Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments
Abstract
1. Introduction
2. Pathophysiological Evidence of the Shared Role of Visceral Obesity in the Occurrence of Cancer and CVD
2.1. Inflammation
2.2. Adipokines
2.3. Insulin, Insulin Resistance and Insulin-Like Growth Factors (IGF)
2.4. Sex Hormones and Lipid Profile
2.5. Fibroblast Growth Factor
2.6. Alterations in DNA Methylation
2.7. Other Biochemical and Metabolic Factors
3. Pharmacological Treatment of Visceral Obesity
3.1. Peroxisome Proliferator-Activated Receptor Gamma (PPARγ)
3.2. Growth Hormone Treatment
3.3. Metformin
3.4. Cycloxyganase Inhibitors
3.5. Statins
3.6. Ezetimibe
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.; Mookadam, F.; Krasushi, R.A.; Ahmed, H. Association Between Obesity and Cardiovascular Outcomes. JAMA Netw. Open 2018, 1, e183788. [Google Scholar] [CrossRef] [PubMed]
- Barberio, A.M.; Alareeki, A.; Viner, B.; Pader, J.; Vena, J.E.; Arora, P.; Friedenreich, C.M.; Brenner, D.R. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat. Commun. 2019, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Ferreira, C.C.D.C.; Pagotto, V.; Santos, A.S.E.A.D.C.; Velasquez-Melendez, G. Total and central obesity in elderly associated with a marker of undernutrition in early life—Sitting height-to-stature ratio: A nutritional paradox. Am. J. Hum. Biol. 2017, 29, e22977. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Kliemann, N.; Noll, M.; Sarrafzadegan, N.; Oliveira, C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes. Rev. 2020, 1, obr.13088. [Google Scholar] [CrossRef] [PubMed]
- Fund, W.C.R. Body Fatness and Weight Gain and the Risk of Cancer. American Institute for Cancer Research, 2018. Available online: https://www.wcrf.org/sites/default/files/Body-fatness-and-weight-gain_0.pdf (accessed on 24 November 2020).
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.P. Link between obesity and cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 8753–8754. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Hernández Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.V.; Cobucci, R.N.O.; Jatobá, C.A.N.; de Medeiros Fernandes, T.A.A.; de Azevedo, J.W.V.; de Araújo, J.M.G. The Role of the Mediators of Inflammation in Cancer Development. Pathol. Oncol. Res. 2015, 21, 527–534. [Google Scholar] [CrossRef]
- Masoudkabir, F.; Sarrafzadegan, N.; Gotay, C.; Ignaszewski, A.; Krahn, A.D.; Davis, M.K.; Franco, C.; Mani, A. Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis 2017, 263, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2. [Google Scholar] [CrossRef]
- Lu, H. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Munn, L.L. Cancer and inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1370. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Evers, N.; Awazawa, M.; Nicholls, H.T.; Bronneke, H.S.; Dietrich, A.; Mauer, J.; Bluher, M.; Bruning, J.C. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 2016, 5, 328–339. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.-L.; et al. Reduction of Macrophage Infiltration and Chemoattractant Gene Expression Changes in White Adipose Tissue of Morbidly Obese Subjects After Surgery-Induced Weight Loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef]
- Chen, B.; Lam, K.S.L.; Wang, Y.; Wu, D.; Lam, M.C.; Shen, J.; Wong, L.; Hoo, R.L.; Zhang, J.; Xu, A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem. Biophys. Res. Commun. 2006, 341, 549–556. [Google Scholar] [CrossRef]
- Wueest, S.; Rapold, R.A.; Rytka, J.M.; Schoenle, E.J.; Konrad, D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 2009, 52, 541–546. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Walter, L. Role of Matrix Metalloproteinases in Inflammation/Colitis-Associated Colon Cancer. Immunogastroenterology 2013, 2, 22. [Google Scholar] [CrossRef]
- Wu, L.-J.; Li, H.-X.; Luo, X.-T.; Lu, R.-Z.; Ma, Y.-F.; Wang, R.; Zhang, J.; Yang, D.-Q.; Yu, H.; Liu, J. STAT3 activation in tumor cell-free lymph nodes predicts a poor prognosis for gastric cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 1140–1146. [Google Scholar] [PubMed]
