PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome
Abstract
1. Introduction
2. Results
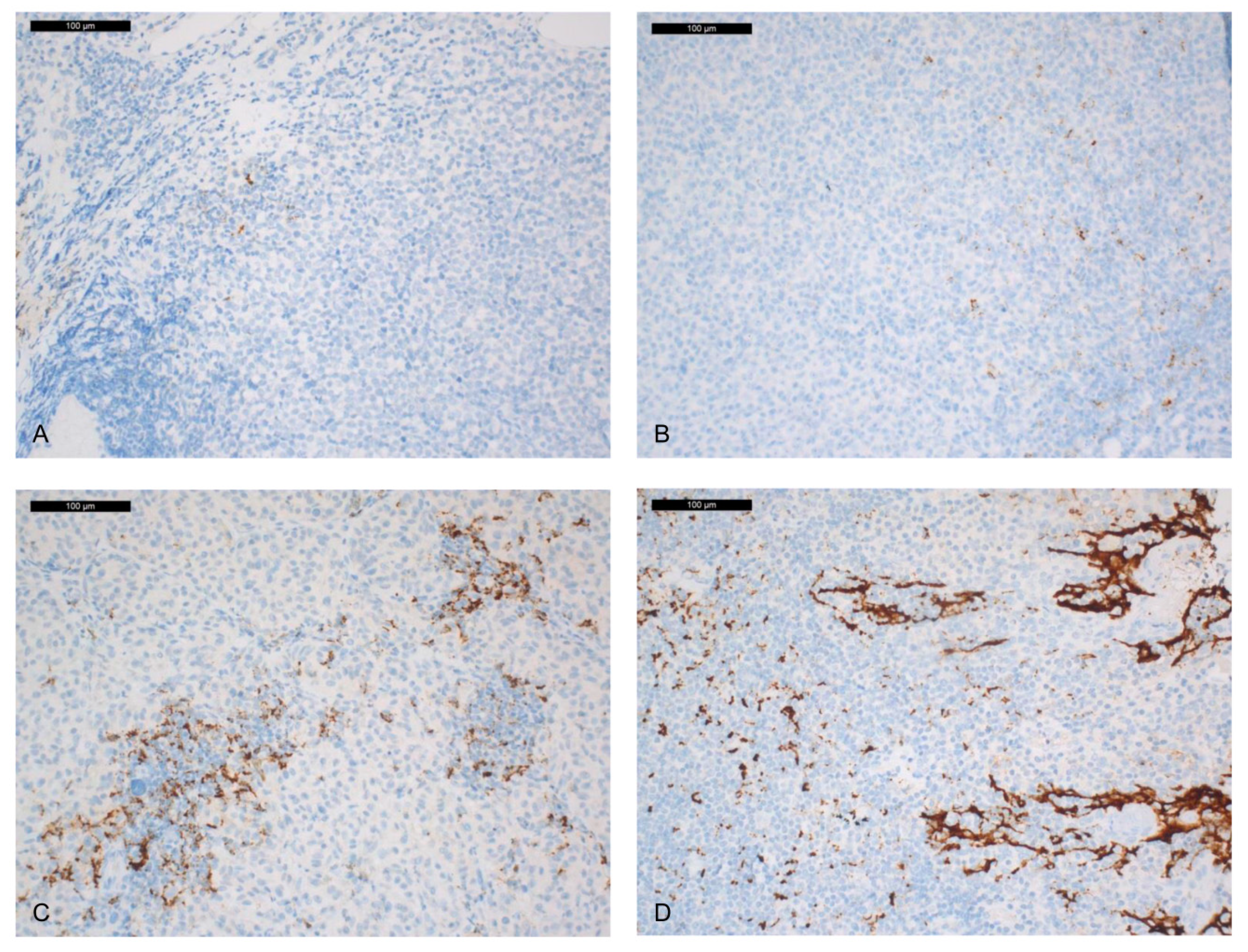
2.1. Immunohistochemical PD-L1 Expression
2.2. CD8+ PD-1 and NKP46 Tumour-Infiltrating Lymphocyte (TIL) Expression
2.3. BRAFV600E and NRASQ61R Mutations
2.4. