Abstract
Connexins (Cx) are members of a protein family that forms intercellular channels localised in gap junction (GJ) plaques and single transmembrane channels called hemichannels. They participate in intercellular communication or communication between the intracellular and extracellular environments. Connexins affect cell homeostasis, growth and differentiation by enabling the exchange of metabolites or by interfering with various signalling pathways. Alterations in the functionality and the expression of connexins have been linked to the occurrence of many diseases. Connexins have been already linked to cancers, cardiac and brain disorders, chronic lung and kidney conditions and wound healing processes. Connexins have been shown either to suppress cancer tumour growth or to increase tumorigenicity by promoting cancer cell growth, migration and invasiveness. A better understanding of the complexity of cancer biology related to connexins and intercellular communication could result in the design of novel therapeutic strategies. The modulation of connexin expression may be an effective therapeutic approach in some types of cancers. Therefore, one important challenge is the search for mechanisms and new drugs, selectively modulating the expression of various connexin isoforms. We performed a systematic literature search up to February 2020 in the electronic databases PubMed and EMBASE. Our search terms were as follows: connexins, hemichannels, cancer and cancer treatment. This review aims to provide information about the role of connexins and gap junctions in cancer, as well as to discuss possible therapeutic options that are currently being studied.
Keywords:
connexin; hemichannel; gap junction; intercellular communication; cancer; cancer treatment 1. Introduction
Connexins (Cx) are members of a protein family that forms intercellular channels localised in gap junction (GJ) plaques and single transmembrane channels called hemichannels [,]. Cx are expressed in almost every tissue of the human body (i.e., epithelial cells, smooth muscle cells, myoblasts, dendritic cells), except for red blood cells, spermatocytes and striated muscle cells [,]. In the majority of cases, each tissue type expresses more than one connexin, therefore creating an extensive spectrum of intercellular communication due to the possibility of heteromeric channels formed by compatible Cx [,,,,]. In humans, 20 connexin isoforms have been described. The connexin family shows high similarity in terms of the amino acid composition as well as in the transmembrane domains. Based on the differences and similarities in the structure of individual connexins, they have been divided into subfamilies. Currently, five sub-families are described: GJA, GJB, GJC, GJD and GJE. This division is based on the structural similarity of genes, their homology and sequence as well as the length of the connexin cytoplasmic domain [].
Connexins occur both in the form of cell-to-cell plasma membrane domains known as gap junctions (GJ) and single-membrane hemichannels []. Gap junction intercellular communication (GIJC) is responsible for the direct communication and exchange of small cytosolic molecules between contacting cells in a relatively non-selective manner. Therefore, GJs are involved in various physiological functions—i.e., the maintenance of cell homeostasis, growth and cell differentiation [], glandular secretion [], angiogenesis [], neuronal migration [] and stem cell development []. Hemichannels, however, remain free and unopposed, and their physiological role is to exchange molecules between the cytosol and the extracellular space [,,]. Hemichannels play a crucial role in autocrine and paracrine signalling pathways [].
The expression and physiological functions of connexins are well-documented, and the main challenge regarding connexins and gap junctions in general concerns both their roles in the pathophysiological mechanisms of various diseases and as novel interventional targets. Connexins have been already linked to cancers, cardiac and brain disorders, chronic lung and kidney conditions and wound healing processes. In cancers, connexins may be involved in alteration of intracellular communication, can interfere with signalling pathways or modulate cells by autocrine and paracrine mechanisms.
This review provides an overview of the role of Cx in cancer pathogenesis and progression and as possible targets for cancer treatment.
2. Structure and Life Cycle of Connexin Proteins
Connexin proteins form hexagonal structures termed connexons or hemichannels in the plasma membrane []. Connexons of contacting cells bind together to form the gap junction—i.e., a hydrophilic pore that enables direct cytosol-to-cytosol communication and the passage of molecules between neighbouring cells []. This docking process is supported by the presence of different adhesion proteins. Connexons consist of connexins, either the same or different, which create homomeric or heteromeric hemichannels, respectively.
Depending on the composition of connexons, intercellular channels of gap junctions could be classified as one of four classes: (1) homomeric-homotypic, which consist of two identical connexons formed by only one type of connexin; (2) homomeric-heterotypic, built from different connexons, each formed of the same type of protein; (3) heteromeric-homotypic of two identical connexons with at least two isoforms of connexins; (4) heteromeric-heterotypic, built from different connexons, each formed with two or more isoforms of connexins [,,,]. The type of gap junction has an impact on its biophysical properties [,,].
All connexins share similar topology: they consist of four hydrophobic transmembrane domains (TM1-TM4) connected by two extracellular loops (E1-E2) and one cytoplasmic loop (CL). Moreover, connexins contain cytoplasmic C- and N-terminal regions [,,]. The extracellular loops are responsible for docking processes [,,,]. The N-terminus participates in the oligomerisation of connexins and connexin trafficking []. It also produces a selectivity signal that allows selective interactions and the docking of connexin proteins [,]. The C-terminus plays a role in the phosphorylation of connexins: Cx31, Cx32, Cx37, Cx40, Cx43, Cx45, Cx47 and Cx50 [,,]. The C-terminus also takes part in oligomerisation processes and affects the chemical and electrical conductivity of gap junction channels [,,]. It has been shown that the C-terminus can also regulate intercellular Ca2+ flow by binding calmodulin []. Moreover, the C-terminus and CL are binding sites for different structural proteins—i.e., zonulin 1 and 2, claudin 1, as well as protein components of adherens junctions [,,]. This enables the formation of an advanced complex of proteins resulting in increased stabilisation and regulated intercellular communication via signalling pathways [,].
Five decades after their discovery, the expression and physiological functions of Cx are well-documented. The main challenge regarding Cx and GJs in general concerns their role in the pathophysiological mechanisms of various diseases and as novel interventional targets. Cx were linked to cancers since shortly after their discovery, and it was hypothesised that altered gap junctional communication promotes cancer incidence and tumour proliferation [,,]. A study by Loewenstein and Kanno was the first to reveal the absence of electrical coupling in rat liver tumour cells in comparison to healthy hepatocytes []. This phenomenon was later confirmed in human thyroid cancer [] and cultured mammalian cancer cells []. Since then, knowledge of the role of Cx in cancer biology has widely expanded. It is now an established fact that Cx display various functions depending on the cancer type and disease stage, as well as on their isoform [,]. The underlying mechanisms encompass their roles in cell-to-cell communication as GJ components or, independent of GJs, as modulators of heterogeneous signalling pathways, including autocrine/paracrine pathways []. The complexity of Cx involvement in cancer biology provides a vast field of research for the use of Cx-targeting drugs as novel therapeutic strategies in cancer management.
3. The Dual Role of Connexins in Cancers
3.1. Connexins as Tumour Suppressors
In vivo and in vitro observations have indicated a role for GJs in the process of tumorigenesis. Data presented in recent decades have shown that GJIC is generally lost in rapidly dividing cells to prevent the exchange of essential metabolites between those cells and their non-dividing neighbours [,]. GJIC depletion has been widely observed in solid tumours, confirming the negative correlation between connexin expression and tumour growth []. This observation served as the basis for the hypothesis that Cx could be tumour suppressors. This role has been confirmed in various studies performed in knock-out (KO) models. For instance, Cx32 KO mice are more susceptible to spontaneous [] or chemically-induced liver tumours [], and these results were compared with previously published data on liver tumours in rodents and humans [,]. In these studies, GJIC was lost due to the absence of Cx32 expression or its ectopic cytoplasmic localisation. Moreover, in Cx32 KO mice, the formation of multiple tumour types was augmented following irradiation by X-rays []. In a recent study, overexpression of Cx43 demonstrated an antimetastatic effect on MDA-MB-231 breast cancer cells in vitro and in vivo and in human breast cancer tissues []. Cx32 is also responsible for the inhibition of hepatocellular carcinoma invasion and metastasis []. The restoration of GJIC function generally results in a decrease in tumour growth, as demonstrated by the use of chemopreventive agents (i.e., flavonoids or carotenoids) or cDNA transfection [,,,]. The effect, however, is dependent on the transfected Cx subtype [].
Unfortunately, it is not clear how Cx modulate cell growth. Studies on various cancer cell lines led to the conclusion that overexpression of Cx is linked to an elongation of the G1 phase of the cell cycle, resulting in decreased cell proliferation [,]. The mechanism that underlies G1/S cycle arrest is related to the accumulation of p27 []. Elevated expression of p27 follows the flux of its enhancer, the cyclic 3′,5′-adenosine monophosphate (cAMP), via the GJ-dependent pathway []. Additionally, p27 levels increase due to the degradation of S-phase kinase-associated protein 2 (Skp2), a protein that is responsible for p27 ubiquitination and degradation, which is driven by Cx43 itself [,]. Cx43 increases the instability of Skp2 protein and suppresses its expression [,]. The connexin-derived control of nuclear processes occurs through the inhibition or activation of other cell cycle regulators. Cx43, as a single entity, promotes the accumulation of p27, inhibits the activity of cyclin-dependent kinases (CDK), cyclin D1 and Skp2 and acts as a sequestrator of various growth modulators. Among them are CCN3, overexpressed in nephroblastoma, PTEN (phosphatase and tensin homolog), C-terminal Src kinase (Csk) or proto-oncogene tyrosine-protein kinase (c-Src) [].
The specific permeability of various Cx plays a role in GJIC-mediated growth control mechanisms. In an osteoblastic model, the transition of second messengers through GJ leads to the activation of ERK and PI3/Akt pathways. The cAMP Cx-mediated redistribution between contacting cells results in prolongation of the G2/M cell cycle phase. Other molecules can also pass through GJs—e.g., microRNAs (miRNAs) [,].
Cx can also control tumour growth through channel-independent mechanisms. The cytoplasmic domains of Cx, especially the C-terminus, can interfere with other cytosolic/membrane proteins []. In this setting, they can modulate cell growth by adjusting channel permeability, and by interfering with plasma membrane-nucleus signalling pathways. Phosphorylation of the Y247, Y265 and Y313 residues on the Cx43 CT domain in a Src-related manner causes channel closure [,]. In addition to broad cytoplasmic localisation, CT-Cx43 also localises to the nucleus. It is, therefore, suggested that nuclear localisation of CT-Cx43 may exert effects on gene expression and growth []. As for the second mechanism, CT regions can modulate the activity of their protein partners. Such a correlation has been described for Skp-2 and Cx50 [], β-catenin and Cx43 [], discs large homologue 1 (Dlgh1) and Cx32 [] and Cx43 []. Cx32 and Dlgh1 together play a role in cancer progression in an oncoprotein E6-related manner [,]. Interaction of Cx43 with β-catenin in the nucleus downregulates genes involved in metastasis [,]. Similar mechanisms occur for other structural proteins—i.e., ZO-1 [,]. Another tumour suppressive role of CT domains has been described recently, as the 266–283 region in the CT domain of Cx43 binds to c-Src and its inhibitors CKS and PTEN, inhibiting the oncogenic activity of c-Src []. Hemichannels are also linked to the modulation of cell-proliferation; however, the exact role of this is yet to be elucidated [].
As explained above, decreased Cx expression or loss of function is commonly observed in cancer. At the transcriptional level, connexin expression is lowered due to alterations in the activity of transcription factors and epigenetic control of connexin mRNA [,]. Epigenetic silencing is mainly related to histone acetylation and the activity of histone acetyltransferase and deacetylase that promote and inhibit transcription, respectively, and to promoter hypermethylation by DNA methyltransferase []. Methylation of connexin genes has been linked to the loss of Cx43 expression in HeLa cells [] and small-cell lung cancer [], Cx32 in renal cell carcinoma [] and Cx45 in colon cancer []. Another mechanism includes the post-transcriptional modification of connexin mRNA by cancer-related microRNAs. In the literature, the inhibitory effects of the mi-R-221/222 complex and miR-125b on Cx43 have been described in glioma [] and that of miR-20a in prostate cancer []. Furthermore, alterations in the synthesis of truncated forms of Cx—i.e., GJA1-20k—in Smad3/ERM-related pathways [] are believed to play a role of chaperone proteins, enabling the trafficking of regular Cx to the cell wall, with their synthesis being mainly moderated by mTOR and Mnk1/2 signalling [,,]. Post-translational modifications include phosphorylation, acetylation, ubiquitination and SUMOylation, with phosphorylation being the most widely studied process in cancers []. Various kinases and phosphatases—i.e., MAPK (mitogen-activated protein kinase), PKC and PKA (protein C and A kinases) and cdc2/cyclin B—modulate Cx43 phosphorylation, leading to the inhibition of GJIC [,]. Phosphorylation interacts with connexin trafficking, stabilisation of the cell membrane and protein-to-protein communication [,,].
Finally, some studies have been performed on the coding regions of Cx genes or their promoters []. However, in contrast to classical suppressors—i.e., p53 and Rb—mutations in Cx genes are rare. One example can be found in the literature: in colon adenocarcinoma, mutations have been documented in the CT region of Cx43. Those mutations led to a shift in the reading frame for all cases and were only related to invasive tumours []. Nonetheless, further studies are required to verify this hypothesis.
3.2. Connexins as Promoters of Cancer Progression and Metastasis
Recent studies suggest that connexin expression may promote tumour malignancy under certain conditions []. Some Cx act as promoters of invasion and metastasis, especially in advanced stages of cancer. In some cancer cells, restoration of Cx promotes migration and invasion [,], intravasation and extravasation [,], metastatic growth [,] and resistance to therapy [].
Increased expression of Cx43 and its membrane localisation has been observed in various cancer types []. Cx43-composed GJs between cancer cells and astrocytes have been linked to increased growth of brain metastases through cGAMP signalling []. Cx43 levels are increased in metastatic lymph nodes compared to primary site tumours []. Moreover, the levels of Cx43 miRNA are also increased in metastases [], and metastatic tumours display Cx43 positivity, even when primary breast tumours are Cx43 negative []. The same observations have been made for Cx26 []. Metastatic breast cancer cells have been shown to display increased levels of Cx26 compared to primary tumours, and surface Cx26 has been observed only in metastatic cells []. In colorectal cancer, lung metastases also display increased levels of Cx26 compared to primary tumours []. Furthermore, Cx26-GJIC was noted to participate in cancer cell migration in a HeLa cell model [] in which HeLa cells overexpressed Cx26, enabling single-cell migration []. Therefore, it was concluded that migration might occur due to the inhibition of cell–cell adhesion. Chandrasekhar et al. showed that PKA is necessary for Cx26-induced tumour growth inhibition []. Activation of PKA was associated with a redistribution of cAMP in the cells, but Cx43 and Cx32 failed to mediate this redistribution. In a study conducted by Chen et al. on mice, it was found that protocahedrin is strongly associated with breast and lung cancer cells, which connects them with astrocytes in the brain and through Cx43-GJ enables the transfer of second messenger cGAMP to astrocytes. As a result, metastatic cells are capable of growth and become resistant to chemotherapy []. Studies performed in recent years suggest that selected connexins—i.e., Cx43, Cx26 and Cx32—could serve as biomarkers in prognosing the course of cancers or exploited for therapeutic strategies in cancer management [].
GJIC between cancer and endothelial cells is also linked to cancer progression []. This concept was first introduced for melanoma []. BL6 cells expressing Cx26 were shown to form coupling with endothelial cells, in contrast to Cx26-deprived F10 cells. BL6 subclones were associated with a higher metastatic potential than F10 subclones. Expression of Cx26 was low in melanoma cells that were located in the basal layer and considerably upregulated in invading cells. The authors concluded that Cx26 might be involved in the intra- and extravasation of melanoma cells due to the formation of GJ with endothelium. This conclusion was later verified in breast cancer and brain metastases []. Breast cancer cells formed functional GJ with brain endothelium in a Cx43-related manner. Upregulation of Cx43 was crucial for extravasation and co-opting blood vessels. Concordant results were obtained in a study on malignant glioma cells, which formed Cx43-mediated GJIC with neighbouring astrocytes, leading to the promotion of tumour invasion []. However, in a recently published report, hypoxia-induced internalisation and degradation of Cx43 and Cx26 were linked to increased proliferation and migration of non-small lung cancer cells [].
The term “cancer stem cells (CSCs)” accounts for the subpopulation of cancer cells that present pro-tumorigenic properties [,]. Some authors even suggest that CSCs play a crucial role in tumour occurrence, progression and metastasis, as the introduction of CSCs into healthy subjects results in the development of specific tumours []. CSCs have been found to contain Cx []. However, the effect of Cx on CSCs depends both on their membrane and cytoplasmic localisation. In cancer, Cx participate in intercellular communication in the form of hemichannels and GJs, or act as cytoplasmic proteins through kinases and transcription factors []. The role of Cx in breast cancer stem cells (BCSC) has been well-documented [,]. In triple-negative breast cancer, upregulation of Cx26 has been observed. However, the pro-tumorigenic effect of Cx26-mediated BCSC relied upon its connection to focal adhesion kinase (FAK) and the transcription factor NANOG, instead of its channel-forming properties. The mentioned complex was found to interact outside the nucleus to phosphorylate NANOG []. In glioma stem cells (GSCs), higher levels of Cx46 wwere identified in comparison to regular cancer cells, and this correlated with proliferation and self-renewal []. In contrast to Cx26 in breast cancer, the role of Cx46 in GSCs is membrane- and GJIC-related [,]. In another study, decreased levels of Cx43 were described for GSCs [] and related to increased capacity for self-renewal, proliferation and tumour formation []. Moreover, Cx43 restoration resulted in a reversal of these effects []. In a study on hepatocellular carcinoma cells, the accumulation of cytoplasmic Cx32 promoted tumour progression and metastasis via cancer stem cell self-renewal []. Table 1 shows clinical relevance of connexins in some types of cancer.

Table 1.
Clinical relevance of connexins in some types of cancer.
Figure 1 Shows connexin–protein interactions influencing carcinogenesis.
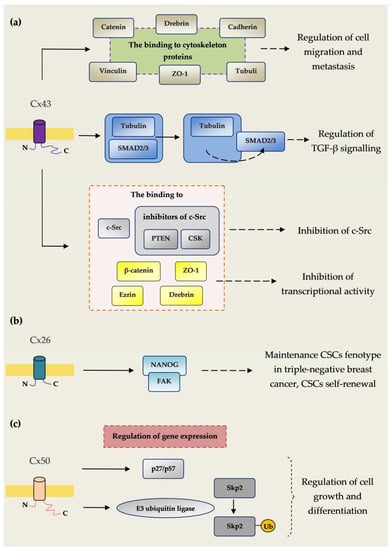
Figure 1.
Connexin–protein interactions influencing carcinogenesis. (a) The binding of Cx43 to cytoskeleton proteins tubulin, cadherins, catenins, vinculin, ZO-1 and drebrin regulates cell migration and metastasis. Cx43 inhibits the connection of Smad2/3 with tubulin, causing the secretion of Smad2/3, which regulates pathways associated with TGF-β. TGF-β signalling plays an important role in many cancers such breast, colon, lung, pancreatic and prostate cancer. Cx43 enhances c-Src blockade, and by a connection with c-Src as well as CSK and PTEN, which are c-Src endogenous inhibitors. C-Src tyrosine kinase is a proto-oncogene involved in many cellular pathways such as cell migration, proliferation and survival. The dysregulation of c-Src leads to malignant transformation and has been observed in several cancer types. C-Src tyrosine kinase also plays an important role in resistance to chemotherapy. Cx43 inhibits in the nucleus the transcriptional activity of β-catenin, drebrin, ezrin and ZO-1 regulating the expression of genes controlling the process of carcinogenesis. (b) Cx26 plays an important role in maintenance of the cancer stem cell (CSC) phenotype in triple-negative breast cancer. Cx26 enhances CSC self-renewal by interaction with the pluripotency transcription factor NANOG and focal adhesion kinase (FAK). (c) Cx50 regulates the expression of the cyclin-dependent kinase inhibitor p27/p57 and E3 ubiquitin ligase Skp2. Cx50 enhances auto-ubiquitination and subsequent degradation of Skp2. Through this mechanism, Cx50 regulates the expression of mediators regulating cell growth and differentiation [].
3.3. Role of Connexins in Chemo- and Radiotherapy
3.3.1. Resistance to Chemotherapy
Cx-related resistance to anti-cancer treatment has been recently reported []. Cancer cells could be resistant to radio- or chemotherapy through GJIC-dependent and independent mechanisms [,]. In a study on glioma cells [], the protective role of neighbouring astrocytes was described in relation to chemoresistance. The protective effect was demonstrated following treatment with temozolomide, cisplatin and fluorouracil. The authors emphasised that the chemoprotective effects of astrocytes relied upon direct contact between astrocytes and glioma cells and was GJ-related. Cx43 was shown to play a crucial role in this phenomenon. A similar observation was made for melanoma brain metastases []. The authors revealed that astrocyte-related chemoprotection occurred through Cx43-GJ []. Sequestration of Ca2+ has been listed as the most probable mechanism by which astrocytes display their protective function on cancer cells []. In another study, breast and lung cancer cells have been shown to establish GJs from Cx43 and protocadherin 7 with astrocytes in the brain []. This has been linked to the transfer of cGAMP from cancer cells to astrocytes and the release of inflammatory molecules interferon α (INFα) and tumour necrosis factor α (TNFα) via the STING pathway, resulting in increased tumour growth and chemoresistance []. A study of lung cancer cells and Cx30.3 (GJB4) revealed the increased metastatic potential and enhanced chemoresistance towards gemcitabine and etoposide in a Src-related manner []. Interestingly, pro-oncogenic properties occurred despite the absence of GJs, indicating the presence of GJIC-independent mechanisms of chemoresistance []. Another study of non-small lung cancer revealed a correlation between resistance towards gefitinib and the upregulation of Cx26 []. However, chemoresistance occurred via a GJIC-independent pathway and was an outcome of increased PI3K/Akt phosphorylation []. The upregulation of Cx32 has also been linked to increased cisplatin resistance in human ovarian cancer cells via the modulation of drug efflux transporters and activation of the EGFR-protein kinase B signalling pathway []. The same Cx has been shown to facilitate hepatocellular carcinoma progression by increasing chemoresistance via modulating Src and favouring EGFR phosphorylation [].
3.3.2. Resistance to Radiotherapy
In a study performed in 1981 [], a correlation was detected between GJIC and radioresistance in spheroids. In 2013, different responses of human cells to ionising radiation were observed based on the type of Cx involved []. Irradiated HeLa cells expressing Cx32, but not Cx26, showed an increased radioresistance rate compared to controls. It has not been ultimately settled whether these effects depend on GJIC or not. Some authors suggest that the opposing effect of irradiation on HeLa cells expressing different Cx could depend upon differential interactions with other proteins—i.e., cytoskeletal proteins, β-catenin, kinases, as well as with molecules that communicate through different GJs. Moreover, the effect could also depend upon the character of the ionising radiation—i.e., in relation to LET (linear energy transfer). High-LED particles—i.e., iron ions or α-particles—display more chemically active species than low-LED particles, and iron ions also generate secondary radiation that is assumed to interact with signalling pathways that are Cx-mediated. However, the authors pointed out that studies using lower doses of irradiation should be performed to further confirm this thesis. In another study on Cx30 and malignant astrocytic gliomas, the suppressive effect of the studied Cx molecules was confirmed in relation to tumour growth []. However, in the same study, Cx30 was shown to display a protective effect on glioblastoma cells in terms of radiation-mediated DNA damage []. These radioprotective properties were due to the translocation of mitochondrial heat shock protein 90 and increased production of ATP []. In a study by Osswald et al., it was confirmed that glioblastoma cells that established functional Cx43-mediated GJs displayed decreased levels of intracellular Ca2+ and were less sensitive towards radiation compared to unconnected cells [].
3.3.3. The Bystander Effect
Discovery of X-rays and ionising radiation was followed by its application in inducing proliferating cancer cell damage due to the generation of single- and double-stranded DNA breaks []. In recent years, it has been revealed that ionising radiation destroys not only targeted cells but also cells that have not been directly irradiated [,]. This so-called ”bystander effect” is assumed to occur thanks to signal transduction via GJs []. This phenomenon has been described in various studies []. The bystander effect plays a role in resistance mechanisms to radiotherapy with a positive correlation between GJIC and radiotherapy resistance confirmed in cultured multi-cell spheroids []. In another study, the restoration of Cx30 resulted in decreased radiation-induced mortality and DNA damage in GBM cells []. Some authors list other factors besides irradiation that could induce the bystander effect [,]. Reactive oxygen species, reactive nitrogen species, Ca2+, protein factors and various cytokines can spread via GJ to neighbouring cells to induce damage [,,]. The concept of the bystander effect could result in newer therapeutic strategies, as understanding of the underlying biology (blockage or restoration of GJ) could produce either a protective effect on surrounding cells or enhance damage to malignant cells [,].
4. Therapeutic Strategies Involving Connexins
The complex role of Cx and GJs in cancer biology provides a broad spectrum of possible therapeutic strategies. A few approaches have been proposed in recent decades; however, further exploration is required to establish their potential. Downregulation of Cx or GJIC has been commonly observed in cancer cells, especially in early-stage cancers. Restoration of GJIC and increased Cx expression has been proposed by some as a possible therapeutic approach []. Many natural-based or synthetic chemical compounds exhibit the potential to modify Cx and/or GJIC [,].
Retinoids, vitamin D, carotenoids, flavonoids, green tea catechins, red wine resveratrol or statins are among the molecules that could reverse GJIC/Cx deficiency [,]. For instance, lycopene, one of the carotenoids present in many fruits and vegetables, was found to inhibit cell proliferation in the breast tumour cell line MCF-7 by stimulating GJIC functionality and increasing Cx43 expression []. High levels of mevalonate have been observed in various cancers, so inhibition of the mevalonate producer β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) reductase could result in cancer regression []. In a study of transformed E9 mouse lung cells, lovastatin increased GJIC but not Cx43 expression in cancer cells through the inhibition of protein kinase C []. In a study on simvastatin, its augmentative effect on GJs composed of Cx43 was revealed in Leydig tumour cells []. In the same study, upregulation of GJs by simvastatin sensitised cancer cells to the chemotherapeutic drug etoposide []. On the contrary, upregulation of Cx43 and GJIC by simvastatin attenuated the toxicity of cisplatin on normal Sertoli cells [].
Chemotherapeutic agents also display modulating potential towards Cx or GJIC expression []. Fukushima et al. showed that therapy with a combination of a Cx43-expressing plasmid and docetaxel enhanced tumour suppression in human prostate cancer cell cultures by increasing the activity of apoptotic molecules and downregulating Bcl-2 expression []. This study was the first to indicate the beneficial effect of combined therapy. In 2009, Sato et al. described augmentation of carboplatin-induced toxicity in the mesothelioma cell line H28 via the upregulation of Cx43 []. The underlying mechanism depended on Cx-related suppression of c-Src []. Cx43 has also been able to enhance sunitinib toxicity in H28 cells through activation of Bax and phosphorylation of c-Jun N-terminal kinase []. Eicosapentaenoic acids have been described to promote chemosensitivity towards to 5-fluorouracil in melanoma models by increasing Cx43 expression []
Other therapeutic approaches embrace the blockage of GJIC and Cx by various molecules, as Cx facilitate tumour growth and metastasis in some cases [,]. Oleamide, an organic amide derived from oleic acid, has an inhibitory effect on pulmonary and hepatic metastases due to the modulation of cancer cell extravasation; this was demonstrated using MDA-MB-231 human breast cancer cells in vitro and a xenograft murine model in vivo. []. In a study by Chen et al., both tonabersat and meclofenamate displayed inhibitory potential on brain metastases from lung and breast cancer in mouse models by blocking GJs between cancer cells and astrocytes []. Addition of either compound to carboplatin therapy further augmented the therapeutic properties []. A clinical trial concerning the effect of meclofenamate treatment in recurrent or progressive brain metastases from solid tumours is currently being conducted in humans (NCT02429570). In a bone metastasis model, Ca2+ signalling from osteogenic cells to cancer cells through GJs played a pivotal role in the formation of bone metastases, alongside the mTOR signalling pathway. Blockage of calcium flow between cells by GJ inhibitors (carbenoxolone, arsenic trioxide) reduced the progression of bone metastases in mice, implicating these agents as potential therapeutic candidates [,]. Carbon monoxide (CO) is another compound that could inhibit hemichannels in the treatment of cancer []. It has been observed that CO donors (CORM-2) display inhibitory potential on Cx46 hemichannels in Xenopus laevis oocytes in a dose-related manner [,,]. The same observation has been made for Cx46 and Cx43 hemichannels in HeLa cells [,,]. However, the exact mechanism is yet to be fully determined.
Another promising strategy concerns the usage of connexin mimetic peptides and specific antibodies to regulate GJ and connexin expression [,,]. This strategy exceeds that which was previously described, as it is more specific and may reduce the number and severity of adverse effects [,]. A αCT1- the Cx43-C-terminal mimicking peptide has been shown to block the ZO-1 interference with Cx43 sensitised temozolomide-resistant human glioblastoma cells to temozolomide treatment []. Moreover, the combination of the Cx43 hemichannel inhibitor and temozolomide resulted in an augmented therapeutic effect []. In another study, targeting Cx40 with a mimetic peptide decreased vascularisation and tumour growth in mice []. In a different context, the mimicking peptides also enhanced GJIC. αCT1 application enhanced GJ activity and reduced tumour proliferation in breast cancer cell lines []. Addition of αCT1 to tamoxifen for the treatment of ER-positive and to lapatinib for the management of HER2-positive breast cancer cells led to more positive effects []. In another study, the cell-penetrating Cx-based peptide TAT-Cx43266-283 displayed anti-tumour properties in human glioblastoma models by reducing migration and the invasive properties of glioblastoma stem cells []. Moreover, monoclonal anti-Cx43 antibodies have been successfully used to reduce tumour growth as monotherapy [] and in combination with standard chemo- and radiotherapy []. In a recently published study, the role of Cx43 and GJIC in melanoma cell killing by cytotoxic T lymphocytes was demonstrated []. The authors suggested possible immune therapeutic strategies in the treatment of selected cancers based on Cx43 levels [].
Most of the abovementioned therapeutic strategies are not directional towards Cx and GJIC and, therefore, are responsible for a number of side effects depending on the receptors they interact with. Currently, effort should be placed on targeted drug delivery to the tumour location or its surroundings in order to minimise deleterious interactions with healthy cells. Connexin targeting nanocarriers are a promising approach. In a study by Baklaushev et al., cisplatin-loaded nanogels were conjugated with monoclonal antibodies to Cx and BSAT1 for the treatment of rat glioma []. The nanogels induced a significant decrease in tumour growth, most probably due to antibody-mediated adhesion of cisplatin-loaded nanogels to the tumour site and their more efficient internalisation. In a study by Chekhonin et al., PEGylated immunoliposomal nanocontainers combined with monoclonal antibodies against the second extracellular loop of Cx43 have been used in the therapy of malignant intracranial C6 gliomas in mice []. Table 2 summarises known approaches to target connexins and gap junctions in the treatment of cancer with chemical modulators—i.e., enhancers and inhibitors, as well as connexin-specific targeted drugs. Figure 2 shows connexin synthesis steps as therapeutic targets.

Table 2.
Therapeutic strategies targeting connexins in cancer management.
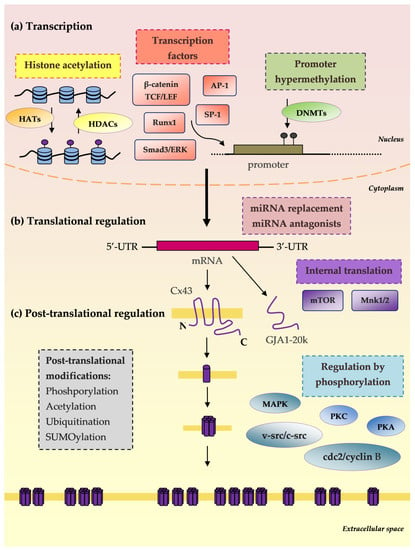
Figure 2.
Connexin synthesis steps as therapeutic targets. (a) Transcription: Histone acetylation: histone acetyltransferase enzymes (HATs), histone deacetylases (HDACs); Transcription factors: Runx1, Ap-1, Sp-1, β-catenin, TCF/LEF; Promoter hypermethylation: DNA methyltransferase enzymes (DNMTs). (b) mRNA translational regulation: microRNA replacement; microRNA antagonists; Internal translation: mTOR and Mnk1/2; GJA1-20k (truncated forms of Cx43): Smad3/ERK-dependent repression of GJA1-20k reduces Cx43 gap junctions. (c) Post-translational regulation: Phosphorylation: mitogen-activated protein kinase (MAPK), protein kinase C (PKC), protein kinase A (PKA), cdc2/cyclin B and v-Src/c-Src; Acetylation; Ubiquitination; SUMOylation [].
5. Conclusions and Future Perspectives
Gap junctions and hemichannels formed by connexins are responsible for cell–cell communication, the passage of small molecules between contacting cells and between cells and the extracellular milieu. They can also display activities independently of GJIC, thus impacting the maintenance of cell homeostasis. The complexity of Cx biology has been a foundation for exploring the role of Cx and GIJC in the onset of various diseases, including cancer. The exact role of Cx in cancer initiation and development is yet to be elucidated. To date, it has been established that Cx can either promote or suppress tumour growth, depending on the connexin isoform or cancer stage/type. As a result, investigations have focused on tissue-specific roles of specific Cx in relation to cancer. In various studies, utilisation of connexin modulators as a therapeutic option in cancer management is currently being verified. However, connexin modulators affect not only cancer cells, resulting in cancer depletion, but also normal, non-cancerogenic cells, thereby producing serious side-effects. There is a need for further development of specific targets toward cancer-related Cx, as well as combination treatment with standard therapeutics.
The effect of connexins on tumour growth depends on the connexin isoform, the stage of the cancer and the type of tissue. Previous studies have shown anti-tumorigenic properties of connexins and reduced connexin expression in tumours, especially Cx43, Cx26 and Cx32 [,,,,,]. Reduced expression of connexins was found mainly in breast and lung tumours, but also in other types of cancers. It has been shown that an experimental increase in connexin expression in cancer cells decreases tumour growth, while the inhibition of connexin expression enhances the growth and invasion of cancer cells [,,,]. However, some studies have shown that connexins may also exert pro-tumorigenic effects by increasing the migration and invasion of cancer cells and may increase chemoresistance [,,,]. In prostate cancer cells, high expression of Cx43 was correlated with a more aggressive phenotype and a high incidence of osteolytic metastases []. Overexpression of Cx32 in hepatocellular carcinoma was associated with advanced stage, poor tumour differentiation and worse prognosis []. Cisplatin resistance in women with ovarian cancer was correlated with the upregulation of Cx32 expression [].
Identifying mechanisms by which different connexins impact different stages of cancer is of particular interest and needs further verification. The exact role of channel-dependent and independent pathways in cancer cell growth and differentiation also requires elucidation in the context of targeted treatment. The contribution of pannexins, a family of proteins showing similar properties to connexin hemichannels, should also be taken into consideration. However, the determination of connexin and pannexin function could remain a challenge. The recognition of novel and tumour-specific connexin modulators that would minimise the adverse effects of blocking or enhancing GJ in other tissues is another issue of great importance.
The multidirectional involvement of connexin and GJIC in the process of carcinogenesis is associated with a broad spectrum of therapeutic challenges. The modulation of connexin expression and GJIC may be a useful therapeutic method in some types of cancers. However, connexins may both inhibit and facilitate the process of carcinogenesis, in particular metastasis. Therefore, there are different clinical situations in which the inhibition of connexins may be associated with different therapeutic results. The blockade of connexins can be achieved through different mechanisms; therefore, the critical challenge is the search for a mechanism and new drugs selectively modulating the expression of various connexin isoforms.
Additional studies should be performed to explain the mechanisms by which the various connexins regulate the growth, differentiation and invasion of cancer cells. These studies require the use of more specific tools. Further efforts are needed to identify more specific connexin inhibitors such as peptides and peptidomimetics, which have proven very promising. Another critical challenge is to investigate the molecular basis of regulation of different connexin isoforms to explain how the dysregulation of these processes contributes to abnormal cellular connexin location at various stages of cancer progression. Identifying these research challenges will prepare the ground for new diagnostic and therapeutic achievements. It is likely that, with appropriate research including functional studies and bioinformatics, some isoforms of connexins could be found to be markers of particular cancers.
In the coming years, research will focus on the mechanisms regulating the expression of various isoforms of connexins, especially in tumour cells as well as in as well as in other cell types involved in tumour progression such as endothelial cells, fibroblasts, vascular smooth muscle cells and macrophages. Therefore, further studies are needed to explain the role of connexin isoforms in metastasis and angiogenesis in tumours. The critical question is the role of connexins during tumour progression as well as the regulation of connexin expression in tumours. Modulation of connexin expression may be a treatment option in some cancers. Many compounds may decrease or increase the permeability of gap junctions or influence the expression of connexins. Locking certain gap junctions in cancer cells may inhibit their proliferation and growth. Some chemotherapeutics used in clinical practice such as docetaxel may modulate the expression of connexins and may inhibit GJIC [].
There are several connexin-specific peptides such as Gap19, Gap 24 and Gap27/40 []. Unfortunately, their mechanisms of action remain incompletely understood. It is also essential to better understand their pharmacological parameters, side effects, toxicity and mechanisms of anti-tumour action. The challenge is to understand which functions of connexins are most appropriate for therapeutic strategies. Inhibiting gap junctions and hemichannel functions are the most straightforward methods []. It has been shown that some peptides such as the Cx43-specific peptide L2 can inhibit hemichannels while simultaneously keeping Cx43 gap junctions in an open state []. Thanks to this, we can develop therapeutic strategies for specific connexin functions in a more personalised way. These therapeutic strategies influencing connexin functions may enhance the sensitivity of tumour cells to chemotherapy and increase its effectiveness. By inhibiting connexin function and tumour cell communication, a progressively lower dose of chemotherapy can be used, limiting side effects while maintaining efficacy against cancer cells [].
Targeting connexins in cancer treatment could be a turning point in cancer management. However, future studies need to concentrate on in situ observations by identifying the precise molecular mechanisms behind the role of Cx in cancer biology, and on designing randomised control trials for particular therapeutic options. For now, it seems that anti-connexin strategies could be more efficient in the treatment of specific tumours rather than globally, as well as in combination treatment with other therapeutic agents rather than alone. Currently, the greatest challenges are to determine the roles of several connexin isoforms in carcinogenesis and metastasis and to continue the search for new selective connexin modulators.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naus, C.C.; Giaume, C. Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J. Transl. Med. 2016, 14, 330. [Google Scholar] [CrossRef]
- Vinken, M. Connexin hemichannels: Novel mediators of toxicity. Arch. Toxicol. 2015, 89, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Angelillo-Scherrer, A.; Fontana, P.; Burnier, L.; Roth, I.; Sugamele, R.; Brisset, A.; Morel, S.; Nolli, S.; Sutter, E.; Chassot, A.; et al. Connexin 37 limits thrombus propensity by downregulating platelet reactivity. Circulation 2011, 124, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Lampe, P.D. Therapeutic strategies targeting connexins. Nat. Rev. Drug Discov. 2018, 17, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W.; Naus, C.C.; Lampe, P.D. SnapShot: Connexins and Disease. Cell 2017, 170, 1260-1260. [Google Scholar] [CrossRef]
- Stauffer, K.A. The gap junction proteins beta 1-connexin (connexin-32) and beta 2-connexin (connexin-26) can form heteromeric hemichannels. J. Biol. Chem. 1995, 270, 6768–6772. [Google Scholar]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect Biol. 2009, 1, a002576. [Google Scholar] [CrossRef]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef]
- Meda, P.; Chanson, M.; Pepper, M.; Giordano, E.; Bosco, D.; Traub, O.; Willecke, K.; el Aoumari, A.; Gros, D.; Beyer, E.C.; et al. In vivo modulation of connexin 43 gene expression and junctional coupling of pancreatic B-cells. Exp. Cell Res. 1991, 192, 469–480. [Google Scholar] [CrossRef]
- Wallez, Y.; Huber, P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta Biomembr. 2008, 1778, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Fushiki, S.; Perez Velazquez, J.L.; Zhang, L.; Bechberger, J.F.; Carlen, P.L.; Naus, C.C.G. Changes in neuronal migration in neocortex of connexin43 null mutant mice. J. Neuropathol. Exp. Neurol. 2003, 62, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological role of connexin intercellular channels and hemichannels. Arch. Biochem. Biophys. 2012, 524, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.C.; Leybaert, L. Hunting for connexin hemichannels. FEBS Lett. 2014, 588, 1205–1211. [Google Scholar] [CrossRef]
- Bavamian, S.; Klee, P.; Britan, A.; Populaire, C.; Caille, D.; Cancela, J.; Charollais, A.; Meda, P. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes. Metab. 2007, 9, 118–132. [Google Scholar] [CrossRef]
- Ramachandra, S.; Xie, L.H.; John, S.A.; Subramaniam, S.; Lal, R. A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS ONE 2007, 2, e712. [Google Scholar]
- Aasen, T.; Leithe, E.; Graham, S.V.; Kameritsch, P.; Mayán, M.D.; Mesnil, M.; Pogoda, K.; Tabernero, A. Connexins in cancer: Bridging the gap to the clinic. Oncogene 2019, 38, 4429–4451. [Google Scholar] [CrossRef]
- Rutkowski, R.; Kosztyla-Hojna, B.; Kanczuga-Koda, L.; Sulkowska, M.; Sulkowski, S.; Rutkowski, K. Structure and physiological function of connexin proteins. Postep. Hig. Med. Dosw. 2008, 62, 632–641. [Google Scholar]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef]
- Krysko, D.V.; Leybaert, L.; Vandenabeele, P.; D’Herde, K. Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 2005, 10, 459–469. [Google Scholar] [CrossRef]
- Meşe, G.; Richard, G.; White, T.W. Gap junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2516–2524. [Google Scholar] [CrossRef]
- Segretain, D.; Falk, M.M. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim. Biophys. Acta 2004, 1662, 3–21. [Google Scholar] [CrossRef]
- Spray, D.C.; Ye, Z.C.; Ransom, B.R. Functional connexin “hemichannels”: A critical appraisal. Glia 2006, 54, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.G. Connexin domains relevant to the chemical gating of gap junction channels. Braz. J. Med. Biol. Res. 1997, 30, 577–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sáez, J.C.; Brañes, M.C.; Corvalán, L.A.; Eugenín, E.A.; González, H.; Martínez, A.D.; Palisson, F. Gap junctions in cells of the immune system: Structure, regulation and possible functional roles. Braz. J. Med. Biol. Res. 2000, 33, 447–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Söhl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.I.; Zhou, L.; Zhu, X.; Nicholson, B.J. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. J. Cell Biol. 1998, 140, 1187–1197. [Google Scholar] [CrossRef]
- Perkins, G.; Goodenough, D.; Sosinsky, G. Three-dimensional structure of the gap junction connexon. Biophys. J. 1997, 72, 533–544. [Google Scholar] [CrossRef]
- Martin, P.E.M.; Mambetisaeva, E.T.; Archer, D.A.; George, C.H.; Evans, W.H. Analysis of gap junction assembly using mutated connexins detected in Charcot-Marie-Tooth X-linked disease. J. Neurochem. 2000, 74, 711–720. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 2007, 217, 35–41. [Google Scholar] [CrossRef]
- Lampe, P.D.; Lau, A.F. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 2000, 384, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Salat-Canela, C.; Sesé, M.; Peula, C.; Ramón, Y.; Cajal, S.; Aasen, T. Internal translation of the connexin 43 transcript. Cell Commun. Signal 2014, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Evans, W.H. Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc. Res. 2004, 62, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Martin, P.E.M.; Evans, W.H. Assembly of gap junction channels: Mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. 2001, 268, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B. Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 2004, 62, 233–245. [Google Scholar] [CrossRef]
- Duffy, H.S.; Delmar, M.; Spray, D.C. Formation of the gap junction nexus: Binding partners for connexins. J. Physiol. Paris 2002, 96, 243–249. [Google Scholar] [CrossRef]
- Kojima, T.; Murata, M.; Go, M.; Spray, D.C.; Sawada, N. Connexins induce and maintain tight junctions in epithelial cells. J. Membr. Biol. 2007, 217, 13–19. [Google Scholar] [CrossRef]
- Yotti, L.; Chang, C.; Trosko, J. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science 1979, 206, 1089–1091. [Google Scholar] [CrossRef]
- Trosko, J.E.; Jone, C.; Chang, C.C. Oncogenes, inhibited intercellular communication and tumor promotion. Princess Takamatsu Symp. 1983, 14, 101–113. [Google Scholar]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and the control of tissue growth: Lack of communication between cancer cells. Nature 1966, 209, 1248–1249. [Google Scholar] [CrossRef]
- Jamakosmanović, A.; Loewenstein, W.R. Intercellular communication and tissue growth. Thyroid cancer. J. Cell Biol. 1968, 38, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Borek, C.; Higashino, S.; Loewenstein, W.R. Intercellular communication and tissue growth: IV. Conductance of membrane junctions of normal and cancerous cells in culture. J. Membr. Biol. 1969, 1, 274–293. [Google Scholar] [CrossRef]
- Mesnil, M.; Crespin, S.; Avanzo, J.L.; Zaidan-Dagli, M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 2005, 1719, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V.; Jiang, J.X.; Mesnil, M. Connexins and pannexins: Important players in tumorigenesis, metastasis and potential therapeutics. Int. J. Mol. Sci. 2018, 19, 1645. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.S.; Boonstra, J.; Burghardt, R.C. Reduced cell-cell communication between mitotic and nonmitotic coupled cells. Exp. Cell Res. 1992, 198, 1–7. [Google Scholar] [CrossRef]
- Vinken, M.; Vanhaecke, T.; Papeleu, P.; Snykers, S.; Henkens, T.; Rogiers, V. Connexins and their channels in cell growth and cell death. Cell Signal 2006, 18, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, I.; Makino, T.; Suzuki, Y.; Kai, K.; Teranishi, M.; Takasaki, W.; Furuhama, K. Background lesions during a 24-month observation period in connexin 32-deficient mice. J. Vet. Med. Sci. 2013, 75, 207–210. [Google Scholar] [CrossRef]
- Temme, A.; Buchmann, A.; Gabriel, H.D.; Nelles, E.; Schwarz, M.; Willecke, K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin. Curr. Biol. 1997, 7, 713–716. [Google Scholar] [CrossRef]
- Oyamada, M.; Krutovskikh, V.A.; Mesnil, M.; Partensky, C.; Berger, F.; Yamasaki, H. Aberrant expression of gap junction gene in primary human hepatocellular carcinomas: Increased expression of cardiac-type gap junction gene connexin. Mol. Carcinog. 1990, 3, 273–278. [Google Scholar] [CrossRef]
- Krutovskikh, V.; Mazzoleni, G.; Mironov, N.; Omori, Y.; Aguelon, A.M.; Mesnil, M.; Berger, F.; Partensky, C.; Yamasaki, H. Altered homologous and heterologous gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin. Int. J. Cancer 2007, 56, 87–94. [Google Scholar] [CrossRef]