- Porporato, P.E.; Filigheddu, N.; Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell. Res. 2018, 28, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.J.; Ma, W.; Starost, M.F.; Lago, C.U.; Lim, P.K.; Sack, M.N.; Kang, J.-G.; Wang, P.-Y.; Hwang, P.M. Ambient Oxygen Promotes Tumorigenesis. PLoS ONE 2011, 6, e19785. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Döppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell. Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, K.K.Y.; Vanhoutte, P.M.; Lam, K.S.L.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Konduru, S.K.; Merchant, N.; Ramsoondar, J.; Rampersad, C.K.; Rajitha, B.; Mukund, V.; Kancherla, J.; Hammond, A.; Barik, T.K.; et al. Adiponectin, Its role in obesity-associated colon and prostate cancers. Crit. Rev. Oncol. Hematol. 2017, 116, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Nedvídková, J.; Smitka, K.; Kopský, V.H.V. Adiponectin, an adipocyte-derived protein. Physiol. Res. 2005, 54, 133–140. [Google Scholar]
- Mohammadi, S.; Arefhosseini, S.R.; Ebrahimi-Mamaeghani, M.; Fallah, P.; Bazi, Z. Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manag. 2015, 11, 55. [Google Scholar] [CrossRef]
- Kanhai, D.A.; Kranendonk, M.E.; Uiterwaal, C.S.P.M.; van der Graaf, Y.; Kappelle, L.J.; Visseren, F.L.J. Adiponectin and incident coronary heart disease and stroke. A systematic review and meta-analysis of prospective studies. Obes. Rev. 2013, 14, 555–567. [Google Scholar] [CrossRef]
- Ouchi, N.; Ohishi, M.; Kihara, S.; Funahashi, T.; Nakamura, T.; Nagaretani, H.; Kumada, M.; Ohashi, K.; Okamoto, Y.; Nishizawa, H.; et al. Association of Hypoadiponectinemia With Impaired Vasoreactivity. Hypertension 2003, 42, 231–234. [Google Scholar] [CrossRef]
- Xie, L.; Wang, Y.; Wang, S.; Wu, N.; Chen, Y.; Yan, J. Adiponectin induces growth inhibition and apoptosis in cervical cancer HeLa cells. Biologia (Bratisl) 2011, 66. [Google Scholar] [CrossRef]
- Gelsomino, L.; Naimo, G.D.; Catalano, S.; Mauro, L.; Andò, S. The Emerging Role of Adiponectin in Female Malignancies. Int. J. Mol. Sci. 2019, 20, 2127. [Google Scholar] [CrossRef]
- Mauro, L.; Naimo, G.D.; Gelsomino, L.; Malivindi, R.; Bruno, L.; Pellegrino, M.; Tarallo, R.; Memoli, D.; Weisz, A.; Panno, M.S.; et al. Uncoupling effects of estrogen receptor α on LKB1/AMPK interaction upon adiponectin exposure in breast cancer. FASEB J. 2018, 32, 4343–4355. [Google Scholar] [CrossRef] [PubMed]
- Mauro, L.; Pellegrino, M.; Giordano, F.; Ricchio, E.; Rizza, P.; De Amicis, F.; Catalano, S.; Bonofiglio, D.; Panno, M.L.; Andò, S. Estrogen receptor-α drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015, 29, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Karbaschian, Z.; Hosseinzadeh-Attar, M.J.; Giahi, L.; Golpaie, A.; Masoudkabir, F.; Talebpour, M.; Kosari, F.; Karbaschian, N.; Hoseini, M.; Mazaherioun, M. Portal and systemic levels of visfatin in morbidly obese subjects undergoing bariatric surgery. Endocrine 2013, 44, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.; Soltani, D.; Sobh-Rakhshankhah, A.; Jafari, S.; Boroumand, M.A.; Goudarzi, V.; Vasheghani-Farahani, A.; Masoudkabir, F. Visfatin as marker of isolated coronary artery ectasia and its severity. Cytokine 2019, 113, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Romacho, T.; Sánchez-Ferrer, C.F.; Peiró, C. Visfatin/Nampt: An Adipokine with Cardiovascular Impact. Mediators Inflamm. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; San Hipólito-Luengo, A.; Gómez-Cerezo, J.F.; et al. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef]
- Wang, P.; Xu, T.-Y.; Guan, Y.-F.; Su, D.-F.; Fan, G.-R.; Miao, C.-Y. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: Role of nicotinamide mononucleotide. Cardiovasc. Res. 2009, 81, 370–380. [Google Scholar] [CrossRef]
- Kim, S.-R.; Bae, Y.-H.; Bae, S.-K.; Choi, K.-S.; Yoon, K.-H.; Koo, T.H.; Jang, H.-O.; Yun, I.; Kim, K.-W.; Kwon, Y.-G.; et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-κB activation in endothelial cells. Biochim. Biophys. Acta Mol. Cell. Res. 2008, 1783, 886–895. [Google Scholar] [CrossRef]
