Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe
Abstract
1. Introduction
2. Results
2.1. Localization of Cathepsin V in Human Thyroid Tissue
2.2. Cathepsin V Is Associated with the Plasma Membrane of Thyroid Epithelial Cells
2.3. The Chimeric Protein hCV-eGFP Undergoes N-Linked Glycosylation in Thyroid Epithelial Cells
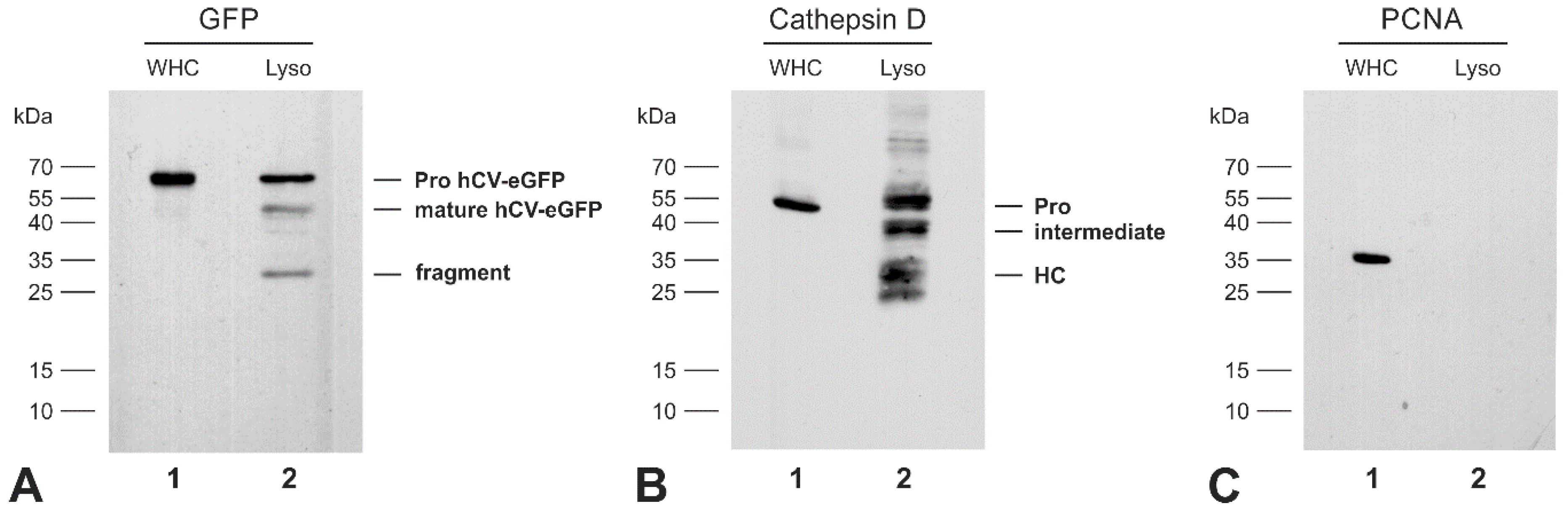
2.4. The Transport Route of the Chimeric Protein hCV-eGFP in Thyroid Epithelial Cells
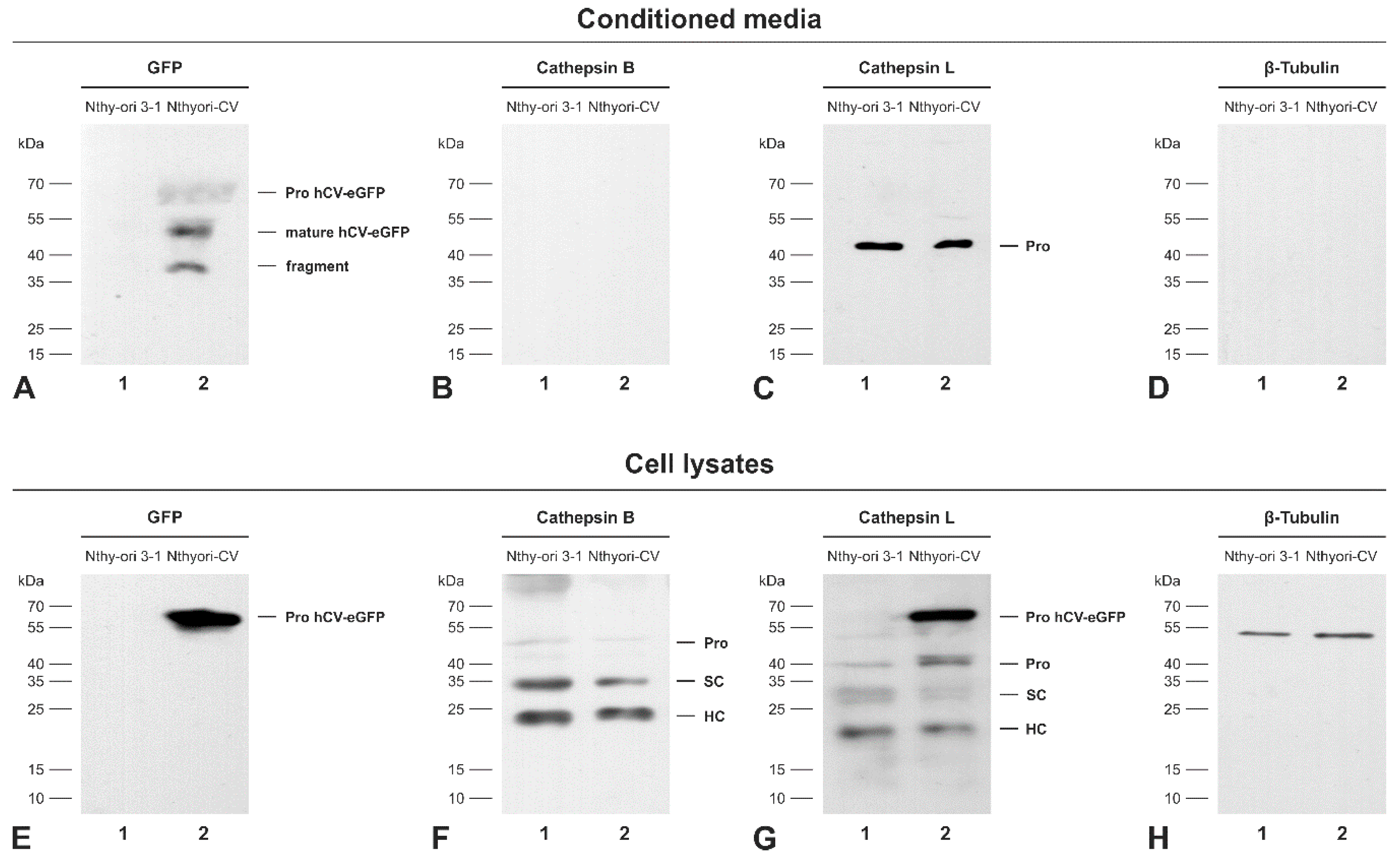
2.5. The Chimeric Protein hCV-eGFP Is Secreted from Thyroid Epithelial Cells
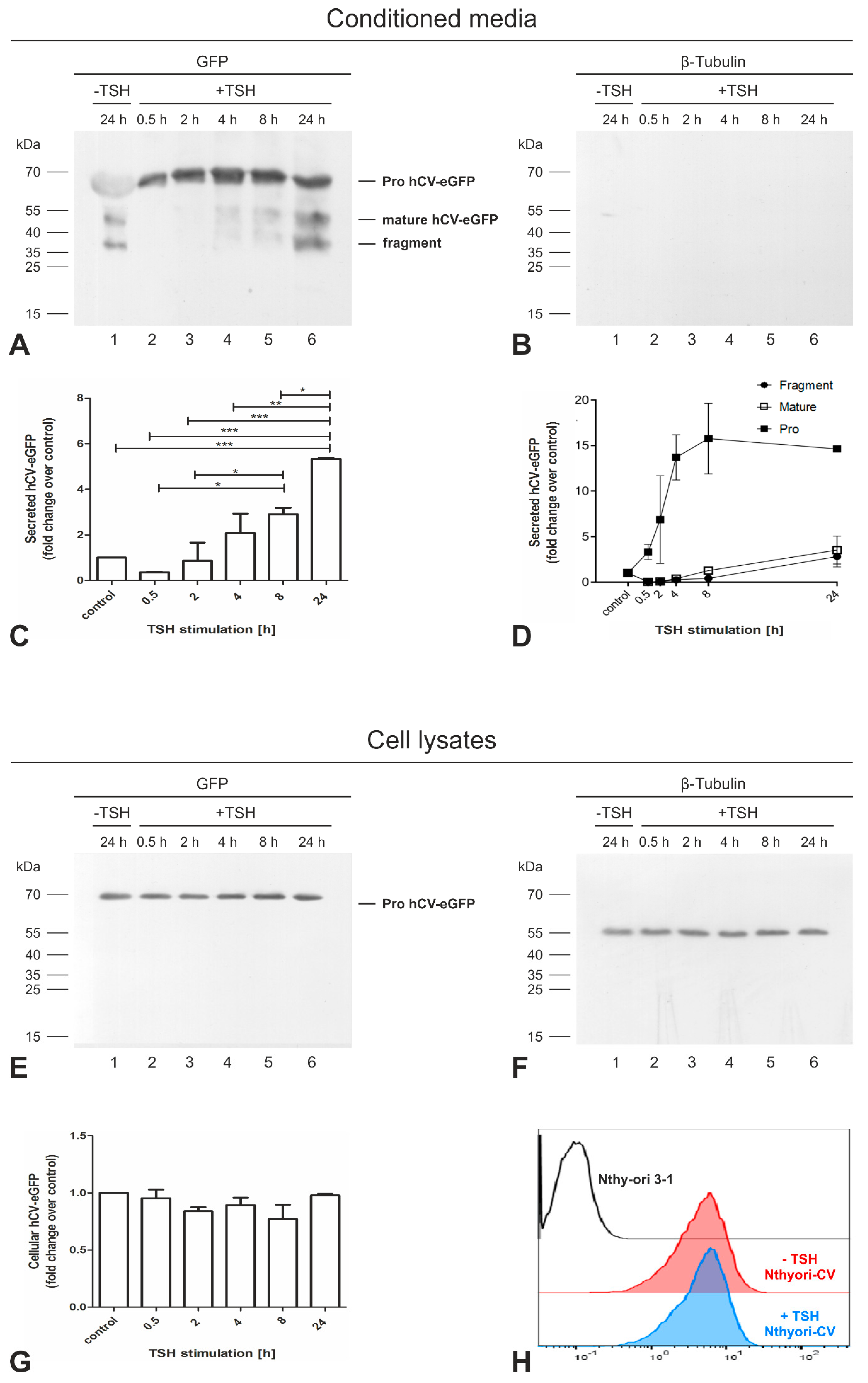
2.6. Secretion of the Proform of hCV-eGFP Is Triggered by TSH Stimulation
2.7. The Activity-Based Probe DCG-04 Recognizes the Proform of eGFP-Tagged Cathepsin V Chimeric Protein
3. Discussion
3.1. Trafficking of Cathepsins to Endo-Lysosomes
3.2. Transport Routes of Cathepsins to the Extracellular Space
3.3. Mechanisms of Procathepsins Activation
3.4. Perspectives
4. Materials and Methods
4.1. Cell Culture
4.2. Flow Cytometry Analysis
4.3. Indirect Immunofluorescence and Image Acquisition
4.4. Subcellular Fractionation
4.5. TCA Protein Precipitation from Conditioned Media
4.6. Detection of DCG-04-Labeled Cysteine Peptidases
4.7. Enzymatic de-Glycosylation of N-Linked Glycoproteins
4.8. SDS-PAGE and Immunoblotting
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ca2+ | free calcium |
| CD-MPR | cation-dependent mannose-6-phosphate receptor |
| CMF-PBS | calcium- and magnesium-free PBS |
| eGFP | enhanced green fluorescent protein |
| hCV-eGFP | human full-length cathepsin V tagged with eGFP |
| M6P | mannose-6 phosphate |
| Nthyori-CV | Nthy-ori 3-1 transduced with hCV-eGFP |
| Lamp1 | lysosome-associated membrane protein 1 |
| PBS | phosphate-buffered saline |
| PCNA | proliferating cell nuclear antigen |
| PDI | ER-resident protein disulfide isomerase |
| PLCβ | Gαq-phospholipase Cβ |
| rER | rough endoplasmic reticulum |
| RPMI | Roswell Park Memorial Institute medium |
| SCTP | Stratum corneum thiol protease |
| TH | thyroid hormone |
| Tg | thyroglobulin |
| TGN | trans-Golgi network |
| TSH | thyroid stimulating hormone |
| TSHR | TSH receptors |
References