- King, T.J.; Lampe, P.D. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 2004, 25, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Kazan, J.; El-Saghir, J.; Saliba, J.; Shaito, A.; Jalaleddine, N.; El-Hajjar, L.; Al-Ghadban, S.; Yehia, L.; Zibara, K.; El-Sabban, M. Cx43 Expression Correlates with Breast Cancer Metastasis in MDA-MB-231 Cells In Vitro, In a Mouse Xenograft Model and in Human Breast Cancer Tissues. Cancers 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, N.; Zhu, J.; Hong, X.T.; Liu, H.; Ou, Y.R.; Su, F.; Wang, R.; Li, Y.M.; Wu, Q. Downregulated connexin32 promotes EMT through the Wnt/β-catenin pathway by targeting Snail expression in hepatocellular carcinoma. Int. J. Oncol. 2017, 50, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zheng, X.; Wu, X.; Wang, S.; Wang, Y.; Xing, F. All- trans retinoic acid reverses epithelial-mesenchymal transition in paclitaxel-resistant cells by inhibiting nuclear factor kappa B and upregulating gap junctions. Cancer Sci. 2019, 110, 379–388. [Google Scholar] [CrossRef]
- Lee, H.J. Growth-Suppressing Activity of the Transfected Cx26 on BICR-M1Rk Breast Cancer Cell Line. J. Microbiol. Biotechnol. 2011, 21, 477–482. [Google Scholar] [CrossRef]
- Wu, D.; Li, B.; Liu, H.; Yuan, M.; Yu, M.; Tao, L.; Dong, S.; Tong, X. In vitro inhibited effect of gap junction composed of Cx43 in the invasion and metastasis of testicular cancer resistanced to cisplatin. Biomed. Pharmacother. 2018, 98, 826–833. [Google Scholar] [CrossRef]
- Mesnil, M.; Krutovskikh, V.; Piccoli, C.; Elfgang, C.; Traub, O.; Willecke, K.; Ymasaki, H. Negative Growth Control of HeLa Cells by Connexin Genes: Connexin Species Specificity. Cancer Res. 1995, 55, 629–639. [Google Scholar]
- Vinken, M.; Decrock, E.; Leybaert, L.; Bultynck, G.; Himpens, B.; Vanhaecke, T.; Rogiers, V. Non-channel functions of connexins in cell growth and cell death. Biochim. Biophys. Acta 2012, 1818, 2002–2008. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Morita, I.; Ikeda, M.; Ma, K.W.; Murota, S. Connexin43 suppresses proliferation of osteosarcoma U2OS cells through post-transcriptional regulation of p. Oncogene 2002, 20, 4138–4149. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Nakayama, K.; Nakayama, K.I.; Morita, I. A novel route for connexin 43 to inhibit cell proliferation: Negative regulation of S-phase kinase-associated protein [Skp 2]. Cancer Res. 2003, 61, 7044–7047. [Google Scholar]
- Peng, Y.; Wang, X.; Guo, Y.; Peng, F.; Zheng, N.; He, B.; Ge, H.; Tao, L.; Wang, Q. The pattern of cell-to-cell transfer of micro RNA by gap junction and its effect on the proliferation of glioma cells. Cancer Sci. 2019, 110, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Warn-Cramer, B.J.; Kurata, W.E.; Lau, A.F. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 2001, 154, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, H.; Cannon, A.; Trease, A.J.; Spagnol, G.; Zheng, H.; Radio, S.; Patel, K.; Batra, S.; Sorgen, P.L. Phosphorylation of Cx43 residue Y313 by Src contributes to blocking the interaction with Drebrin and disassembling gap junctions. J. Mol. Cell Cardiol. 2019, 126, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Doble, B.W.; Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell Biochem. 2003, 242, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Gu, S.; Yu, X.S.; White, T.W.; Banks, E.A.; Jiang, J.X. Connexin Controls Cell-Cycle Exit and Cell Differentiation by Directly Promoting Cytosolic Localization and Degradation of E3 Ligase Skp. Dev. Cell 2015, 35, 483–496. [Google Scholar] [CrossRef]
- Sirnes, S.; Bruun, J.; Kolberg, M.; Kjenseth, A.; Lind, G.E.; Svindland, A.; Brech, A.; Nesbakken, A.; Lothe, R.A.; Leithe, E.; et al. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int. J. Cancer 2012, 131, 570–581. [Google Scholar] [CrossRef]
- Duffy, H.S.; Iacobas, I.; Hotchkiss, K.; Hirst-Jensen, B.J.; Bosco, A.; Dandachi, N.; Dermietzel, R.; Sorgen, P.L.; Spray, D.C. The Gap Junction Protein Connexin32 Interacts with the Src Homology 3/Hook Domain of Discs Large Homolog. J. Biol. Chem. 2007, 282, 9789–9796. [Google Scholar] [CrossRef]
- MacDonald, A.I.; Sun, P.; Hernandez-Lopez, H.; Aasen, T.; Hodgins, M.B.; Edward, M.; Roberts, S.; Massimi, P.; Thomas, M.; Banks, L.; et al. A functional interaction between the MAGUK protein hDlg and the gap junction protein connexin 43 in cervical tumour cells. Biochem. J. 2012, 446, 9–21. [Google Scholar] [CrossRef]
- Penes, M.C.; Li, X.; Nagy, J.I. Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB)-MsY3 in glial cells and colocalization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur. J. Neurosci. 2005, 22, 404–418. [Google Scholar] [CrossRef]
- González-Sánchez, A.; Jaraíz-Rodríguez, M.; Domínguez-Prieto, M.; Herrero-González, S.; Medina, J.M.; Tabernero, A. Connexin43 recruits PTEN and Csk to inhibit c-Src activity in glioma cells and astrocytes. Oncotarget 2016, 7, 49819–49833. [Google Scholar] [CrossRef]
- Song, D.; Liu, X.; Liu, R.; Yang, L.; Zuo, J.; Liu, W. Connexin 43 hemichannel regulates H9c2 cell proliferation by modulating intracellular ATP and [Ca2+]. Acta Biochim. Biophys. Sin. (Shanghai) 2010, 42, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Sirnes, S.; Honne, H.; Ahmed, D.; Danielsen, S.A.; Rognum, T.O.; Meling, G.I.; Leithe, E.; Rivedal, E.; Ragnhild, A.L.; Lind, G.E. DNA methylation analyses of the connexin gene family reveal silencing of GJC1 (Connexin45) by promoter hypermethylation in colorectal cancer. Epigenetics 2011, 6, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Omori, Y.; Zaidan-Dagli, M.L.; Mironov, N.; Mesnil, M.; Krutovskikh, V. Genetic and epigenetic changes of intercellular communication genes during multistage carcinogenesis. Cancer Detect. Prev. 1999, 23, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jinn, Y.; Inase, N. Connexin 43, E-cadherin, beta-catenin and ZO-1 expression, and aberrant methylation of the connexin 43 gene in NSCLC. Anticancer Res. 2010, 30, 2271–2288. [Google Scholar] [PubMed]
- Yano, T.; Ito, F.; Yamasaki, H.; Hagiwara, K.; Ozasa, H.; Nakazawa, H.; Toma, H. Epigenetic inactivation of connexin 32 in renal cell carcinoma from hemodialytic patients. Kidney Int. 2004, 65, 1519–1521. [Google Scholar] [CrossRef]
- Jin, Z.; Xu, S.; Yu, H.; Yang, B.; Zhao, H.; Zhao, G. miR-125b Inhibits Connexin43 and Promotes Glioma Growth. Cell Mol. Neurobiol. 2013, 33, 1143–1148. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.H.; Song, B.; Xiong, E.Q.; Chen, Z.W.; Zhou, Z.S.; Sue, P.Y. Suppression of CX43 expression by miR-20a in the progression of human prostate cancer. Cancer Biol. Ther. 2012, 13, 890–898. [Google Scholar] [CrossRef]
- Smyth, J.W.; Shaw, R.M. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013, 5, 611–618. [Google Scholar] [CrossRef]
- Ul-Hussain, M.; Olk, S.; Schoenebeck, B.; Wasielewski, B.; Meier, C.; Prochnow, N.; May, C.; Galozzi, S.; Zoidl, G.; Dermietzel, R. Internal ribosomal entry site (IRES) activity generates endogenous carboxyl-terminal domains of Cx43 and is responsive to hypoxic conditions. J. Biol. Chem. 2014, 289, 20979–20990. [Google Scholar] [CrossRef]
- Johnstone, S.R.; Billaud, M.; Lohman, A.W.; Taddeo, E.P.; Isakson, B.E. Post-translational modifications in connexins and pannexins. J. Membr. Biol. 2012, 245, 319–332. [Google Scholar] [CrossRef]
- Johnson, K.E.; Mitra, S.; Katoch, P.; Kelsey, L.S.; Johnson, K.R.; Mehta, P.P. Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Mol. Biol. Cell 2013, 24, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Leithe, E.; Kjenseth, A.; Sirnes, S.; Stenmark, H.; Brech, A.; Rivedal, E. Ubiquitylation of the gap junction protein connexin-43 signals its trafficking from early endosomes to lysosomes in a process mediated by Hrs and Tsg. J. Cell Sci. 2009, 122, 3883–3893. [Google Scholar] [CrossRef] [PubMed]
- Dubina, M.V.; Iatckii, N.A.; Popov, D.E.; Vasil’ev, S.V.; Krutovskikh, V.A. Connexin 43, but not connexin 32, is mutated at advanced stages of human sporadic colon cancer. Oncogene 2002, 21, 4992–4996. [Google Scholar] [CrossRef] [PubMed]
- Kotini, M.; Mayor, R. Connexins in migration during development and cancer. Dev. Biol. 2015, 401, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Defamie, N.; Chepied, A.; Mesnil, M. Connexins, gap junctions and tissue invasion. FEBS Lett. 2014, 588, 1331–1338. [Google Scholar] [CrossRef]
- Ito, A.; Katoh, F.; Kataoka, T.R.; Okada, M.; Tsubota, N.; Asada, H.; Yoshikawa, K.; Maeda, S.; Kitamura, Y.; Yamasaki, H.; et al. A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J. Clin. Investig. 2000, 105, 1189–1197. [Google Scholar] [CrossRef]
- Saito-Katsuragi, M.; Asada, H.; Niizeki, H.; Katoh, F.; Masuzawa, M.; Tsutsumi, M.; Kuniyasu, H.; Akihiko, I.; Hiroshi, N.; Miyagawa, S. Role for connexin 26 in metastasis of human malignant melanoma. Cancer 2007, 110, 1162–1172. [Google Scholar] [CrossRef]
- Lamiche, C.; Clarhaut, J.; Strale, P.O.; Crespin, S.; Pedretti, N.; Bernard, F.X.; Naus, C.C.; Vincent, C.C.; Foster, L.J.; Defamine, N.; et al. The gap junction protein Cx43 is involved in the bone-targeted metastatic behaviour of human prostate cancer cells. Clin. Exp. Metastasis 2012, 29, 111–122. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Q.; Guo, Y.; Ge, H.; Fu, Y.; Wang, X.; Tao, L. Cx32 exerts anti-apoptotic and pro-tumor effects via the epidermal growth factor receptor pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 145. [Google Scholar] [CrossRef]
- Wu, J.I.; Wang, L.H. Emerging roles of gap junction proteins connexins in cancer metastasis, chemoresistance and clinical application. J. Biomed. Sci. 2019, 26, 8. [Google Scholar] [CrossRef]