- Lin, T.-C. The role of visfatin in cancer proliferation, angiogenesis, metastasis, drug resistance and clinical prognosis. Cancer Manag. Res. 2019, 11, 3481–3491. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, W.; Chen, X.; Wang, S.; Wang, X.; Xu, H.; Li, L. The NAMPT/E2F2/SIRT1 axis promotes proliferation and inhibits p53-dependent apoptosis in human melanoma cells. Biochem. Biophys. Res. Commun. 2017, 493, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, C.; Zhang, Y.; Gao, Y.; Teng, F.; Tian, W.; Yang, W.; Yan, Y.; Xue, F. Visfatin stimulates endometrial cancer cell proliferation via activation of PI3K/Akt and MAPK/ERK1/2 signalling pathways. Gynecol. Oncol. 2016, 143, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kim, W.-H.; Shin, S.-H.; Kim, J.Y.; Yun, M.R.; Park, K.J.; Park, H.-Y. Visfatin exerts angiogenic effects on human umbilical vein endothelial cells through the mTOR signaling pathway. Biochim. Biophys. Acta Mol. Cell. Res. 2011, 1813, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-H.; Park, H.-J.; Kim, S.-R.; Kim, J.-Y.; Kang, Y.; Kim, J.-A.; Wee, H.-J.; Kageyama, R.; Jung, J.S.; Bae, M.-K.; et al. Notch1 mediates visfatin-induced FGF-2 up-regulation and endothelial angiogenesis. Cardiovasc. Res. 2011, 89, 436–445. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Lee, J.S.; Ho, G.Y.; Going, S.B.; Beebe-Dimmer, J.; Manson, J.E.; Chlebowski, R.T.; Rohan, T. Metabolic Obesity Phenotypes and Risk of Breast Cancer in Postmenopausal Women. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1730–1735. [Google Scholar] [CrossRef]
- Liang, X.; Margolis, K.L.; Hendryx, M.; Rohan, T.E.; Groessl, E.J.; Thomson, C.A.; Kroenke, C.H.; Simon, M.S.; Lane, D.; Stefanick, M.; et al. Metabolic Phenotype and Risk of Colorectal Cancer in Normal-Weight Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. 2017, 26, 155–161. [Google Scholar] [CrossRef]
- Greenberg, A.S.; McDaniel, M.L. Identifying the links between obesity, insulin resistance and beta-cell function: Potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur. J. Clin. Invest. 2002, 32, 24–34. [Google Scholar] [CrossRef]
- Frayn, K.N. Visceral fat and insulin resistance—Causative or correlative? Br. J. Nutr. 2000, 83, S71–S77. [Google Scholar] [CrossRef]
- Paneni, F.; Costantino, S.; Cosentino, F. Insulin Resistance, Diabetes, and Cardiovascular Risk. Curr. Atheroscler. Rep. 2014, 16, 419. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Minireview: IGF, Insulin, and Cancer. Endocrinology 2011, 152, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, M.; Karimi, R.; Vaseghi, G. Different effects of metformin and insulin on primary and secondary chemoprevention of colorectal adenoma in diabetes type 2: Traditional and Bayesian meta-analysis. EXCLI J. 2018, 17, 45–56. [Google Scholar] [PubMed]
- Lukanova, A.; Söderberg, S.; Stattin, P.; Palmqvist, R.; Lundin, E.; Biessy, C.; Rinaldi, S.; Riboli, E.; Hallmans, G.; Kaaks, R. Nonlinear relationship of insulin-like growth factor (IGF)-I and IGF-I/IGF-binding protein-3 ratio with indices of adiposity and plasma insulin concentrations (Sweden). Cancer Causes Control 2002, 13, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, R.; Ibarra, C.; Vicencio, J.M.; Jaimovich, E.; Lavandero, S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 2014, 25, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Burgers, A.M.G.; Biermasz, N.R.; Schoones, J.W.; Pereira, A.M.; Renehan, A.G.; Zwahlen, M.; Egger, M.; Dekkers, O.M. Meta-analysis and dose-response metaregression: Circulating insulin-like growth factor I (IGF-I) and mortality. J. Clin. Endocrinol. Metab. 2011, 96, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Zhang, J.; Zheng, C.; Zhu, H.; Yu, H.; Fan, L. Circulating Insulin-Like Growth Factor-1 Level and Ovarian Cancer Risk. Cell. Physiol. Biochem. 2016, 38, 589–597. [Google Scholar] [CrossRef]