- Brömme, D.; Li, Z.; Barnes, M.; Mehler, E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry 1999, 38, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Deveraux, Q.; Turk, B.; Sali, A. Comprehensive search for cysteine cathepsins in the human genome. Biol. Chem. 2004, 385, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A. Stratum corneum thiol protease (SCTP): A novel cysteine protease of late epidermal differentiation. Arch. Dermatol. Res. 1999, 291, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Adachi, W.; Kawamoto, S.; Ohno, I.; Nishida, K.; Kinoshita, S.; Matsubara, K.; Okubo, K. Isolation and characterization of human cathepsin V: A major proteinase in corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1789–1796. [Google Scholar]
- Santamaría, I.; Velasco, G.; Cazorla, M.; Fueyo, A.; Campo, E.; López-Otín, C. Cathepsin L2, a novel human cysteine proteinase produced by breast and colorectal carcinomas. Cancer Res. 1998, 58, 1624–1630. [Google Scholar]
- Tolosa, E.; Li, W.; Yasuda, Y.; Wienhold, W.; Denzin, L.K.; Lautwein, A.; Driessen, C.; Schnorrer, P.; Weber, E.; Stevanovic, S.; et al. Cathepsin V is involved in the degradation of invariant chain in human thymus and is overexpressed in myasthenia gravis. J. Clin. Investig. 2003, 112, 517–526. [Google Scholar] [CrossRef]
- Jing, J.; Wang, S.; Ma, J.; Yu, L.; Zhou, H. Elevated CTSL2 expression is associated with an adverse prognosis in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 4035–4043. [Google Scholar]
- Al-Hashimi, A.; Venugopalan, V.; Sereesongsaeng, N.; Tedelind, S.; Pinzaru, A.M.; Hein, Z.; Springer, S.; Weber, E.; Führer, D.; Scott, C.J.; et al. Significance of nuclear cathepsin V in normal thyroid epithelial and carcinoma cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118846. [Google Scholar] [CrossRef]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef]
- Jordans, S.; Jenko-Kokalj, S.; Kühl, N.M.; Tedelind, S.; Sendt, W.; Brömme, D.; Turk, D.; Brix, K. Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem. 2009, 10, 23. [Google Scholar] [CrossRef]
- Weber, J.; McInnes, J.; Kizilirmak, C.; Rehders, M.; Qatato, M.; Wirth, E.K.; Schweizer, U.; Verrey, F.; Heuer, H.; Brix, K. Interdependence of thyroglobulin processing and thyroid hormone export in the mouse thyroid gland. Eur. J. Cell Biol. 2017, 96, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Qatato, M.; Szumska, J.; Skripnik, V.; Rijntjes, E.; Köhrle, J.; Brix, K. Canonical TSH Regulation of Cathepsin-Mediated Thyroglobulin Processing in the Thyroid Gland of Male Mice Requires Taar1 Expression. Front. Pharmacol. 