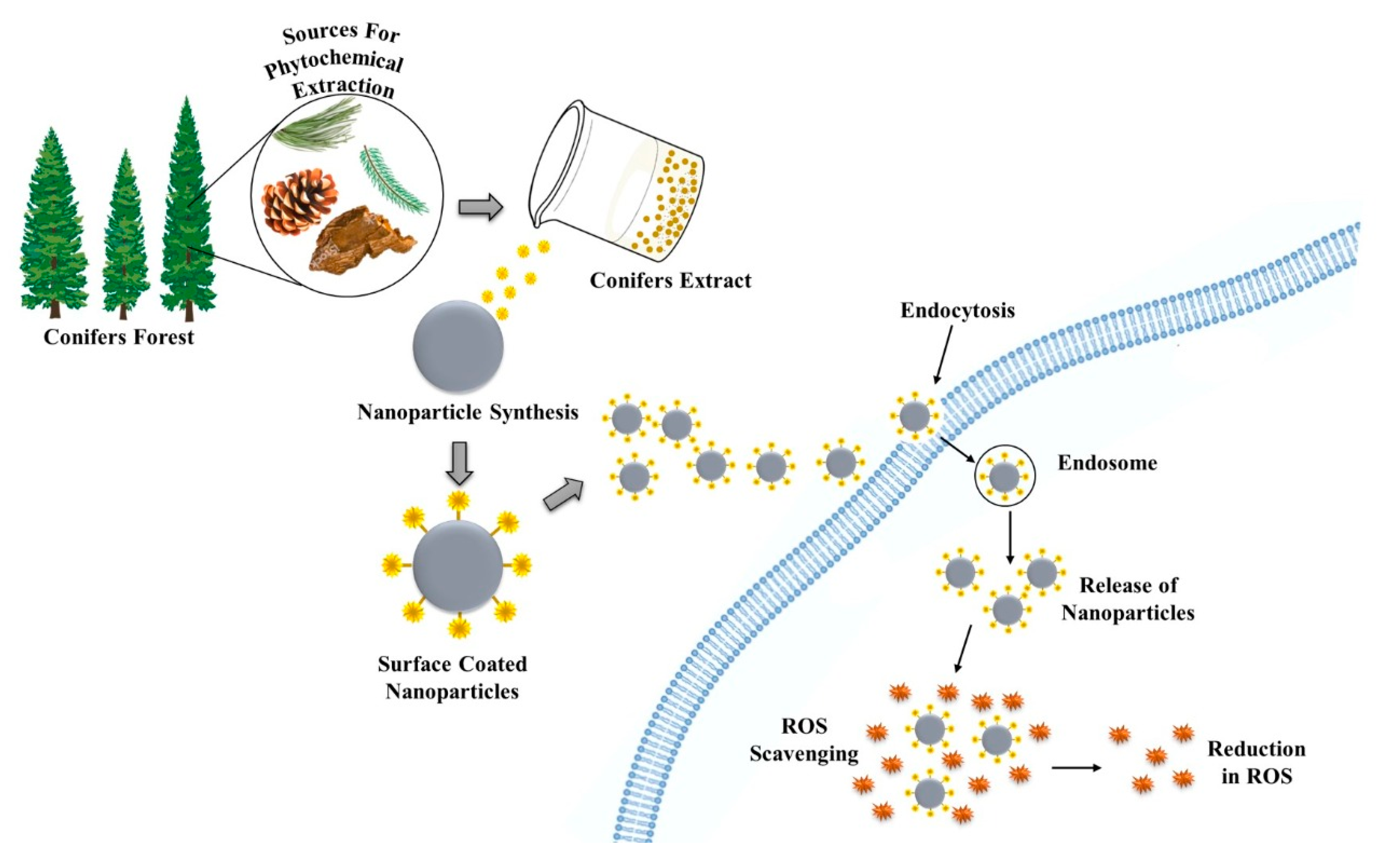
5.1.3. Antioxidant
5.2. Other Activities
6. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A549 | Human Lung Carcinoma Epithelial Cells |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | Acetylcholinesterase |
| AFM | Atomic force microscopy |
| Ag+ | Silver Ions |
| AgNPs | Silver Nanoparticles |
| ATR-FTIR | Attenuated total reflection-Fourier-transform infrared spectroscopy |
| AuNPs | Gold Nanoparticles |
| BChE | Butyrylcholinesterase |
| Caov-4 | Human Ovarian Cancer Cell Line |
| CSH | Cupressus Sempervirens Var. Horizantalis |
| CSP | Cupressus Sempervirens Var. Pyramidalis |
| CuNPs | Copper Nanoparticles |
| DLS | Dynamic Light Scattering |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2,1-diphenyl-1-picrylhydrazyl |
| EDX | Energy-dispersive X-ray spectroscopy |
| Eos | Essential Oils |
| FeNPs | Iron Nanoparticles |
| FESEM | Field Emission Electron Microscope |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| GSH | Glutathione |
| GSNPs | Green Synthesized Nanoparticles |
| H2O2 | Hydrogen peroxide |
| HEK293 | Human Embryonic Kidney Cell Line |
| HeLa | Human Cells Derived From Cervical Cancer Cell Line |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| HSA | Human Serum Albumin |
| HT1080 | Human Fibrosarcoma Cell Line |
| IC50 | Half Maximal Inhibitory Concentration |
| K562 | Immortalised Myelogenous Leukemia Cell Line |
| KB | Ubiquitous KERATIN-Forming Tumour Cell Line Hela |
| L929 | Mouse Fibroblast Cell Line |
| LS174T | Human Caucasian colon adenocarcinoma cell line |
| MDA-MB231 | Epithelial, Human Breast Cancer Cell Line |
| MCF-7 | Breast Cancer Cell Line |
| MIC | Minimum Inhibitory Concentration |
| MNPs | Metal Nanoparticles |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ND | Not determined |
| NPs | Nanoparticles |
| NS | Not Specified |
| O2− | Superoxide Ion |
| OH• | Hydroxyl Radical |
| PBMCs | Peripheral Blood Mononuclear Cells |
| Pd/RPN | Palladium/Residue of the Pine Needle |
| ROS | Reactive Oxygen Species |
| RT | Room Temperature |
| SEM | Scanning Electron Microscopy |
| SMMC-7721 | Hepatocellular Carcinoma Cell Line |
| TEM | Transmission Electron Microscopy |
| TYRO | Tyrosinase |
| UV–Vis | Ultraviolet-Visible Spectroscopy |
| WHO | World Health Organization |
| XRD | X-Ray Powder Diffraction |
| ZnO NPs | Zinc Oxide Nanoparticles |
| ZVI@AgNPs | Zero Valent Iron Coated Silver Nanoparticles |
References
- Bhattacharyya, D.; Singh, S.; Satnalika, N.; Khandelwal, A.; Jeon, S.-H. Nanotechnology, big things from a tiny world: A review. Int. J. u-e-Serv. Sci. Technol. 2009, 2, 29–38. [Google Scholar]
- Baker, S.; Satish, S. Endophytes: Toward a vision in synthesis of nanoparticle for future therapeutic agents. Int. J. Bio-Inorg. Hybd. Nanomat. 2012, 1, 67–77. [Google Scholar]
- Sarmast, M.K.; Salehi, H. Silver Nanoparticles: An Influential Element in Plant Nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 1–24. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures & Nanomaterials: Synthesis, Properties & Applications; Imperial College Press: London, UK, 2004; ISBN 1860944809. [Google Scholar]
- Kavitha, K.S.; Baker, S.; Rakshith, D.; Kavitha, H.U.; Yashwantha Rao, H.C.; Harini, B.P.; Satish, S. Plants as green source towards synthesis of nanoparticles. Int. Res. J. Biol. Sci. 2013, 2, 66–76. [Google Scholar]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuca, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Mehta, M.; Sharma, P.; Kaur, S.; Dhanjal, D.S.; Singh, B.; Vyas, M.; Gupta, G.; Chellappan, D.K.; Nammi, S.; Singh, T.G.; et al. Plant-based drug delivery systems in respiratory diseases. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Academic Press: Cambrige, MA, USA, 2020; pp. 517–539. [Google Scholar]
- Ndeh, N.T.; Maensiri, S.; Maensiri, D. The effect of green synthesized gold nanoparticles on rice germination and roots. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035008. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 104197. [Google Scholar] [CrossRef]