- Morris, P.G.; Hudis, C.A.; Giri, D.; Morrow, M.; Falcone, D.J.; Zhou, X.K.; Du, B.; Brogi, E.; Crawford, C.B.; Kopelovich, L.; et al. Inflammation and Increased Aromatase Expression Occur in the Breast Tissue of Obese Women with Breast Cancer. Cancer Prev. Res. 2011, 4, 1021–1029. [Google Scholar] [CrossRef]
- Shi, R.; Yu, H.; McLarty, J.; Glass, J. IGF-I and breast cancer: A meta-analysis. Int. J. Cancer 2004, 111, 418–423. [Google Scholar] [CrossRef]
- Svensson, J.; Carlzon, D.; Petzold, M.; Karlsson, M.K.; Ljunggren, Ö.; Tivesten, Å.; Mellström, D.; Ohlsson, C. Both Low and High Serum IGF-I Levels Associate with Cancer Mortality in Older Men. J. Clin. Endocrinol. Metab. 2012, 97, 4623–4630. [Google Scholar] [CrossRef]
- Camhi, S.M.; Bray, G.A.; Bouchard, C.; Greenway, F.L.; Johnson, W.D.; Newton, R.L.; Ravussin, E.; Ryan, D.H.; Smith, S.R.; Katzmarzyk, P.T. The Relationship of Waist Circumference and BMI to Visceral, Subcutaneous, and Total Body Fat: Sex and Race Differences. Obesity 2011, 19, 402–408. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.L.; Anderson, C.A.M.; Allison, M.A.; Ouyang, P.; Szklo, M.; Vaidya, D.; Woodward, M.; Golden, S.H. Association Between Sex Hormones and Adiposity: Qualitative Differences in Women and Men in the Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2015, 100, E596–E600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sairam, M.R. Sex hormone imbalances and adipose tissue dysfunction impacting on metabolic syndrome; a paradigm for the discovery of novel adipokines. Horm. Mol. Biol. Clin. Investig. 2014, 17. [Google Scholar] [CrossRef] [PubMed]
- von Hafe, P.; Pina, F.; Pérez, A.; Tavares, M.; Barros, H. Visceral fat accumulation as a risk factor for prostate cancer. Obes. Res. 2004, 12, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D.; Gunter, M.J.; Murphy, N.; Rohan, T.E.; Strickler, H.D. The Relation of Obesity-Related Hormonal and Cytokine Levels with Multiple Myeloma and Non-Hodgkin Lymphoma. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Casimirri, F.; De Iasio, R.; Mesini, P.; Boschi, S.; Chierici, R.; Flamia, R.; Biscotti, M.; Vicennati, V. Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J. Clin. Endocrinol. Metab. 1995, 80, 654–658. [Google Scholar]
- Frasor, J.; Danes, J.M.; Komm, B.; Chang, K.C.N.; Lyttle, C.R.; Katzenellenbogen, B.S. Profiling of Estrogen Up- and Down-Regulated Gene Expression in Human Breast Cancer Cells: Insights into Gene Networks and Pathways Underlying Estrogenic Control of Proliferation and Cell Phenotype. Endocrinology 2003, 144, 4562–4574. [Google Scholar] [CrossRef]
- Rothenberger, N.; Somasundaram, A.; Stabile, L. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Suba, Z. Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: Preventive and therapeutic implications. Onco. Targets. Ther. 2014, 7, 147. [Google Scholar] [CrossRef]
- Zittermann, S.I.; Issekutz, A.C. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am. J. Pathol. 2006, 168, 835–846. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H. Pathophysiological roles of FGF signaling in the heart. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef]
- Korc, M.; Friesel, R.E. The role of fibroblast growth factors in tumor growth. Curr. Cancer Drug Targets 2009, 9, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Benham, V.; Bullard, B.; Kearney, T.; Hsia, H.C.; Gibbon, D.; Demireva, E.Y.; Lunt, S.Y.; Bernard, J.J. Fibroblast growth factor receptor is a mechanistic link between visceral adiposity and cancer. Oncogene 2017, 36, 6668–6679. [Google Scholar] [CrossRef] [PubMed]
- Benham, V.; Chakraborty, D.; Bullard, B.; Bernard, J.J. A role for FGF2 in visceral adiposity-associated mammary epithelial transformation. Adipocyte 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Hu, X.; Ma, X.; Luo, Y.; Xu, Y.; Xiong, Q.; Pan, X.; Xiao, Y.; Bao, Y.; Jia, W.-P. Associations of serum fibroblast growth factor 23 levels with obesity and visceral fat accumulation. Clin. Nutr. 2018, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Masai, H.; Joki, N.; Sugi, K.; Moroi, M. A preliminary study of the potential role of FGF-23 in coronary calcification in patients with suspected coronary artery disease. Atherosclerosis 2013, 226, 228–233. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Januzzi, J.L.; Isakova, T.; Laliberte, K.; Smith, K.; Collerone, G.; Sarwar, A.; Hoffmann, U.; Coglianese, E.; Christenson, R.; et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009, 119, 2545–2552. [Google Scholar] [CrossRef]
- Hsu, H.J.; Wu, M.-S. Fibroblast growth factor 23: A possible cause of left ventricular hypertrophy in hemodialysis patients. Am. J. Med. Sci. 2009, 337, 116–122. [Google Scholar] [CrossRef]
- Ix, J.H.; Katz, R.; Kestenbaum, B.R.; De Boer, I.H.; Chonchol, M.; Mukamal, K.J.; Rifkin, D.; Siscovick, D.S.; Sarnak, M.J.; Shlipak, M.G. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J. Am. Coll. Cardiol. 2012, 60, 200–207. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, C.; Huang, W.; Zhang, J.; Xia, M.; Zhang, Y.; Ling, W. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. PLoS ONE 2013, 8, e72545. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, Y. DNA methylation in human diseases. Genes Dis. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA methylation and DNA methyltransferases. Epigenet. Chromatin 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.H.; Marsit, C.J.; Kelsey, K.T. Global Methylation in Exposure Biology and Translational Medical Science. Environ. Health Perspect. 2011, 119, 1528–1533. [Google Scholar] [CrossRef]
- Keller, M.; Hopp, L.; Liu, X.; Wohland, T.; Rohde, K.; Cancello, R.; Klös, M.; Bacos, K.; Kern, M.; Eichelmann, F.; et al. Genome-wide DNA promoter methylation and transcriptome analysis in human adipose tissue unravels novel candidate genes for obesity. Mol. Metab. 2017, 6, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Castellano, D.; Moreno-Indias, I.; Sánchez-Alcoholado, L.; Ramos-Molina, B.; Torres, J.A.; Morcillo, S.; Ocaña-Wilhelmi, L.; Tinahones, F.J.; Moreno-Indias, I.; Cardona, F. Altered Adipose Tissue DNA Methylation Status in Metabolic Syndrome: Relationships Between Global DNA Methylation and Specific Methylation at Adipogenic, Lipid Metabolism and Inflammatory Candidate Genes and Metabolic Variables. J. Clin. Med. 2019, 8, 87. [Google Scholar] [CrossRef]
- Turcot, V.; Tchernof, A.; Deshaies, Y.; Pérusse, L.; Bélisle, A.; Marceau, S.; Biron, S.; Lescelleur, O.; Biertho, L.; Vohl, M.-C. LINE-1 methylation in visceral adipose tissue of severely obese individuals is associated with metabolic syndrome status and related phenotypes. Clin. Epigenet. 2012, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Lynam-Lennon, N.; Connaughton, R.M.; Carr, E.; Mongan, A.M.; O’Farrell, N.J.; Porter, R.K.; Brennan, L.; Pidgeon, G.P.; Lysaght, J.; Reynolds, J.; et al. Excess visceral adiposity induces alterations in mitochondrial function and energy metabolism in esophageal adenocarcinoma. BMC Cancer 2014, 14, 907. [Google Scholar] [CrossRef]
- Vernochet, C.; Damilano, F.; Mourier, A.; Bezy, O.; Mori, M.A.; Smyth, G.; Rosenzweig, A.; Larsson, N.; Kahn, C.R. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J. 2014, 28, 4408–4419. [Google Scholar] [CrossRef]
- Li, M.; Cheung, B.M.Y. Pharmacotherapy for obesity. Br. J. Clin. Pharmacol. 2009, 68, 804–810. [Google Scholar] [CrossRef]
- Abbas, A.; Blandon, J.; Rude, J.; Elfar, A.; Mukherjee, D. PPAR- γ Agonist in Treatment of Diabetes: Cardiovascular Safety Considerations. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 124–134. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Mahankali, A.; Matsuda, M.; Mahankali, S.; Hardies, J.; Cusi, K.; Mandarino, L.; DeFronzo, R. Effect of Pioglitazone on Abdominal Fat Distribution and Insulin Sensitivity in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2002, 87, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Wolski, K.; Nicholls, S.J.; Nissen, S.E. Pioglitazone and Risk of Cardiovascular Events in Patients with Type 2 Diabetes Mellitus. JAMA 2007, 298, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K. Rosiglitazone Revisited. Arch Intern Med. 2010, 170. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Nicolosi, M.L.; Giuliano, S.; Bellomo, M.; Belfiore, A.; Malaguarnera, R. PPAR-γ Agonists As Antineoplastic Agents in Cancers with Dysregulated IGF Axis. Front. Endocrinol. (Lausanne) 2017, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, P.-P.; Sun, X.-C.; Hu, T.-T. Thiazolidinediones and risk of colorectal cancer in patients with diabetes mellitus: A meta-analysis. Saudi J. Gastroenterol. 2018, 24, 75. [Google Scholar]