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Szumska, J.; Weber, J.; Qatato, M.; Venugopalan, V.; Al-Hashimi, A.; Rehders, M. Auto-Regulation of the Thyroid Gland Beyond Classical Pathways. Exp. Clin. Endocrinol. Diabetes 2020, 128, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Linke, M.; Tepel, C.; Herzog, V. Cysteine proteinases mediate extracellular prohormone processing in the thyroid. Biol. Chem. 2001, 382, 717–725. [Google Scholar] [CrossRef]
- Clark, A.A.; Dotson, C.D.; Elson, A.E.T.; Voigt, A.; Boehm, U.; Meyerhof, W.; Steinle, N.I.; Munger, S.D. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015, 29, 164–172. [Google Scholar] [CrossRef]
- Lemoine, N.R.; Mayall, E.S.; Jones, T.; Sheer, D.; McDermid, S.; Kendall-Taylor, P.; Wynford-Thomas, D. Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br. J. Cancer 1989, 60, 897–903. [Google Scholar] [CrossRef]
- Piotrowska, U.; Mackiewicz, U.; Czarnocka, B. TSH Signal Transduction in Thyroid Cells of Nthy-Ori 3–1 Line; Borgis: Prague, Czech Republic, 2011. [Google Scholar]
- Szumska, J.; Batool, Z.; Al-Hashimi, A.; Venugopalan, V.; Skripnik, V.; Schaschke, N.; Bogyo, M.; Brix, K. Treatment of rat thyrocytes in vitro with cathepsin B and L inhibitors results in disruption of primary cilia leading to redistribution of the trace amine associated receptor 1 to the endoplasmic reticulum. Biochimie 2019, 166, 270–285. [Google Scholar] [CrossRef]
- Linke, M.; Herzog, V.; Brix, K. Trafficking of lysosomal cathepsin B-green fluorescent protein to the surface of thyroid epithelial cells involves the endosomal/lysosomal compartment. J. Cell Sci. 2002, 115, 4877–4889. [Google Scholar] [CrossRef]
- Linke, M.; Jordans, S.; Mach, L.; Herzog, V.; Brix, K. Thyroid stimulating hormone upregulates secretion of cathepsin B from thyroid epithelial cells. Biol. Chem. 2002, 383, 773–784. [Google Scholar] [CrossRef]
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine cathepsins: Cellular roadmap to different functions. Biochimie 2008, 90, 194–207. [Google Scholar] [CrossRef]
- Erickson, A.H.; Isidoro, C.; Mach, L.; Mort, J.S. Cathepsins: Getting in Shape for Lysosomal Proteolysis. In Proteases: Structure and Function; Brix, K., Stöcker, W., Eds.; Springer: Vienna, Austria, 2013; pp. 127–173. [Google Scholar]