- Gernandt, D.; Willyard, A.; Syring, J.; Liston, A. The Conifers (Pinophyta). Genet. Genom. Breed. Conifers 2011, 29–67. [Google Scholar] [CrossRef]
- Farjon, A. Coniferous Trees. In Forests and Forest Plants-Vol II; Owens, J.N., Lund, H.G., Eds.; Eolss Publisher Co. Ltd.: Oxford, UK, 2009; pp. 39–58. [Google Scholar]
- Mourey, A.; Canillac, N. Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control. 2002, 13, 289–292. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Islam, M.T.; Jayasena, V.; Sharma, B.; Sharma, S.; Sharma, P.; Kuča, K.; Bhardwaj, P. Review on essential oils, chemical composition, extraction, and utilization of some conifers in Northwestern Himalayas. Phytotherapy Res. 2020, 34, 2889–2910. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Bhardwaj, P.; Kaur, S. Medicinal Value of Secondary Metabolites of Pines grown in Himalayan Region of India. Res. J. Biotech. 2020, 15, 131–140. [Google Scholar]
- Abdillahi, H.; Stafford, G.; Finnie, J.; Van Staden, J. Ethnobotany, phytochemistry and pharmacology of Podocarpus sensu latissimo (s.l.). South Afr. J. Bot. 2010, 76, 1–24. [Google Scholar] [CrossRef]
- Aslam, M.S.; Choudhary, B.; Uzair, M.; Ijaz, A. Phytochemical and Ethno-Pharmacological Review of the Genus Araucaria—Review. Trop. J. Pharm. Res. 2013, 12, 651–659. [Google Scholar] [CrossRef]
- Kumar, B.; Rani, R.; Das, S.; Das, S. Phytoconstituents and therapeutic potential of Thuja occidentalis. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 354–362. [Google Scholar]
- Tumen, I.; Deniz, F.S.S.; Orhan, I.E. Evaluation of possible in vitro neurobiological effects of two varieties of Cupressus sempervirens (Mediterranean cypress) through their antioxidant and enzyme inhibition actions. Turk. J. Biochem. 2012, 37, 5–13. [Google Scholar] [CrossRef]
- Branco, C.S.; Rodrigues, T.S. Chemical Constituents and Biological Activities of Araucaria angustifolia (Bertol.) O. Kuntze: A Review. J. Org. Inorg. Chem. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Medical importance of Cupressus sempervirens—A review. IOSR J. Pharm. 2016, 6, 66–76. [Google Scholar]
- Naser, B.; Bodinet, C.; Tegtmeier, M.; Lindequist, U. Thuja occidentalis (Arbor vitae): A review of its pharmaceutical, pharmacological and clinical properties. Evid. Based Complement. Altern. Med. 2005, 2, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Hong, Y.D.; Kim, E.; Baik, K.-S.; Yoon, D.H.; Kim, S.; Lee, M.-N.; Rho, H.S.; Shin, S.S.; et al. Src/Syk/IRAK1-targeted anti-inflammatory action of Torreya nucifera butanol fraction in lipopolysaccharide-activated RAW264.7 cells. J. Ethnopharmacol. 2016, 188, 167–176. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, J.-R.; Han, J.-M.; Jang, K.C.; Sok, D.-E.; Jeong, T.-S. Antioxidant Activities of Abietane Diterpenoids Isolated fromTorreya nuciferaLeaves. J. Agric. Food Chem. 2006, 54, 5369–5374. [Google Scholar] [CrossRef]
- Yu, S.; Yan, H.; Zhang, L.; Shan, M.; Chen, P.-D.; Ding, A.; Li, S.F.Y. A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules 2017, 22, 299. [Google Scholar] [CrossRef]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of Silver Nanoparticles Using Taxus yunnanensis Callus and Their Antibacterial Activity and Cytotoxicity in Human Cancer Cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomater. 2020, 10, 1146. [Google Scholar] [CrossRef]
- Liu, G.; Bai, X.; Lv, H. Green synthesis of supported palladium nanoparticles employing pine needles as reducing agent and carrier: New reusable heterogeneous catalyst in the Suzuki coupling reaction. Appl. Organomet. Chem. 2016, 31, e3587. [Google Scholar] [CrossRef]