- Yan, H.; Xie, H.; Ying, Y.; Li, J.; Wang, X.; Xu, X.; Zheng, X. Pioglitazone use in patients with diabetes and risk of bladder cancer: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 1627–1638. [Google Scholar] [CrossRef]
- Monami, M.; Dicembrini, I.; Mannucci, E. Thiazolidinediones and cancer: Results of a meta-analysis of randomized clinical trials. Acta Diabetol. 2014, 51, 91–101. [Google Scholar] [CrossRef]
- Beauregard, C.; Utz, A.L.; Schaub, A.E.; Nachtigall, L.; Biller, B.M.K.; Miller, K.K.; Klibanski, A. Growth Hormone Decreases Visceral Fat and Improves Cardiovascular Risk Markers in Women with Hypopituitarism: A Randomized, Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2008, 93, 2063–2071. [Google Scholar] [CrossRef]
- Lewitt, M.S. The Role of the Growth Hormone/Insulin-Like Growth Factor System in Visceral Adiposity. Biochem. Insights 2017, 10, 117862641770399. [Google Scholar] [CrossRef]
- Livingstone, C.; Borai, A. Insulin-like growth factor-II: Its role in metabolic and endocrine disease. Clin. Endocrinol. 2014, 80, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Deodati, A.; Ferroli, B.B.; Cianfarani, S. Association between growth hormone therapy and mortality, cancer and cardiovascular risk: Systematic review and meta-analysis. Growth Horm. IGF Res. 2014, 24, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, Q.; Li, Y.; Fu, J.; Huang, X.; Shen, L. Growth hormone replacement therapy reduces risk of cancer in adult with growth hormone deficiency: A meta-analysis. Oncotarget 2016, 7. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes. Ann. Intern Med. 2016, 164, 740. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Viswanath, A.K. Medical Management of Diabesity: Do We Have Realistic Targets? Curr. Diab. Rep. 2017, 17, 4. [Google Scholar] [CrossRef]
- Tokubuchi, I.; Tajiri, Y.; Iwata, S.; Hara, K.; Wada, N.; Hashinaga, T.; Nakayama, H.; Mifune, H.; Yamada, K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 2017, 12, e0171293. [Google Scholar] [CrossRef]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Effects on Micro and Macrovascular Complications in Type 2 Diabetes. Cardiovasc. Drugs Ther. 2008, 22, 215–224. [Google Scholar] [CrossRef]
- Luo, F.; Das, A.; Chen, J.; Wu, P.; Li, X.; Fang, Z. Metformin in patients with and without diabetes: A paradigm shift in cardiovascular disease management. Cardiovasc. Diabetol. 2019, 18, 54. [Google Scholar] [CrossRef]
- Chen, Q.; Thompson, J.; Hu, Y.; Das, A.; Lesnefsky, E.J. Metformin attenuates ER stress–induced mitochondrial dysfunction. Transl. Res. 2017, 190, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The beneficial effects of metformin on cancer prevention and therapy: A comprehensive review of recent advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, C.; Berrino, F.; Venturelli, E.; Abbà, C.; Biglia, N.; Brucato, T.; Cogliati, P.; Danese, S.; Donadio, M.; Zito, G.; et al. Metformin Decreases Circulating Androgen and Estrogen Levels in Nondiabetic Women with Breast Cancer. Clin. Breast Cancer 2013, 13, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhong, X.; Gao, P.; Shi, J.; Wu, Z.; Guo, Z.; Wang, Z.; Song, Y.-X. The Potential Effect of Metformin on Cancer: An Umbrella Review. Front. Endocrinol. (Lausanne) 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Farb, M.G.; Tiwari, S.; Karki, S.; Ngo, D.T.; Carmine, B.; Hess, D.T.; Zuriaga, M.A.; Walsh, K.; Fetterman, J.L.; Hamburg, N.M.; et al. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity 2014, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Antman, E.M.; Bennett, J.S.; Daugherty, A.; Furberg, C.; Roberts, H.; Taubert, K.A. Use of Nonsteroidal Antiinflammatory Drugs. Circulation 2007, 115, 1634–1642. [Google Scholar] [CrossRef]