- Brix, K.; McInnes, J.; Al-Hashimi, A.; Rehders, M.; Tamhane, T.; Haugen, M.H. Proteolysis mediated by cysteine cathepsins and legumain-recent advances and cell biological challenges. Protoplasma 2015, 252, 755–774. [Google Scholar] [CrossRef]
- Brix, K. Host Cell Proteases: Cathepsins. In Activation of Viruses by Host Proteases; Böttcher-Friebertshäuser, E., Garten, W., Klenk, H.D., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 249–276. [Google Scholar]
- Ong, P.C.; McGowan, S.; Pearce, M.C.; Irving, J.A.; Kan, W.T.; Grigoryev, S.A.; Turk, B.; Silverman, G.A.; Brix, K.; Bottomley, S.P.; et al. DNA accelerates the inhibition of human cathepsin V by serpins. J. Biol. Chem. 2007, 282, 36980–36986. [Google Scholar] [CrossRef]
- Pratt, M.R.; Sekedat, M.D.; Chiang, K.P.; Muir, T.W. Direct measurement of cathepsin B activity in the cytosol of apoptotic cells by an activity-based probe. Chem. Biol. 2009, 16, 1001–1012. [Google Scholar] [CrossRef]
- Tedelind, S.; Poliakova, K.; Valeta, A.; Hunegnaw, R.; Yemanaberhan, E.L.; Heldin, N.E.; Kurebayashi, J.; Weber, E.; Kopitar-Jerala, N.; Turk, B.; et al. Nuclear cysteine cathepsin variants in thyroid carcinoma cells. Biol. Chem. 2010, 391, 923–935. [Google Scholar] [CrossRef]
- Tamhane, T.; Lllukkumbura, R.; Lu, S.; Maelandsmo, G.M.; Haugen, M.H.; Brix, K. Nuclear cathepsin L activity is required for cell cycle progression of colorectal carcinoma cells. Biochimie 2016, 122, 208–218. [Google Scholar] [CrossRef]
- Hämälistö, S.; Stahl, J.L.; Favaro, E.; Yang, Q.; Liu, B.; Christoffersen, L.; Loos, B.; Guasch Boldú, C.; Joyce, J.A.; Reinheckel, T.; et al. Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation. Nat. Commun. 2020, 11, 229. [Google Scholar] [CrossRef]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef]
- Staudt, C.; Puissant, E.; Boonen, M. Subcellular Trafficking of Mammalian Lysosomal Proteins: An Extended View. Int. J. Mol. Sci. 2016, 18, 47. [Google Scholar] [CrossRef]
- Gieselmann, V.; Pohlmann, R.; Hasilik, A.; Von Figura, K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J. Cell Biol. 1983, 97, 1–5. [Google Scholar] [CrossRef]
- Coutinho, M.F.; Prata, M.J.; Alves, S. Mannose-6-phosphate pathway: A review on its role in lysosomal function and dysfunction. Mol. Genet. Metab. 2012, 105, 542–550. [Google Scholar] [CrossRef]
- Bieberich, E. Synthesis, Processing, and Function of N-glycans in N-glycoproteins. Adv. Neurobiol. 2014, 9, 47–70. [Google Scholar]