- Chen, Q.; Boire, A.; Jin, X.; Valiente, M.; Er, E.E.; Lopez-Soto, A.; Leni, J.; Patwa, R.; Shah, H.; Xu, K.; et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kanczuga-Koda, L.; Sulkowski, S.; Lenczewski, A.; Koda, M.; Wincewicz, A.; Baltaziak, M.; Sulkowska, M. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J. Clin. Pathol. 2006, 59, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Ming, J.; Yang, L.; Du, J.Z.; Wang, N.; Luo, H.J. Mechanism of Regulatory Effect of MicroRNA-206 on Connexin 43 in Distant Metastasis of Breast Cancer. Chin. Med. J. 2016, 129, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Ezumi, K.; Yamamoto, H.; Murata, K.; Higashiyama, M.; Damdinsuren, B.; Nakamura, Y.; Kyo, N.; Okami, J.; Ngan, C.Y.; Takemasa, I.; et al. Aberrant Expression of Connexin 26 Is Associated with Lung Metastasis of Colorectal Cancer. Clin. Cancer Res. 2008, 14, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Polusani, S.R.; Kalmykov, E.A.; Chandrasekhar, A.; Zucker, S.N.; Nicholson, B.J. Cell coupling mediated by connexin 26 selectively contributes to reduced adhesivity and increased migration. J. Cell Sci. 2016, 129, 4399–4410. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Kalmykov, E.A.; Polusani, S.R.; Mathis, S.A.; Zucker, S.N.; Nicholson, B.J. Intercellular redistribution of cAMP underlies selective suppression of cancer cell growth by connexin. PLoS ONE 2013, 8, e82335. [Google Scholar] [CrossRef] [PubMed]
- Zefferino, R.; Piccoli, C.; Gioia, S.D.; Capitanio, N.; Conese, M. Gap Junction Intercellular Communication in the Carcinogenesis Hallmarks: Is This a Phenomenon or Epiphenomenon? Cells 2019, 8, 896. [Google Scholar] [CrossRef]
- Stoletov, K.; Strnadel, J.; Zardouzian, E.; Momiyama, M.; Park, F.D.; Kelber, J.A.; Pizzzo, D.P.; Hoffman, R.; VandenBerg, S.R.; Klemke, R.L. Role of connexins in metastatic breast cancer and melanoma brain colonization. J. Cell Sci. 2013, 126, 904–913. [Google Scholar] [CrossRef]
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- Zeng, S.G.; Lin, X.; Liu, J.C.; Zhou, J. Hypoxia-induced internalization of connexin 26 and connexin 43 in pulmonary epithelial cells is involved in the occurrence of non-small cell lung cancer via the P53/MDM2 signaling pathway. Int. J. Oncol. 2019, 55, 845–859. [Google Scholar] [CrossRef]
- Toledo-Guzmán, M.E.; Bigoni-Ordóñez, G.D.; Ibáñez Hernández, M.; Ortiz-Sánchez, E. Cancer stem cell impact on clinical oncology. World J. Stem. Cells 2018, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Hainz, N.; Tschernig, T.; Meier, C. Facets of Communication: Gap Junction Ultrastructure and Function in Cancer Stem Cells and Tumor Cells. Cancers (Basel) 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, P.S.; Sinyuk, M.; Turaga, S.M.; Mulkearns-Hubert, E.E.; Hale, J.S.; Rao, V.; Demelash, A.; Saygin, C.; China, A.; Alban, T.J.; et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat. Commun. 2018, 9, 578. [Google Scholar] [CrossRef]
- Hitomi, M.; Deleyrolle, L.P.; Mulkearns-Hubert, E.E.; Jarrar, A.; Li, M.; Sinyuk, M.; Otvos, B.; Brunet, S.; Flavahan, W.A.; Hubert, C.G.; et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015, 11, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.; Xiao, H.L.; Jiang, X.F.; Wang, Q.L.; Li, Y.; Yang, X.J.; Ping, Y.F.; Duan, J.J.; Jiang, J.Y.; Ye, X.Z.; et al. Connexin 43 Reverses Malignant Phenotypes of Glioma Stem Cells by Modulating E-Cadherin. Stem Cells 2012, 30, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Omori, Y.; Li, Q.; Nishikawa, Y.; Yoshioka, T.; Yoshida, M.; Ishikawa, K.; Enomoto, K. Cytoplasmic accumulation of connexin32 expands cancer stem cell population in human HuH7 hepatoma cells by enhancing its self-renewal. Int. J. Cancer 2011, 128, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Acuña, R.A.; Varas-Godoy, M.; Berthoud, V.M.; Alfaro, I.E.; Retamal, M.A. Connexin-46 contained in extracellular vesicles enhance malignancy features in breast cancer cells. Biomolecules 2020, 10, 676. [Google Scholar] [CrossRef]
- Chasampalioti, M.; Green, A.R.; Ellis, I.O.; Rakha, E.A.; Jackson, A.M.; Spendlove, I.; Ramage, J.M. Connexin 43 is an independent predictor of patient outcome in breast cancer patients. Breast Cancer Res. Treat. 2019, 174, 93–102. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wu, X.; Li, C.; Huang, Y.; Zhou, H.; Cui, Y. Resveratrol Sensitizes Colorectal Cancer Cells to Cetuximab by Connexin 43 Upregulation-Induced Akt Inhibition. Front. Oncol. 2020, 10, 383. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Zhao, Z.; Zhang, C.; Yang, X.; Wang, Y. Connexin 43 enhances paclitaxel cytotoxicity in colorectal cancer cell lines. Exp. Ther. Med. 2017, 14, 1212–1218. [Google Scholar] [CrossRef]
- Chi, Q.; Wang, Z.Y.; Li, H.Y.; Song, D.B.; Xu, H.; Ma, G.; Wang, Z.M.; Li, X.M. Tumor-suppressor microRNA-139-5p restrains bladder cancer cell line ECV-304 properties via targeting Connexin. Chin. Med. J. (Engl.) 2019, 132, 2354–2361. [Google Scholar] [CrossRef]
- Wang, H.; Tian, L.; Liu, J.; Goldstein, A.; Bado, I.; Zhang, W.; Arenkiel, B.R.; Li, Z.; Yang, M.; Du, S.; et al. The Osteogenic Niche Is a Calcium Reservoir of Bone Micrometastases and Confers Unexpected Therapeutic Vulnerability. Cancer Cell 2018, 34, 823–839.e7. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Luo, Y.; Mao, N.; Huang, G.; Teng, C.; Wang, H.; Wu, J.; Liao, X.; Yang, J. Cancer-Associated Fibroblasts Accelerate Malignant Progression of Non-Small Cell Lung Cancer via Connexin 43-Formed Unidirectional Gap Junctional Intercellular Communication. Cell Physiol. Biochem. 2018, 51, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Crespin, S.; Fromont, G.; Wager, M.; Levillain, P.; Cronier, L.; Monvoisin, A.; Defamie, N.; Mesnil, M. Expression of a gap junction protein, connexin43, in a large panel of human gliomas: New insights. Cancer Med. 2016, 5, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Ji, L.; Wang, H.; Wang, W.; Zheng, H.; Zou, J.; Liu, L.; Qi, X.; Liu, Z.; Du, B.; et al. Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing malignancy and inducing M1 polarization. Int. J. Cancer 2017, 141, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, J.H.; Du, Q.Y.; Zhou, Y.C.; Yao, T.J.; Wu, Q.; Liu, J.; Ou, Y.R. Connexin 32 downregulation is critical for chemoresistance in oxaliplatin-resistant HCC cells associated with EMT. Cancer Manag. Res. 2019, 11, 5133–5146. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Ge, H.; Zheng, N.; Peng, F.; Fu, Y.; Tao, L.; Wang, Q. Inhibition of ubiquitin-specific protease 14 promotes connexin 32 internalization and counteracts cisplatin cytotoxicity in human ovarian cancer cells. Oncol. Rep. 2019, 42, 1237–1247. [Google Scholar] [CrossRef]
- Sinyuk, M.; Mulkearns-Hubert, E.E.; Reizes, O.; Lathia, J. Cancer Connectors: Connexins, Gap Junctions, and Communication. Front. Oncol. 2018, 8, 646. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Z.; Ling, F.; Xu, G. Astrocytes protect glioma cells from chemotherapy and upregulate survival genes via gap junctional communication. Mol. Med. Rep. 2016, 13, 1329–1335. [Google Scholar] [CrossRef]
- Lin, Q.; Balasubramanian, K.; Fan, D.; Kim, S.J.; Guo, L.; Wang, H.; Bar-El, M.; Aldape, K.D.; Fidler, I.J. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 2010, 12, 748–754. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wu, J.I.; Tseng, C.W.; Chen, H.J.; Wang, L.H. Gjb4 serves as a novel biomarker for lung cancer and promotes metastasis and chemoresistance via Src activation. Oncogene 2019, 38, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qin, G.; Luo, M.; Chen, J.; Zhang, Q.; Li, L.; Pan, L.; Qin, S. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis. 2015, 6, e1829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, L.; Fan, L.X.; Huang, K.; Luo, H.M.; Ge, H.; Wang, X.; Wang, Q. Cx32 mediates cisplatin resistance in human ovarian cancer cells by affecting drug efflux transporter expression and activating the EGFR-Akt pathway. Mol. Med. Rep. 2019, 19, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Dertinger, H.; Hülser, D. Increased radioresistance of cells in cultured multicell spheroids. I. Dependence on cellular interaction. Radiat Environ. Biophys. 1981, 19, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Autsavapromporn, N.; De Toledo, S.M.; Jay-Gerin, J.P.; Harris, A.L.; Azzam, E.I. Human cell responses to ionizing radiation are differentially affected by the expressed connexins. J. Radiat Res. 2013, 54, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Artesi, M.; Kroonen, J.; Bredel, M.; Nguyen-Khac, M.; Deprez, M.; Schoysman, L.; Poulet, C.; Chakravarti, A.; Kim, H.; Scholtens, D.; et al. Connexin 30 expression inhibits growth of human malignant gliomas but protects them against radiation therapy. Neuro. Oncol. 2015, 17, 392–406. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Prise, K.M.; O’Sullivan, J.M. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer 2009, 9, 351–360. [Google Scholar] [CrossRef]
- Gaillard, S.; Pusset, D.; de Toledo, S.M.; Fromm, M.; Azzam, E.I. Propagation distance of the alpha-particle-induced bystander effect: The role of nuclear traversal and gap junction communication. Radiat. Res. 2009, 171, 513–520. [Google Scholar] [CrossRef]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc. Natl. Acad. Sci. USA 2001, 98, 473–478. [Google Scholar] [CrossRef]