- Taghizadeh, S.-M.; Berenjian, A.; Taghizadeh, S.; Ghasemi, Y.; Taherpour, A.; Sarmah, A.K.; Ebrahiminezhad, A. One-put green synthesis of multifunctional silver iron core-shell nanostructure with antimicrobial and catalytic properties. Ind. Crop. Prod. 2019, 130, 230–236. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Khosropour, A.R.; Razmjou, A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014, 4, 61394–61403. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Maurya, S.; Bhardwaj, A.K.; Gupta, K.K.; Agarwal, S.; Kushwaha, A.; Vk, C.; Pathak, R.K.; Gopal, R.; Uttam, K.N.; Singh, A.K. Green synthesis of silver nanoparticles using Pleurotus and its bactericidal activity. Cell. Mol. Biol. 2016, 62, 131. [Google Scholar]
- Mohamed, M.S.; Kumar, D.S. Effect of Nanoparticles on Plants with Regard to Physiological Attributes. In Plant Nanotechnology; Springer Science and Business Media LLC: Cham, Switzerland, 2016; pp. 119–153. [Google Scholar]
- Baruwati, B.; Varma, R.S. High Value Products from Waste: Grape Pomace Extract A Three-in-One Package for the Synthesis of Metal Nanoparticles. ChemSusChem 2009, 2, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varma, R.S. A Greener Synthesis of Core (Fe, Cu)-Shell (Au, Pt, Pd, and Ag) Nanocrystals Using Aqueous Vitamin C. Cryst. Growth Des. 2007, 7, 2582–2587. [Google Scholar] [CrossRef]
- Mallikarjuna, N.N.; Varma, R.S. Microwave-Assisted Shape-Controlled Bulk Synthesis of Noble Nanocrystals and Their Catalytic Properties. Cryst. Growth Des. 2007, 7, 686–690. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Baruwati, B.; Nadagouda, M.N.; Varma, R.S. Bulk Synthesis of Monodisperse Ferrite Nanoparticles at Water−Organic Interfaces under Conventional and Microwave Hydrothermal Treatment and Their Surface Functionalization. J. Phys. Chem. C 2008, 112, 18399–18404. [Google Scholar] [CrossRef]
- Baruwati, B.; Polshettiwar, V.; Varma, R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009, 11, 926–930. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-Assembly of Metal Oxides into Three-Dimensional Nanostructures: Synthesis and Application in Catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef]
- Lukman, A.I.; Gong, B.; Marjo, C.E.; Roessner, U.; Harris, A.T. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J. Colloid Interface Sci. 2011, 353, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhafri, K.; Ching, C.L.; Philip, K. Phyto-synthesis of silver nanoparticles and its bioactivity response towards nosocomial bacterial pathogens. Biocatal. Agric. Biotechnol. 2019, 18, 101075. [Google Scholar] [CrossRef]
- Wu, T.; Duan, X.; Hu, C.; Wu, C.; Chen, X.; Huang, J.; Liu, J.; Cui, S. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Saipriya, C.; Angalene, J.L.A.; Roshini, S.M.; Cypriyana, P.J.J.; Saigeetha, S.; Raji, P.; Kumar, S.S. Evaluation of Nanotoxicity of Araucaria heterophylla Gum Derived Green Synthesized Silver Nanoparticles on Eudrilus eugeniae and Danio rerio. J. Clust. Sci. 2019, 30, 1017–1024. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Berta, L.; Coman, N.-A.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Nicolescu, A.; Jakab-Farkas, L.; Biró, D.; et al. Antibacterial and Antioxidant Potential of Silver Nanoparticles Biosynthesized Using the Spruce Bark Extract. Nanomaterials 2019, 9, 1541. [Google Scholar] [CrossRef]
- Iravani, S.; Zolfaghari, B. Green Synthesis of Silver Nanoparticles UsingPinus eldaricaBark Extract. BioMed Res. Int. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Masruri, M.; Pangestin, D.N.; Ulfa, S.M.; Riyanto, S.; Srihardyastutie, A.; Rahman, M.F. A Potent Staphylococcus Aureus Growth Inhibitor Of A Dried Flower Extract Of Pinus Merkusii Jungh & De Vriese And Copper Nanoparticle. IOP Conf. Series: Mater. Sci. Eng. 2018, 299, 12072. [Google Scholar] [CrossRef]
- Mariychuk, R.; Fejer, J.; Porubska, J.; Grishchenko, L.M.; Lisnyak, V.V. Green synthesis and characterization of gold triangular nanoprisms using extract of Juniperus communis L. Appl. Nanosci. 2020, 10, 2835–2841. [Google Scholar] [CrossRef]
- Prashanth, S.; Menaka, I.; Muthezhilan, R.; Sharma, N.K. Synthesis of plant-mediated silver nano particles using medicinal plant extract and evaluation of its anti microbial activities. Int. J. Eng. Sci. Technol. 2011, 3, 6235–6250. [Google Scholar]