- Niwa, Y.; Suzuki, T.; Dohmae, N.; Umezawa, K.; Simizu, S. Determination of cathepsin V activity and intracellular trafficking by N-glycosylation. FEBS Lett. 2012, 586, 3601–3607. [Google Scholar] [CrossRef]
- Freeze, H.H.; Kranz, C. Endoglycosidase and glycoamidase release of N-linked glycans. Curr. Protoc. Mol. Biol. 2010, 89, 17. [Google Scholar] [CrossRef]
- Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 2011, 3, a005199. [Google Scholar] [CrossRef]
- Lingeman, R.G.; Joy, D.S.; Sherman, M.A.; Kane, S.E. Effect of carbohydrate position on lysosomal transport of procathepsin L. Mol. Biol. Cell. 1998, 9, 1135–1147. [Google Scholar] [CrossRef][Green Version]
- Collette, J.; Bocock, J.P.; Ahn, K.; Chapman, R.L.; Godbold, G.; Yeyeodu, S.; Erickson, A.H. Biosynthesis and alternate targeting of the lysosomal cysteine protease cathepsin L. Int. Rev. Cytol. 2004, 241, 1–51. [Google Scholar]
- Sleat, D.E.; Zheng, H.; Qian, M.; Lobel, P. Identification of sites of mannose 6-phosphorylation on lysosomal proteins. Mol. Cell Proteom. 2006, 5, 686–701. [Google Scholar] [CrossRef]
- Fisher, P.; Spencer, H.; Thomas-Oates, J.; Wood, A.J.; Ungar, D. Modeling Glycan Processing Reveals Golgi-Enzyme Homeostasis upon Trafficking Defects and Cellular Differentiation. Cell Rep. 2019, 27, 1231–1243.e6. [Google Scholar] [CrossRef]
- Dong, J.M.; Sahagian, G.G. Basis for low affinity binding of a lysosomal cysteine protease to the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 1990, 265, 4210–4217. [Google Scholar]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell. Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Renko, M.; Završnik, J.; Turk, D.; Seeger, M.A.; Vasiljeva, O.; Grütter, M.G.; Turk, V.; Turk, B. Non-invasive in vivo imaging of tumour-associated cathepsin B by a highly selective inhibitory DARPin. Theranostics 2017, 7, 2806–2821. [Google Scholar] [CrossRef] [PubMed]
- Büth, H.; Luigi Buttigieg, P.; Ostafe, R.; Rehders, M.; Dannenmann, S.R.; Schaschke, N.; Stark, H.J.; Boukamp, P.; Brix, K. Cathepsin B is essential for regeneration of scratch-wounded normal human epidermal keratinocytes. Eur. J. Cell Biol. 2007, 86, 747–761. [Google Scholar] [CrossRef]