- Cannon, C.P.; Cannon, P.J. COX-2 Inhibitors and Cardiovascular Risk. Science 2012, 336, 1386–1387. [Google Scholar] [CrossRef]
- Gurpinar, E.; Grizzle, W.E.; Piazza, G.A. COX-Independent Mechanisms of Cancer Chemoprevention by Anti-Inflammatory Drugs. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef]
- Chow, L.W.C.; Loo, W.T.Y.; Toi, M. Current directions for COX-2 inhibition in breast cancer. Biomed. Pharmacother. 2005, 59, S281–S284. [Google Scholar] [CrossRef]
- Tóth, L.; Muszbek, L.; Komáromi, I. Mechanism of the irreversible inhibition of human cyclooxygenase-1 by aspirin as predicted by QM/MM calculations. J. Mol. Graph. Model. 2013, 40, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.; Zacharias-Millward, N.; Menter, D.G.; Davis, J.S.; Lichtenberger, L.; Hawke, D.; Hawk, E.; Vilar, E.; Bhattacharya, P.; Millward, S. Beyond COX-1: The effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017, 36, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Alenghat, F.J.; Davis, A.M. Management of Blood Cholesterol. JAMA 2019, 321, 800. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.S. Statins in relation to adiponectin: A significant association with clinical implications. Atherosclerosis 2016, 253, 270–272. [Google Scholar] [CrossRef][Green Version]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.G.; Singh, P.P.; Murad, M.H.; Iyer, P.G. Statins Are Associated With Reduced Risk of Esophageal Cancer, Particularly in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 620–629. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, W.; Wang, J.; Xie, L.; Li, T.; He, Y.; Deng, Y.; Peng, Q.; Li, S.; Qin, X. Association between statin use and colorectal cancer risk: A meta-analysis of 42 studies. Cancer Causes Control 2014, 25, 237–249. [Google Scholar] [CrossRef]
- Singh, P.P.; Singh, S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1721–1730. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Shen, Y.; Zhou, H.; Shao, Y.; Zhu, W.; Chen, Y. Impact of statin use on cancer-specific mortality and recurrence. Medicine (Baltimore) 2020, 99, e19596. [Google Scholar] [CrossRef]
- Mansourian, M.; Haghjooy-Javanmard, S.; Eshraghi, A.; Vaseghi, G.; Hayatshahi, A.; Thomas, J. Statins Use and Risk of Breast Cancer Recurrence and Death: A Systematic Review and Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. 2016, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.A.P.; Dayspring, T.D.; Toth, P.P. Ezetimibe therapy: Mechanism of action and clinical update. Vasc. Health Risk Manag. 2012, 8, 415. [Google Scholar] [PubMed]
- Takase, H.; Dohi, Y.; Okado, T.; Hashimoto, T.; Goto, Y.; Kimura, G. Effects of ezetimibe on visceral fat in the metabolic syndrome: A randomised controlled study. Eur. J. Clin. Investig. 2012, 42, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Dolezelova, E.; Stein, E.; Derosa, G.; Maffioli, P.; Nachtigal, P.; Sahebkar, A. Effect of ezetimibe on plasma adipokines: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2017, 83, 1380–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Tang, M.; Liu, F.; Xia, P.; Shu, M.; Wu, X. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohnishi, H.; Morimoto, N.; Minami, S.; Ishioka, M.; Watanabe, S.; Tsukui, M.; Takaoka, Y.; Nomoto, H.; Isoda, N.; et al. Ezetimibe suppresses development of liver tumors by inhibiting angiogenesis in mice fed a high-fat diet. Cancer. Sci. 2019, 110, 771–783. [Google Scholar] [CrossRef]
- Masko, E.M.; Alfaqih, M.A.; Solomon, K.R.; Barry, W.T.; Newgard, C.B.; Muehlbauer, M.J.; Valilis, N.A.; Phillips, T.E.; Poulton, S.H.; Freedland, A.R.; et al. Evidence for Feedback Regulation Following Cholesterol Lowering Therapy in a Prostate Cancer Xenograft Model. Prostate 2017, 77, 446–457. [Google Scholar] [CrossRef]
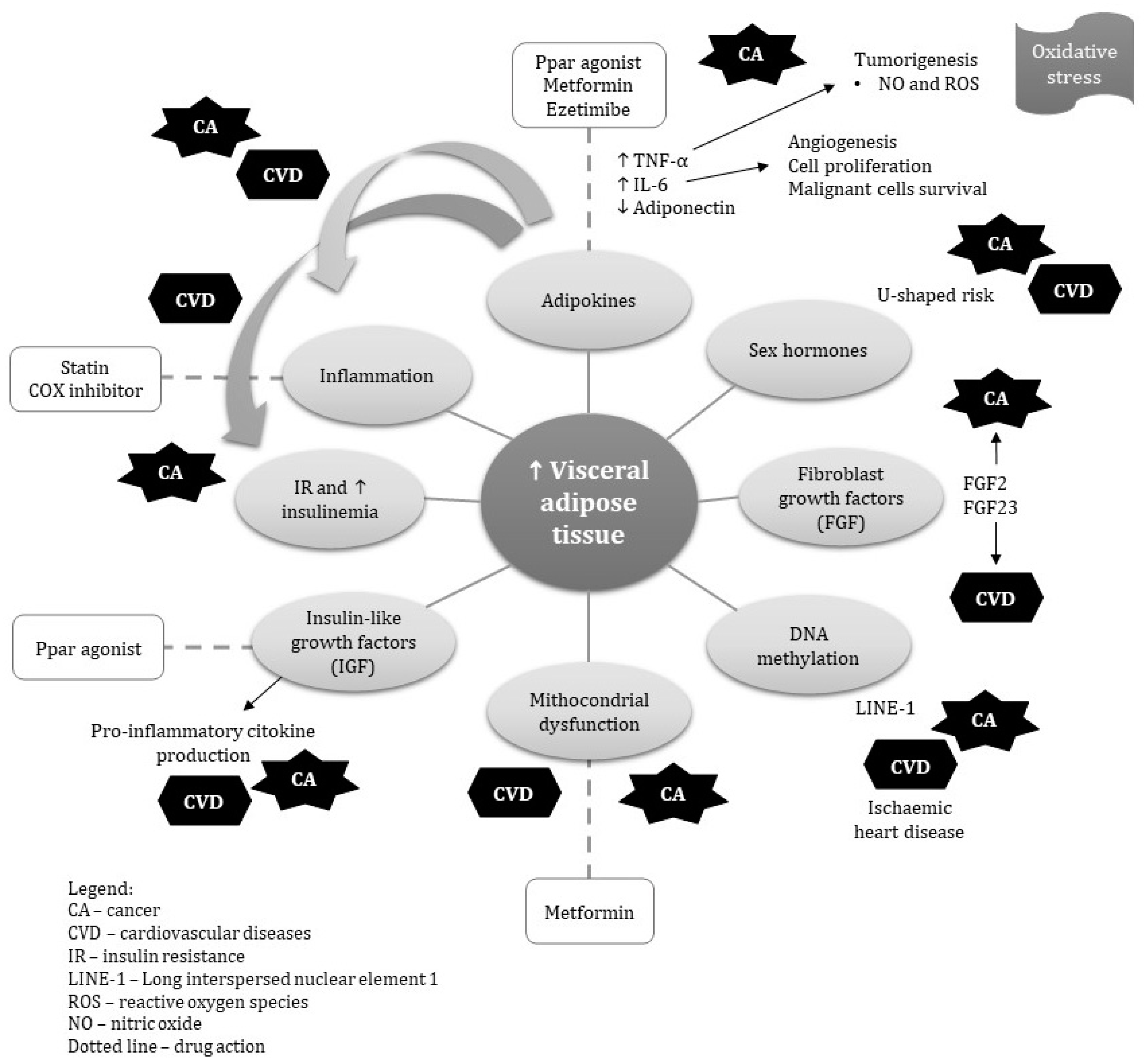