- Velmurugan, P.; Park, J.-H.; Lee, S.-M.; Jang, J.S.; Lee, K.-J.; Han, S.-S.; Lee, S.-H.; Cho, M.; Oh, B.-T. Synthesis and characterization of nanosilver with antibacterial properties using Pinus densiflora young cone extract. J. Photochem. Photobiol. B: Biol. 2015, 147, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Azkiya, N.I.; Masruri, M.; Ulfa, S.M. Green Synthesis of Silver Nanoparticles using Extract ofPinus merkusiiJungh & De Vriese Cone Flower. IOP Conf. Series: Mater. Sci. Eng. 2018, 299, 12070. [Google Scholar] [CrossRef]
- Samrot, A.V.; Angalene, J.L.A.; Roshini, S.M.; Raji, P.; Stefi, S.M.; Preethi, R.; Selvarani, A.J.; Madankumar, A. Bioactivity and Heavy Metal Removal Using Plant Gum Mediated Green Synthesized Silver Nanoparticles. J. Clust. Sci. 2019, 30, 1599–1610. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and in vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Davoodi, D. A rapid biosynthesis route for the preparation of gold nanoparticles by aqueous extract of cypress leaves at room temperature. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2012, 94, 84–88. [Google Scholar] [CrossRef]
- Kheshtzar, R.; Berenjian, A.; Taghizadeh, S.-M.; Ghasemi, Y.; Asad, A.G.; Ebrahiminezhad, A. Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles. Green Process. Synth. 2019, 8, 846–855. [Google Scholar] [CrossRef]
- Hernández, L.G.; Islas, D.A.; Guerrero, M.U.F.; Ortega, P.A.R.; Lechuga, L.G. Use of Extract of Cupressus Goveniana for Synthesis and Stabilization of Nanoparticles Silver. In Proceedings of the TMS 2015 144th Annual Meeting & Exhibition; Springer Science and Business Media LLC: Cham, Switzerland, 2015; pp. 1105–1112. [Google Scholar]
- Ebrahiminezhad, A.; Taghizadeh, S.; Ghasemi, Y. Green Synthesis of Silver Nanoparticles using Mediterranean Cypress (Cupressus sempervirens) Leaf Extract. Am. J. Biochem. Biotechnol. 2017, 13, 1–6. [Google Scholar] [CrossRef]
- Rajput, K.; Bhatt, A.; Agrawal, P.K. Plant mediated biosynthesis, characterization and application of silver nanoparticles by leaves extract of cupressus torulosa. Int. J. Adv. Res. 2016, 4, 1199–1207. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Kilany, M.; Ghramh, H.A.; Khan, K.A.; Islam, S.U. Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol. Sci. 2019, 26, 1689–1694. [Google Scholar] [CrossRef]
- Kanawaria, S.K.; Sankhla, A.; Jatav, P.K.; Yadav, R.S.; Verma, K.S.; Velraj, P.; Kachhwaha, S.; Kothari, S.L. Rapid biosynthesis and characterization of silver nanoparticles: An assessment of antibacterial and antimycotic activity. Appl. Phys. A 2018, 124, 320. [Google Scholar] [CrossRef]
- Bhor, G.L.; Kharate, S.; Nikam, S.; Kulkarni, V.D. Synthesis of Silver Nanoparticles using thuja leaf extract. Res. J. Mater. Sci. 2016, 4, 4–6. [Google Scholar]
- Riat, A.K.; Geyi, D.; Rafi, M.; Kaur, G. Efficacy of Thuja occidentalis plant mediated synthesis of Silver nanoparticles against Culex quinquefasciatus Larvae. Res. J. Pharm. Technol. 2018, 11, 4981. [Google Scholar] [CrossRef]
- Barua, S.; Konwarh, R.; Bhattacharya, S.S.; Das, P.; Devi, K.S.P.; Maiti, T.K.; Mandal, M.; Karak, N. Non-hazardous anticancerous and antibacterial colloidal ‘green’silver nanoparticles. Colloids Surf. B. Biointerfaces 2013, 105, 37–42. [Google Scholar] [CrossRef]
- Barua, S.; Banerjee, P.P.; Sadhu, A.; Sengupta, A.; Chatterjee, S.; Sarkar, S.; Barman, S.; Chattopadhyay, A.; Bhattacharya, S.; Mondal, N.C.; et al. Silver Nanoparticles as Antibacterial and Anticancer Materials Against Human Breast, Cervical and Oral Cancer Cells. J. Nanosci. Nanotechnol. 2017, 17, 968–976. [Google Scholar] [CrossRef]
- Kalpana, D.; Han, J.H.; Park, W.S.; Lee, S.M.; Wahab, R.; Lee, Y.S. Green biosynthesis of silver nanoparticles using Torreya nucifera and their antibacterial activity. Arab. J. Chem. 2019, 12, 1722–1732. [Google Scholar] [CrossRef]