- Brömme, D.; Lecaille, F. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opin. Investig. Drugs 2009, 18, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Vizovišek, M.; Fonović, M.; Turk, B. Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond. Matrix. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Lemansky, P.; Herzog, V. Evidence for extracellularly acting cathepsins mediating thyroid hormone liberation in thyroid epithelial cells. Endocrinology 1996, 137, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Lechan, R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1387–1428. [Google Scholar]
- Eggo, M.C.; Lippes, H.; Burrow, G.N. Control of thyroid secretion: Effects of stimulators of protein kinase C, thyrotropin, and calcium mobilization on secretion of iodinated compounds from sheep thyroid cells. Endocrinology 1992, 130, 2274–2283. [Google Scholar]
- Kero, J.; Ahmed, K.; Wettschureck, N.; Tunaru, S.; Wintermantel, T.; Greiner, E.; Schütz, G.; Offermanns, S. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J. Clin. Investig. 2007, 117, 2399–2407. [Google Scholar] [CrossRef]
- Cavallo-Medved, D.; Dosescu, J.; Linebaugh, B.E.; Sameni, M.; Rudy, D.; Sloane, B.F. Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia 2003, 5, 507–519. [Google Scholar] [CrossRef]
- Jane, D.T.; Morvay, L.; Dasilva, L.; Cavallo-Medved, D.; Sloane, B.F.; Dufresne, M.J. Cathepsin B localizes to plasma membrane caveolae of differentiating myoblasts and is secreted in an active form at physiological pH. Biol. Chem. 2006, 387, 223–234. [Google Scholar] [CrossRef]
- Kirschke, H.; Langner, J.; Wiederanders, B.; Ansorge, S.; Bohley, P.; Cathepsin, L. A new proteinase from rat-liver lysosomes. Eur. J. Biochem. 1977, 74, 293–301. [Google Scholar] [CrossRef]
- Buck, M.R.; Karustis, D.G.; Day, N.A.; Honn, K.V.; Sloane, B.F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 1992, 282, 273–278. [Google Scholar] [CrossRef]
- Novinec, M.; Lenarčič, B.; Baici, A. Probing the activity modification space of the cysteine peptidase cathepsin K with novel allosteric modifiers. PLoS ONE 2014, 9, e106642. [Google Scholar] [CrossRef]
- Wilkinson, R.D.; Young, A.; Burden, R.E.; Williams, R.; Scott, C.J. A bioavailable cathepsin S nitrile inhibitor abrogates tumor development. Mol. Cancer 2016, 15, 29. [Google Scholar] [CrossRef]
- Coulombe, R.; Grochulski, P.; Sivaraman, J.; Ménard, R.; Mort, J.S.; Cygler, M. Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 1996, 15, 5492–5503. [Google Scholar] [CrossRef]