- Verma, N.; Tiku, A.B. Significance and nature of bystander responses induced by various agents. Mutat. Res. 2017, 773, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Fornelli, F.; Leone, A.; Verdesca, I.; Minervini, F.; Zacheo, G. The influence of lycopene on the proliferation of human breast cell line (MCF-7). Toxicol. In Vitro 2007, 21, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Safwat, S.; Ishak, R.A.; Hathout, R.M.; Mortada, N.D. Statins anticancer targeted delivery systems: Re-purposing an old molecule. J. Pharm. Pharmacol. 2017, 69, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Cesen-Cummings, K.; Warner, K.A.; Ruch, R.J. Role of protein kinase C in the deficient gap junctional intercellular communication of K-ras-transformed murine lung epithelial cells. Anticancer Res. 1998, 18, 4343–4346. [Google Scholar] [PubMed]
- Wang, L.; Fu, Y.; Peng, J.; Wu, D.; Yu, M.; Xu, C.; Wang, Q.; Tao, L. Simvastatin-induced up-regulation of gap junctions composed of connexin 43 sensitize Leydig tumor cells to etoposide: An involvement of PKC pathway. Toxicology 2013, 312, 149–157. [Google Scholar] [CrossRef]
- Wang, L.; Peng, J.; Huang, H.; Wang, Q.; Yu, M.; Tao, L. Simvastatin protects Sertoli cells against cisplatin cytotoxicity through enhanced gap junction intercellular communication. Oncol. Rep. 2015, 34, 2133–2141. [Google Scholar] [CrossRef]
- King, T.J.; Bertram, J.S. Connexins as targets for cancer chemoprevention and chemotherapy. Biochim. Biophys. Acta 2005, 1719, 146–160. [Google Scholar] [CrossRef]
- Fukushima, M.; Hattori, Y.; Yoshizawa, T.; Maitani, Y. Combination of non-viral connexin 43 gene therapy and docetaxel inhibits the growth of human prostate cancer in mice. Int. J. Oncol. 2007, 30, 225–231. [Google Scholar] [CrossRef][Green Version]
- Sato, H.; Iwata, H.; Takano, Y.; Yamada, R.; Okuzawa, H.; Nagashima, Y.; Yamaura, K.; Ueno, K.; Yano, T. Enhanced effect of connexin 43 on cisplatin-induced cytotoxicity in mesothelioma cells. J. Pharmacol. Sci. 2009, 110, 466–475. [Google Scholar] [CrossRef]
- Uzu, M.; Sato, H.; Shimizu, A.; Shibata, Y.; Ueno, K.; Hisaka, A. Connexin 43 enhances Bax activation via JNK activation in sunitinib-induced apoptosis in mesothelioma cells. J. Pharmacol. Sci. 2017, 134, 101–107. [Google Scholar] [CrossRef]
- Yang, C.J.; Kuo, C.T.; Wu, L.H.; Chen, M.C.; Pangilinan, C.R.; Phacharapiyangkul, N.; Liu, W.; Chen, Y.H.; Lee, C.H. Eicosapentaenoic acids enhance chemosensitivity through connexin 43 upregulation in murine melanoma models. Int. J. Med. Sci. 2019, 16, 636–643. [Google Scholar] [CrossRef]
- Zibara, K.; Awada, Z.; Dib, L.; El-Saghir, J.; Al-Ghadban, S.; Ibrik, A.; El-Zein, N.; El-Sabban, M. Anti-angiogenesis therapy and gap junction inhibition reduce MDA-MB-231 breast cancer cell invasion and metastasis in vitro and in vivo. Sci. Rep. 2015, 5, 12598. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, L.; Goldstein, A.; Liu, J.; Lo, H.C.; Sheng, K.; Welte, T.; Wong, S.T.C.; Gugala, Z.; Stossi, F.; et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat. Commun. 2017, 8, 15045. [Google Scholar] [CrossRef]
- Retamal, M.A.; León-Paravic, C.G.; Ezquer, M.; Ezquer, F.; Del Rio, R.; Pupo, A.; Martínez, A.D.; González, C. Carbon monoxide: A new player in the redox regulation of connexin hemichannels. IUBMB Life 2015, 67, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: A revision. BMC Cell Biol. 2016, 17 (Suppl. 1), 11. [Google Scholar] [CrossRef] [PubMed]
- León-Paravic, C.G.; Figueroa, V.A.; Guzmán, D.J.; Valderrama, C.F.; Vallejos, A.A.; Fiori, M.C.; Altenberg, G.A.; Reuss, L.; Retamal, M.A. Carbon monoxide (CO) is a novel inhibitor of connexin hemichannels. J. Biol. Chem. 2014, 289, 36150–36157. [Google Scholar] [CrossRef]
- Willebrords, J.; Maes, M.; Yanguas, S.C.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef]
- Murphy, S.F.; Varghese, R.T.; Lamouille, S.; Guo, S.; Pridham, K.J.; Kanabur, P.; Osimani, A.M.; Sharma, S.; Jourdan, J.; Rodgers, C.M.; et al. Connexin 43 Inhibition Sensitizes Chemoresistant Glioblastoma Cells to Temozolomide. Cancer Res. 2016, 76, 139–149. [Google Scholar] [CrossRef]
- Alonso, F.; Domingos-Pereira, S.; Le Gal, L.; Derré, L.; Meda, P.; Jichlinski, P.; Nardelli-Haefliger, D.; Haefliger, J.A. Targeting endothelial connexin40 inhibits tumor growth by reducing angiogenesis and improving vessel perfusion. Oncotarget 2016, 7, 14015–14028. [Google Scholar] [CrossRef]
- Grek, C.L.; Rhett, J.M.; Bruce, J.S.; Abt, M.A.; Ghatnekar, G.S.; Yeh, E.S. Targeting connexin 43 with α-connexin carboxyl-terminal (ACT1) peptide enhances the activity of the targeted inhibitors, tamoxifen and lapatinib, in breast cancer: Clinical implication for ACT. BMC Cancer 2015, 15, 296. [Google Scholar] [CrossRef]
- Jaraíz-Rodríguez, M.; Tabernero, M.D.; González-Tablas, M.; Otero, A.; Orfao, A.; Medina, J.M.; Tabernero, A. A Short Region of Connexin43 Reduces Human Glioma Stem Cell Migration, Invasion, and Survival through Src, PTEN, and FAK. Stem Cell Rep. 2017, 9, 451–463. [Google Scholar] [CrossRef]
- Yusubalieva, G.M.; Baklaushev, V.P.; Gurina, O.I.; Gulyaev, M.V.; Pirogo, Y.A.; Chekhonin, V.P. Antitumor effects of monoclonal antibodies to connexin 43 extracellular fragment in induced low-differentiated glioma. Bull. Exp. Biol. Med. 2012, 153, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Yusubalieva, G.M.; Baklaushev, V.P.; Gurina, O.I.; Zorkina, Y.A.; Gubskii, I.L.; Kobyakov, G.L.; Golanov, A.V.; Goryainov, S.A.; Gorlachev, G.E.; Konovalov, A.N.; et al. Treatment of poorly differentiated glioma using a combination of monoclonal antibodies to extracellular connexin-43 fragment, temozolomide, and radiotherapy. Bull. Exp. Biol. Med. 2014, 157, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Navarrete, M.; Álvarez, J.; Guerrero, I.; Gleisner, M.A.; Tittarelli, A.; Salazar-Onfray, F. Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing. Int. J. Mol. Sci. 2019, 20, 4509. [Google Scholar] [CrossRef] [PubMed]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Yusubalieva, G.M.; Grinenko, N.P.; Gubskiy, I.L.; Melnikov, P.A.; Kardashova, K.S.; Kabanov, A.V.; et al. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT. Drug Deliv. 2015, 22, 276–285. [Google Scholar] [CrossRef]
- Chekhonin, V.P.; Baklaushev, V.P.; Yusubalieva, G.M.; Belorusova, A.E.; Gulyaev, M.V.; Tsitrin, E.B.; Grinenko, N.F.; Gurina, O.I.; Pirogov, Y.A. Targeted delivery of liposomal nanocontainers to the peritumoral zone of glioma by means of monoclonal antibodies against GFAP and the extracellular loop of Cx. Nanomedicine 2012, 8, 63–70. [Google Scholar] [CrossRef]
- Kong, H.; Liu, X.; Yang, L.; Qi, K.; Zhang, H.; Zhang, J.; Huang, Z.; Wang, H. All-trans retinoic acid enhances bystander effect of suicide gene therapy in the treatment of breast cancer. Oncol. Rep. 2016, 35, 1868–1874. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, S.; Zhang, X.; Sun, A.; Tao, F.; Ju, H.; Qian, H. Effects of small interfering RNA interference of connexin 37 on subcutaneous gastric tumours in mice. Mol. Med. Rep. 2014, 10, 2955–2960. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Hua, S.; Dai, Y.; Yuan, Y.; Yang, J.; Deng, J.; Huo, Y.; Chen, X.; Teng, B.; Yu, X.; et al. Roles of Cx43 and AKAP95 in ovarian cancer tissues in G1/S phase. Int. J. Clin. Exp. Pathol. 2015, 8, 14315–14324. [Google Scholar]
- Xie, J.; Wang, X.; Ge, H.; Peng, F.; Zheng, N.; Wang, Q.; Tao, L. Cx32 mediates norepinephrine-promoted EGFR-TKI resistance in a gap junction-independent manner in non-small-cell lung cancer. J. Cell Physiol. 2019, 234, 23146–23159. [Google Scholar] [CrossRef]
- Kanczuga-Koda, L.; Koda, M.; Sulkowski, S.; Wincewicz, A.; Zalewski, B.; Sulkowska, M. Gradual loss of functional gap junction within progression of colorectal cancer—A shift from membranous CX32 and CX43 expression to cytoplasmic pattern during colorectal carcinogenesis. In Vivo 2010, 24, 101–107. [Google Scholar] [PubMed]
- Chen, Y.; Hühn, D.; Knösel, T.; Pacyna-Gengelbach, M.; Deutschmann, N.; Petersen, I. Downregulation of connexin 26 in human lung cancer is related to promoter methylation. Int. J. Cancer 2005, 113, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Shao, Q.; Curtis, H.; Galipeau, J.; Belliveau, D.J.; Wang, T.; Alaoui-Jamali, M.A.; Laird, D.W. Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. J. Biol. Chem. 2002, 277, 29132–29138. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, E.; Shao, Q.; Wang, H.L.; Langlois, S.; Laird, D.W. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006, 66, 9886–9894. [Google Scholar] [CrossRef]
- Zhang, W.; Nwagwu, C.; Le, D.M.; Yong, V.W.; Song, H.; Couldwell, W.T. Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J. Neurosurg. 2003, 99, 1039–1046. [Google Scholar] [CrossRef]
- Wu, W.; Fan, L.; Bao, Z.; Zhang, Y.; Peng, Y.; Shao, M.; Xiang, Y.; Zhang, X.; Wang, Q.; Tao, L. The cytoplasmic translocation of Cx32 mediates cisplatin resistance in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2017, 487, 292–299. [Google Scholar] [CrossRef]
- Tang, N.; Liu, J.; Chen, B.; Zhang, Y.; Yu, M.; Cai, Z.; Chen, H. Effects of gap junction intercellular communication on the docetaxel-induced cytotoxicity in rat hepatocytes. Mol. Med. Rep. 2017, 15, 2689–2694. [Google Scholar] [CrossRef]
- Evans, W.H.; Leybaert, L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun. Adhes 2007, 14, 265–273. [Google Scholar] [CrossRef]
- Iyyathurai, J.; D’hondt, C.; Wang, N.; De Bock, M.; Himpens, B.; Retamal, M.A.; Stehberg, J.; Leybaert, L.; Bultynck, G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: Gap junctions versus hemichannels. Neuropharmacology 2013, 75, 491–505. [Google Scholar] [CrossRef]
- Mulkearns-Hubert, E.E.; Torre-Healy, L.A.; Silver, D.J.; Eurich, J.T.; Bayik, D.; Serbinowski, E.; Hitomi, M.; Zhou, J.; Przychodzen, B.; Zhang, R.; et al. Development of a Cx46 Targeting Strategy for Cancer Stem Cells. Cell Rep. 2019, 27, 1062–1072.e5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).