- Sarli, S.; Ghasemi, N. Optimization of biosynthesized Zn nanoparticles by poisonous Taxus baccata leaves extract and evaluation of their effect on the bacterias and MCF-7 cancer cells. Eurasian Chem. Commun. 2020, 2, 302–318. [Google Scholar] [CrossRef]
- Fernando, S.I.D.; Judan-Cruz, K.G.; De Guia, A.C.M. Biologically synthesized gold nanoparticles (Aunp) using pine (Pinus kesiya) pollen extract show antifungal activity against Candida albicans. Int. J. Agric. Technol. 2017, 13, 2615–2622. [Google Scholar]
- Khan, N.; Khan, I.; Nadhman, A.; Azam, S.; Ullah, I.; Ahmad, F.; Khan, H.A. Pinus wallichiana-synthesized silver nanoparticles as biomedical agents: In-vitro and in-vivo approach. Green Chem. Lett. Rev. 2020, 13, 69–82. [Google Scholar] [CrossRef]
- Das, S.; Das, J.; Samadder, A.; Bhattacharyya, S.S.; Das, D.; Khuda-Bukhsh, A.R. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids Surfaces B: Biointerfaces 2013, 101, 325–336. [Google Scholar] [CrossRef]
- Deshmukh, S.; Patil, S.; Mullani, S.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Bhagat, M. Silver nanoparticles (AgNPs): As nanopesticides and nanofertilizers. MOJ Biol. Med. 2019, 4, 19–20. [Google Scholar]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- Das, P.; Barua, S.; Sarkar, S.; Karak, N.; Bhattacharyya, P.; Raza, N.; Kim, K.-H.; Bhattacharya, S.S. Plant extract–mediated green silver nanoparticles: Efficacy as soil conditioner and plant growth promoter. J. Hazard. Mater. 2018, 346, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Mashwani, Z.-U.-R.; Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Khullar, P.; Goshisht, M.K.; Moudgil, L.; Singh, G.; Mandial, D.; Kumar, H.; Ahluwalia, G.K.; Bakshi, M.S. Mode of Protein Complexes on Gold Nanoparticles Surface: Synthesis and Characterization of Biomaterials for Hemocompatibility and Preferential DNA Complexation. ACS Sustain. Chem. Eng. 2016, 5, 1082–1093. [Google Scholar] [CrossRef]
- Lazarides, A.; Kelly, K.L.; Jensen, T.; Schatz, G. Optical properties of metal nanoparticles and nanoparticle aggregates important in biosensors. J. Mol. Struct. Theochem 2000, 529, 59–63. [Google Scholar] [CrossRef]
- Usman, A.I.; Aziz, A.A.; Abu Noqta, O. Application of Green Synthesis of Gold Nanoparticles: A Review. J. Teknol. 2018, 81, 1–5. [Google Scholar] [CrossRef]
- Anand, K.; Rajamanikandan, R.; Sharma, A.S.; Ilanchelian, M.; Khan, F.I.; Tiloke, C.; Katari, N.K.; Boomi, P.; Balakumar, C.; Saravanan, M.; et al. Human serum albumin interaction, in silico and anticancer evaluation of Pine-Gold nanoparticles. Process Biochem. 2020, 89, 98–109. [Google Scholar] [CrossRef]
- Velmurugan, P.; Lee, S.-M.; Iydroose, M.; Lee, K.-J.; Oh, B.-T. Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Appl. Microbiol. Biotechnol. 2013, 97, 361–368. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.K.; Chakraborty, M.; Maiti, M.K.; Das, S.K.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Chen, L.; Batjikh, I.; Hurh, J.; Han, Y.; Huo, Y.; Ali, H.; Li, J.F.; Rupa, E.J.; Ahn, J.C.; Mathiyalagan, R.; et al. Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik 2019, 184, 324–329. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Espinoza-Gómez, H.; Alonso-Núñez, G.; Rivero, I.A.; Gochi-Ponce, Y.; Flores-López, L.Z. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J. Saudi Chem. Soc. 2017, 21, 341–348. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total. Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.-Y.; Chen, D.-H. Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nanoparticles. Dye. Pigment. 2004, 61, 93–98. [Google Scholar] [CrossRef]
- Sylvester, P.; Westerhoff, P.; Möller, T.; Badruzzaman, M.; Boyd, O. A Hybrid Sorbent Utilizing Nanoparticles of Hydrous Iron Oxide for Arsenic Removal from Drinking Water. Environ. Eng. Sci. 2007, 24, 104–112. [Google Scholar] [CrossRef]
- Parham, H.; Zargar, B.; Shiralipour, R. Fast and efficient removal of mercury from water samples using magnetic iron oxide nanoparticles modified with 2-mercaptobenzothiazole. J. Hazard. Mater. 2012, 205, 94–100. [Google Scholar] [CrossRef]