- Cygler, M.; Sivaraman, J.; Grochulski, P.; Coulombe, R.; Storer, A.C.; Mort, J.S. Structure of rat procathepsin B: Model for inhibition of cysteine protease activity by the proregion. Structure 1996, 4, 405–416. [Google Scholar] [CrossRef]
- Sivaraman, J.; Lalumière, M.; Ménard, R.; Cygler, M. Crystal structure of wild-type human procathepsin K. Protein Sci. 1999, 8, 283–290. [Google Scholar] [CrossRef]
- Jerala, R.; Zerovnik, E.; Kidric, J.; Turk, V. pH-induced conformational transitions of the propeptide of human cathepsin L. A role for a molten globule state in zymogen activation. J. Biol. Chem. 1998, 273, 11498–11504. [Google Scholar] [CrossRef]
- Verma, S.; Dixit, R.; Pandey, K.C. Cysteine Proteases: Modes of Activation and Future Prospects as Pharmacological Targets. Front. Pharmacol. 2016, 7, 107. [Google Scholar] [CrossRef]
- Yadati, T.; Dixit, R.; Pandey, K.C. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 7. [Google Scholar] [CrossRef]
- Almeida, P.C.; Nantes, I.L.; Chagas, J.R.; Rizzi, C.C.; Faljoni-Alario, A.; Carmona, E.; Juliano, L.; Nader, H.B.; Tersariol, I.L. Cathepsin B activity regulation. Heparin-like glycosaminogylcans protect human cathepsin B from alkaline pH-induced inactivation. J. Biol. Chem. 2001, 276, 944–951. [Google Scholar] [CrossRef]
- Fogelfeld, L.; Harel, G.; Beamer, W.G.; Schneider, A.B. Low-molecular-weight iodoproteins in the congenital goiters of cog/cog mice. Thyroid 1992, 2, 329–335. [Google Scholar] [CrossRef]
- Emoto, N.; Kunii, Y.K.; Ashizawa, M.; Oikawa, S.; Shimizu, K.; Shimonaka, M.; Toyoda, A.; Toyoda, H. Reduced sulfation of chondroitin sulfate in thyroglobulin derived from human papillary thyroid carcinomas. Cancer Sci. 2007, 98, 1577–1581. [Google Scholar] [CrossRef]
- Xavier, A.C.; Maciel, R.M.; Vieira, J.G.; Dias-da-Silva, M.R.; Martins, J.R. Insights into the posttranslational structural heterogeneity of thyroglobulin and its role in the development, diagnosis, and management of benign and malignant thyroid diseases. Arch. Endocrinol. Metab. 2016, 60, 66–75. [Google Scholar] [CrossRef]
- Dall, E.; Brandstetter, H. Structure and function of legumain in health and disease. Biochimie 2016, 122, 126–150. [Google Scholar] [CrossRef]
- Pungerčar, J.R.; Caglič, D.; Sajid, M.; Dolinar, M.; Vasiljeva, O.; Požgan, U.; Turk, D.; Bogyo, M.; Turk, V.; Turk, B. Autocatalytic processing of procathepsin B is triggered by proenzyme activity. FEBS J. 2009, 276, 660–668. [Google Scholar] [CrossRef]