| Risk factor | Visceral Obesity | Cardiovascular Disease | Cancer |
|---|---|---|---|
| Inflammation | Reactive oxygen species (ROS) and release of pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) | Metabolic syndrome, type 2 diabetes, hypertension and dyslipidemia, | Promote angiogenesis and sustain proliferative signaling |
| Adipokines | Adipocytes cell differentiation | Effect on nitric oxide synthase (NOS) and ROS, peripheral arterial dysfunction, hypertension, dyslipidemia | Proliferation and inhibits p53-dependent apoptosis |
| Insulin and insulin like growth hormone | Metabolic dysfunction, | Decrease eNOS activation and NO production, which leads to entrance of inflammation to plaque | Promote cell proliferation, differentiation and protection from apoptosis |
| Sex hormones | Estrogen signaling | Plasma HDL cholesterol levels are significantly higher and fasting plasma glucose concentrations | Increase aromatase activity |
| Fibroblast growth factor | Inflammation | Cardiac hypertrophy through the activation of FGF receptor | Transformation of epithelial cell |
| Alterations in DNA methylation | Long interspersed nuclear element activation | Metabolic syndrome, Ischemic heart disease | Cell proliferation |
| Drugs | Direct Target | Action on Visceral Obesity | Action on Cancer | Action on CVD |
|---|---|---|---|---|
| PPARγ agonists | Peroxisome proliferator-activated receptor Gamma | Adipokine /IGF | Reduce circulating insulin, Reduce Angiogenesis Increase Apoptosis | Reduce blood pressure Reduce myocardial infarction and stroke Improve endothelial function |
| Recombinant human growth hormones | Growth hormone | Increase lipolysis/IGF-I | Reduce risk of cancer in adults with growth hormone deficiency | Reduce risk of MI in adults with growth hormone deficiency |
| Metformin | Unknown | Induces anorexia, upregulates adaptive thermogenes, and modulates adipokines Improves mithocondrial dysfunction | Reduces pre-neoplastic and neoplastic cell proliferation Reduces circulating levels of androgen and estrogen | Increases eNOS production Attenuates ER stress-induced mitochondrial dysfunction Reduces cardiac injury through ER-stress |
| NSAIDs | Cycloxyganase | Reduce prostaglandin levels Modulate adiponectin | Reduce inflammation | Reduce platelet aggregation and vasoconstriction |
| Statins | HMG-CoA reductase | Increase circulating adiponectin | Tumor-suppressor Increase Apoptosis | Improve endothelial function Plaque stabilization |
| Ezetimibe | Niemann-Pick C1-like 1 blocker | Modulate adiponectin Decrease insulin resistant which results | Suppress inflammation Inhibiting angiogenesis | Reduces in blood level of TNF-α in patients with hyperlipidemia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparecida Silveira, E.; Vaseghi, G.; de Carvalho Santos, A.S.; Kliemann, N.; Masoudkabir, F.; Noll, M.; Mohammadifard, N.; Sarrafzadegan, N.; de Oliveira, C. Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. Int. J. Mol. Sci. 2020, 21, 9042. https://doi.org/10.3390/ijms21239042
Aparecida Silveira E, Vaseghi G, de Carvalho Santos AS, Kliemann N, Masoudkabir F, Noll M, Mohammadifard N, Sarrafzadegan N, de Oliveira C. Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. International Journal of Molecular Sciences. 2020; 21(23):9042. https://doi.org/10.3390/ijms21239042
Chicago/Turabian StyleAparecida Silveira, Erika, Golnaz Vaseghi, Annelisa Silva de Carvalho Santos, Nathalie Kliemann, Farzad Masoudkabir, Matias Noll, Noushin Mohammadifard, Nizal Sarrafzadegan, and Cesar de Oliveira. 2020. "Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments" International Journal of Molecular Sciences 21, no. 23: 9042. https://doi.org/10.3390/ijms21239042
APA StyleAparecida Silveira, E., Vaseghi, G., de Carvalho Santos, A. S., Kliemann, N., Masoudkabir, F., Noll, M., Mohammadifard, N., Sarrafzadegan, N., & de Oliveira, C. (2020). Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments. International Journal of Molecular Sciences, 21(23), 9042. https://doi.org/10.3390/ijms21239042