- Zargar, B.; Parham, H.; Hatamie, A. Fast removal and recovery of amaranth by modified iron oxide magnetic nanoparticles. Chemosphere 2009, 76, 554–557. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu-Sirriah, S.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Losasso, C.; Belluco, S.; Cibin, V.; Zavagnin, P.; Mičetić, I.; Gallocchio, F.; Zanella, M.; Bregoli, L.; Biancotto, G.; Ricci, A. Antibacterial activity of silver nanoparticles: Sensitivity of different Salmonella serovars. Front. Microbiol. 2014, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Gordon, O.; Slenters, T.V.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver Coordination Polymers for Prevention of Implant Infection: Thiol Interaction, Impact on Respiratory Chain Enzymes, and Hydroxyl Radical Induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef]
- Husen, A. Medicinal Plant Product-Based Fabrication Nanoparticles (Au and Ag) and Their Anticancer Effects; CRC Press: Boca Raton, FL, USA, 2019; pp. 133–147. [Google Scholar]
- Gherbawy, Y.A.; Elhariry, H.M. Endophytic fungi associated with high-altitude Juniperus trees and their antimicrobial activities. Plant Biosyst. 2016, 150, 131–140. [Google Scholar] [CrossRef]
- Khatami, M.; Mortazavi, S.M.; Kishani-Farahani, Z.; Amini, A.; Amini, E.; Heli, H. Biosynthesis of Silver Nanoparticles Using Pine Pollen and Evaluation of the Antifungal Efficiency. Iran. J. Biotechnol. 2017, 15, 95–101. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Dhanjal, D.S.; Nepovimova, E.; Șen, F.; Regassa, H.; Singh, R.; Verma, R.; Kumar, V.; Kumar, D.; et al. Fruit Extract Mediated Green Synthesis of Metallic Nanoparticles: A New Avenue in Pomology Applications. Int. J. Mol. Sci. 2020, 21, 8458. [Google Scholar] [CrossRef]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Wei, L.J.; Gan, S.H. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxidative Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Doria, G.; Baptista, P. Noble Metal Nanoparticles Applications in Cancer. J. Drug Deliv. 2011, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Kumar, S.R.; Tambuwala, M.; Bakshi, H.A.; et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem. Interactions 2019, 309, 108720. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Dhanjal, D.S.; Paudel, K.R.; Singh, B.; Gupta, G.; RajeshKumar, S.; Thangavelu, L.; Tambuwala, M.; Bakshi, H.A.; Chellappan, D.K.; et al. Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: An update. Inflammopharmacology 2020, 28, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Amalinei, R.L.M.; Trifan, A.; Cioanca, O.; Miron, S.D.; Mihai, C.T.; Rotinberg, P.; Miron, A. Polyphenol-rich extract from Pinus sylvestris L. bark--chemical and antitumor studies. Med Surg. J. 2014, 118, 551–557. [Google Scholar]
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant Fortification of the Diet for Anti-Ageing Effects: A Review. Nutrients 2020, 12, 3008. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuca, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Watters, J.L.; A Satia, J.; Kupper, L.L.; A Swenberg, J.; Schroeder, J.C.; Switzer, B.R.; Florin, T.A.; Fryer, G.E.; Miyoshi, T.; Weitzman, M.; et al. Associations of Antioxidant Nutrients and Oxidative DNA Damage in Healthy African-American and White Adults. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1428–1436. [Google Scholar] [CrossRef]
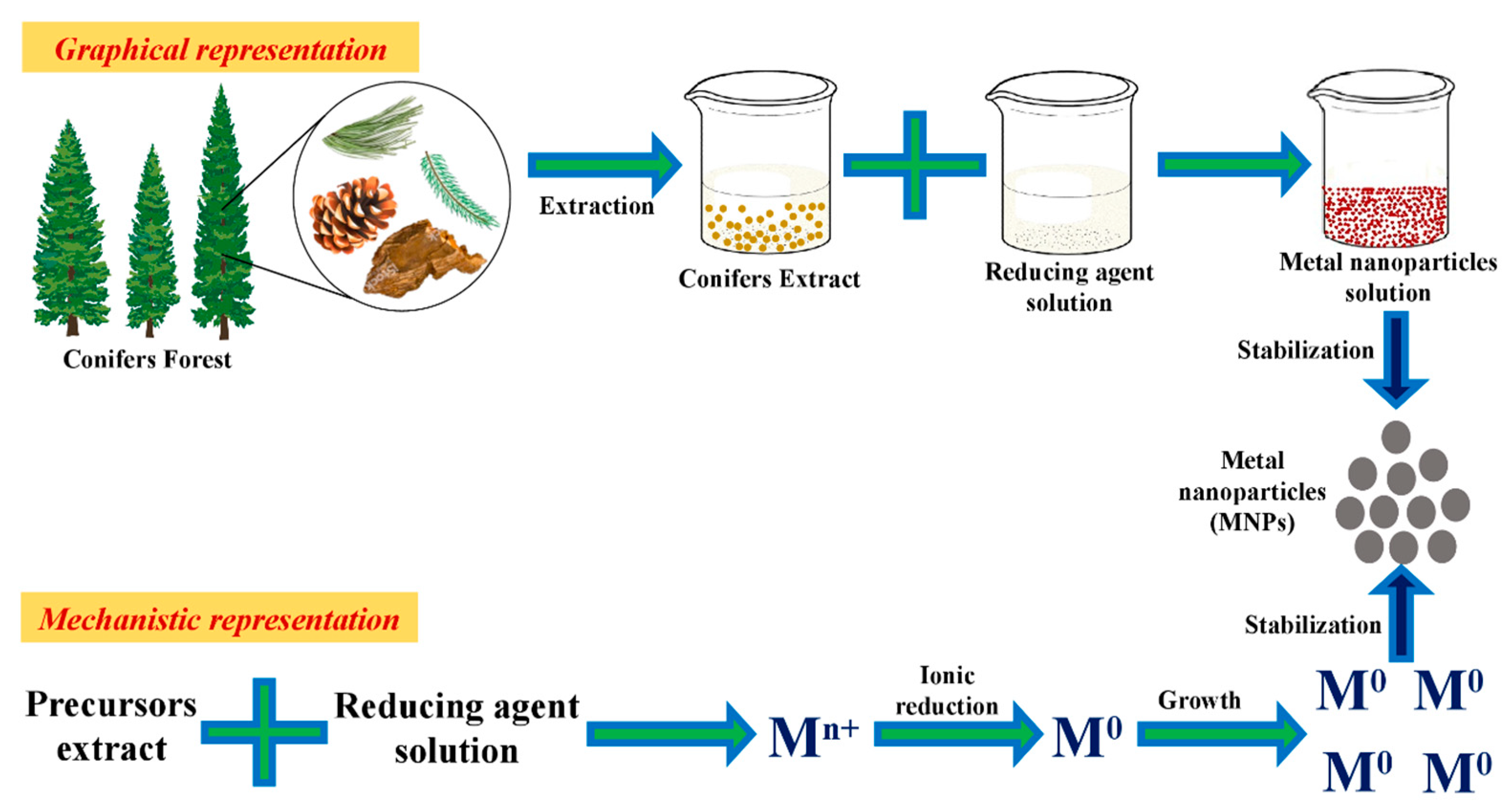
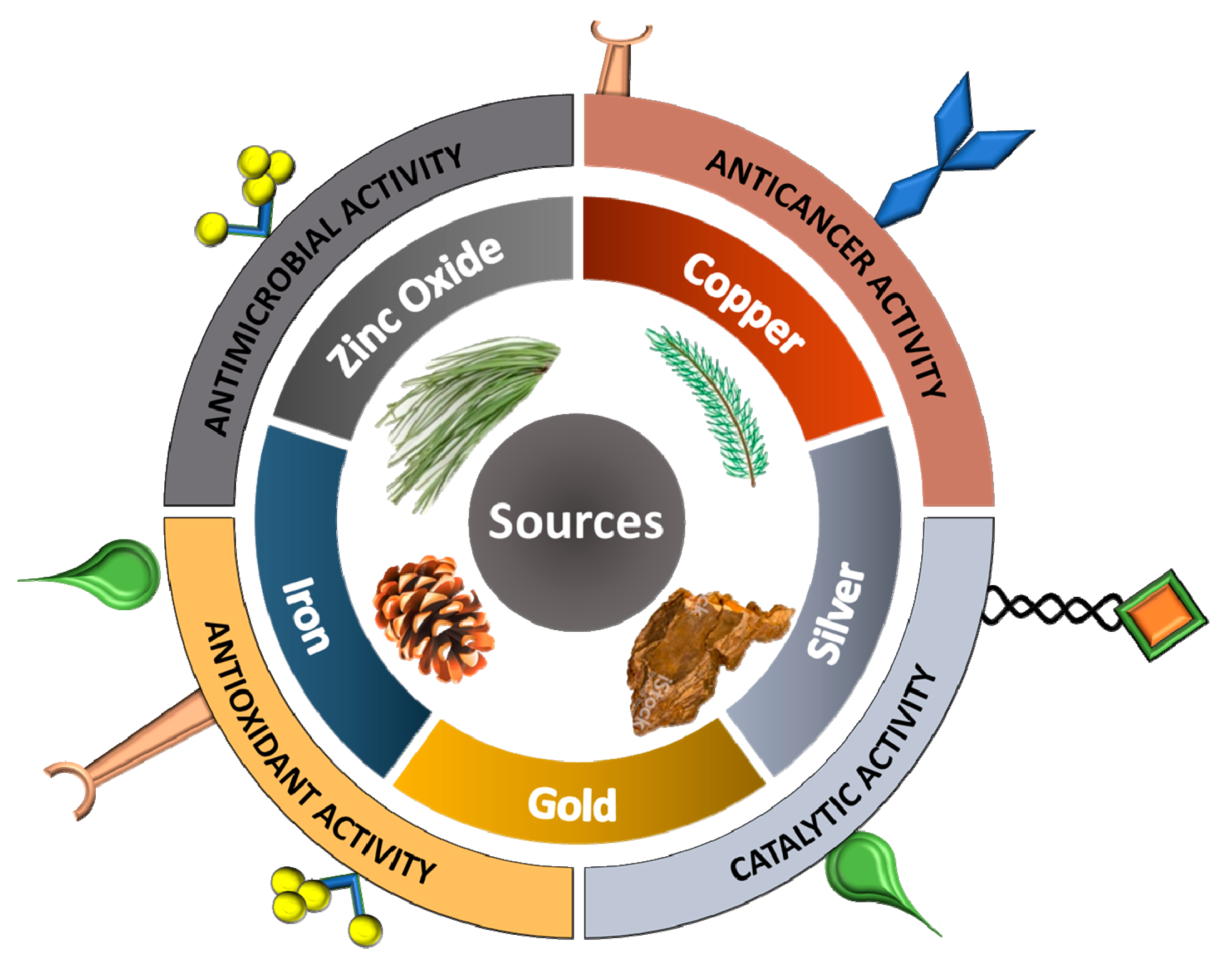

| Types of Conifer | Family | Parts of Plant Used | Types of Metallic NPs | Reducing Agent | Stabilizing Agent | Reaction Time | Reaction Temp | Characterization | Shape | Size (nm) | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Picea abies L. | Pinaceae | Bark | Silver | Phenolic compounds | Phenolic compounds | 3 h | 60 °C | FTIR, UV–Vis, TEM | Sphere and Polygonal | 44 | ND | [51] |