- Rozman, J.; Stojan, J.; Kuhelj, R.; Turk, V.; Turk, B. Autocatalytic processing of recombinant human procathepsin B is a bimolecular process. FEBS Lett. 1999, 459, 358–362. [Google Scholar] [CrossRef]
- Krause, K.; Karger, S.; Sheu, S.Y.; Aigner, T.; Kursawe, R.; Gimm, O.; Schmid, K.W.; Dralle, H.; Fuhrer, D. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J. Endocrinol. 2008, 198, 291–299. [Google Scholar] [CrossRef]
- Neuhoff, V.; Philipp, K.; Zimmer, H.G.; Mesecke, S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe Seylers Z. Physiol. Chem. 1979, 360, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Medzihradszky, K.F.; Burlingame, A.; Bogyo, M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem. Biol. 2000, 7, 569–581. [Google Scholar] [CrossRef]
- Fritzsche, S.; Springer, S. Pulse-chase analysis for studying protein synthesis and maturation. Curr. Protoc. Protein Sci. 2014. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hashimi, A.; Venugopalan, V.; Rehders, M.; Sereesongsaeng, N.; Hein, Z.; Springer, S.; Weber, E.; Führer, D.; Bogyo, M.S.; Scott, C.J.; et al. Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe. Int. J. Mol. Sci. 2020, 21, 9140. https://doi.org/10.3390/ijms21239140
Al-Hashimi A, Venugopalan V, Rehders M, Sereesongsaeng N, Hein Z, Springer S, Weber E, Führer D, Bogyo MS, Scott CJ, et al. Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe. International Journal of Molecular Sciences. 2020; 21(23):9140. https://doi.org/10.3390/ijms21239140
Chicago/Turabian StyleAl-Hashimi, Alaa, Vaishnavi Venugopalan, Maren Rehders, Naphannop Sereesongsaeng, Zeynep Hein, Sebastian Springer, Ekkehard Weber, Dagmar Führer, Matthew S. Bogyo, Christopher J. Scott, and et al. 2020. "Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe" International Journal of Molecular Sciences 21, no. 23: 9140. https://doi.org/10.3390/ijms21239140
APA StyleAl-Hashimi, A., Venugopalan, V., Rehders, M., Sereesongsaeng, N., Hein, Z., Springer, S., Weber, E., Führer, D., Bogyo, M. S., Scott, C. J., Burden, R. E., & Brix, K. (2020). Procathepsin V Is Secreted in a TSH Regulated Manner from Human Thyroid Epithelial Cells and Is Accessible to an Activity-Based Probe. International Journal of Molecular Sciences, 21(23), 9140. https://doi.org/10.3390/ijms21239140