| Pinus eldarica | Pinaceae | Bark | Silver | Bark extract | Bark extract | ND | RT | UV–Vis, TEM | Sphere | 10–40 | ND | [52] |
| Taxus yunnanensis | Taxaceae | Callus | Silver | Callus extract | Callus extract | 120 min | NS | XRD, TEM, FTIR, | Sphere | 6.4–27.2 | More than 2 weeks | [30] |
| Pinus merkusii Jungh Et De Vriese | Pinaceae | Cone/Flower | Copper | Phenolic groups | Phenolic groups | NS | NS | FTIR, SEM | ND | 20–35 | ND | [53] |
| Juniperus communis L. | Cupressaceae | Berry/Cone | Gold | Carbonyl, Carboxyl and Hydroxyl groups | Polyphenols | 24 h | RT | AFM, ATR-FTIR, EDX, UV–Vis, TEM, | Sphere and Triangle | 10–50 | ND | [54,55] |
| Pinus densiflora | Pinaceae | Cone | Silver | Hydroxyl and Carbonyl groups | Phytochemicals | 15 min | RT | UV–Vis, TEM, FTIR, XRD | Oval and Triangular | 40–70 | ND | [56] |
| Pinus merkusii Jungh Et De Vriese | Pinaceae | Cone | Silver | Phenolic hydroxyl groups | Cone flower | 60 min | 60 °C | UV–Vis, TEM, FTIR | Sphere | 8–23 | ND | [57] |
| Araucaria heterophylla | Araucariaceae | Gum | Silver | Gum extract | Gum extract | NS | NS | AFM, EDX, FTIR, SEM, UV–Vis | ND | <25 | ND | [48] |
| Araucaria heterophylla | Araucariaceae | Gum | Silver | Gum extract | Gum extract | NS | RT | UV–Vis, FTIR, SEM, EDX, AFM | Sphere | <30 | ND | [58] |
| Taxus baccata | Taxaceae | Leaf/Needle | Gold | Needle extract | Needle extract | 24 h | 25 °C | UV–Vis, HRTEM, EDX, AFM | Sphere, Semi-sphere, Hexagonal and Triangle | <20 | Several months | [59] |
| Thuja orientalis | Cupressaceae | Leaf/Needle | Gold | Phytochemicals | Phytochemicals | 10 min | RT | EDX, FTIR, XRD, TEM, UV–Vis, | Sphere | 5–94 | ND | [60] |
| Pinus eldarica | Pinaceae | Leaf/Needle | Iron Oxide | Leaf extract | Leaf extract | 30 min | RT | TEM, FTIR, XRD, EDX | Sphere | 8–34 | ND | [61] |
| Cupressus goveniana | Cupressaceae | Leaf/Needle | Silver | Carboxyl and Hydroxyl and Amine groups | Phytochemicals | 60 min | 80 °C | UV–Vis, FTIR, SEM | Sphere | 67–200 | 4 months | [62] |
| Cupressus sempervirens | Cupressaceae | Leaf/Needle | Silver | Carbonyl and Hydroxyl groups | Phytochemicals | 12 h | RT | UV–Vis, TEM, XRD, FTIR | Sphere, Hexahedral, Oval and Triangle | 10–80 | ND | [63] |
| Cupressus torulosa D. Don | Cupressaceae | Leaf/Needle | Silver | Leaf extract | Leaf extract | 24 h | NS | UV–Vis, SEM, XRD, TEM | Sphere | NS | ND | [64] |
| Juniperus procera | Cupressaceae | Leaves | Silver | Phenolic acids, chlorogenic acid, flavonoids, caffeoylquinic acids | Phytochemicals | 24 h | RT | UV–Vis SEM, FTIR | Spherical, cubical | 30–90 | ND | [65] |
| Juniperus chinensis | Cupressaceae | Leaf/Needle | Silver | Proteins | Phytochemicals | 60 min | 100 °C | EDX, XRD, HRTEM, UV–Vis | NS | 18–25 | ND | [46] |
| Taxus baccata | Taxaceae | Leaf/Needle | Silver | Proteins and Terpenoids | Phytochemicals | NS | 10 and 30 °C | UV–Vis, TEM, AFM | Triangular and Hexagonal | 75.1 | Six months | [34] |
| Thuja occidentalis L. | Cupressaceae | Leaf/Needle | Silver | Carbonyl, Carboxyl, Aliphatic and Aromatic amine groups | Carbonyl, Carboxyl, Aliphatic and Aromatic amine groups | 35–40 min | 60 °C | FTIR, UV–Vis, SEM, XRD, TEM | Sphere | <30 | ND | [66] |
| Thuja occidentalis | Cupressaceae | Leaf/Needle | Silver | Hydroxyl and Carbonyl groups | Phytochemicals | 3–4 h | RT | XRD, FTIR | ND | 41.48 | ND | [67] |
| Thuja occidentalis | Cupressaceae | Leaf/Needle | Silver | Leaf extract | Leaf extract | 3–4 h | RT | UV–Vis, FTIR | ND | ND | ND | [68] |
| Thuja occidentalis | Cupressaceae | Leaf/Needle | Silver | Leaf extract | Leaf extract | NS | NS | UV–Vis, XRD, HRTEM | ND | 7–14 | ND | [69] |
| Thuja occidentalis | Cupressaceae | Leaf/Needle | Silver | Leaf extract | Leaf extract | NS | RT | UV–Vis, XRD, TEM | ND | 10–15 | ND | [70] |
| Torreya nucifera | Taxaceae | Leaf/Needle | Silver | Proteins | Proteins | 24 hrs | 20 °C | FTIR, XRD, TEM, UV–Vis, | Sphere | 10–125 | ND | [71] |
| Taxus baccata | Taxaceae | Leaf/Needle | Zinc Oxide | Proteins | Proteins | NS | NS | UV–Vis, SEM, TEM, FTIR | Hexagonal | 20–27.64 | ND | [72] |
| Pinus kesiya | Pinaceae | Pollen | Gold | Pollen extract | Pollen extract | 60 min | RT | UV–Vis | ND | ND | 3 months | [73] |
| Pinus wallichiana | Pinaceae | Stem | Silver | Hydroxyl groups | Phytochemicals | 30 min | 55 °C | EDX, FTIR, SEM, UV–Vis, XRD | Sphere | 10–30 | ND | [74] |
| Abies spectabilis | Pinaceae | NS | Gold | Polyphenols | Polyphenols | 24 h | 29 °C | AFM, DLS, EDX, FTIR, UV–Vis, XRD, | Sphere | 20–200 | ND | [47] |
| Thuja occidentalis | Cupressaceae | NS | Silver | Carbonyl groups | Flavonoids, Terpenoids and Thiamines | 10 min | 27 °C | DLS, TEM, XRD, UV–Vis, | Sphere | 122.8 | ND | [75] |
| Types of Conifer | Family | Applications | Ref. |
|---|---|---|---|
| Silver NPs | |||
| Juniperus chinensis | Cupressaceae | Antibacterial activity against B. subtilis, E. coli, P. aeruginosa, and S. aureus | [46] |
| Juniperus procera | Cupressaceae | Antimicrobial, cellular proliferation/cytotoxicity | [65] |
| Pinus densiflora | Pinaceae | Antibacterial activity against Bacillus cereus, Brevibacterium linens, Propionibacterium acnes and Staphylococcus epidermidis | [56] |
| Pinus thunbergii | Pinaceae | Antibacterial activity against Bacillus thuringiensis, Bacillus megaterium, Burkholderia glumae, Pseudomonas syringae and Xanthomonas oryzae | [81] |
| Pinus wallichiana | Pinaceae | Antibacterial activity against Acinetobacter baumannii; Antioxidant activity; Antipyretic activity | [74] |
| Taxus baccata | Taxaceae | Anti-cancerous activity against human breast (MCF-7) cell line | [72] |
| Taxus yunnanensis | Taxaceae | Antibacterial activity against E. coli, S. aureus, Salmonella paratyphi and B. subtilis; Anti-cancerous activity against SMMC-7721, A549, LS174T andMCF-7 cell line | [30] |
| Thuja occidentalis | Cupressaceae | Plant growth promoter and soil conditioner | [79] |
| Thuja occidentalis | Cupressaceae | Antibacterial activity against B. subtilis, E. coli, and Pseudomonas putida; Antifungal activity against Aspergillus niger, Alternaria alternata and Fusarium spp. | [66] |
| Thuja occidentalis | Cupressaceae | Antibacterial activity against E. coli, S. aureus; Anti-cancerous activity against HeLa, MDA-MB 231, and MCF 7 cell line | [69] |
| Thuja occidentalis | Cupressaceae | Antibacterial activity against B. subtilis, S. aureus, S. typhimurium, L. monocytogenes and P. aeruginosa; | [70] |
| Gold NPs | |||
| Abies spectabilis | Pinaceae | Anti-cancerous activity against bladder cancer (T24) cell line | [47] |
| Pinus kesiya | Pinaceae | Antifungal activity against Candida albicans | [73] |
| Taxus baccata | Taxaceae | Anti-cancerous activity against MCF-7, HeLa and Caov-4 cell lines | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, K.; Dhanjal, D.S.; Sharma, A.; Nepovimova, E.; Kalia, A.; Thakur, S.; Bhardwaj, S.; Chopra, C.; Singh, R.; Verma, R.; et al. Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications. Int. J. Mol. Sci. 2020, 21, 9028. https://doi.org/10.3390/ijms21239028
Bhardwaj K, Dhanjal DS, Sharma A, Nepovimova E, Kalia A, Thakur S, Bhardwaj S, Chopra C, Singh R, Verma R, et al. Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications. International Journal of Molecular Sciences. 2020; 21(23):9028. https://doi.org/10.3390/ijms21239028
Chicago/Turabian StyleBhardwaj, Kanchan, Daljeet Singh Dhanjal, Anirudh Sharma, Eugenie Nepovimova, Anu Kalia, Shabnam Thakur, Sonali Bhardwaj, Chirag Chopra, Reena Singh, Rachna Verma, and et al. 2020. "Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications" International Journal of Molecular Sciences 21, no. 23: 9028. https://doi.org/10.3390/ijms21239028
APA StyleBhardwaj, K., Dhanjal, D. S., Sharma, A., Nepovimova, E., Kalia, A., Thakur, S., Bhardwaj, S., Chopra, C., Singh, R., Verma, R., Kumar, D., Bhardwaj, P., & Kuča, K. (2020). Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications. International Journal of Molecular Sciences, 21(23), 9028. https://doi.org/10.3390/ijms21239028












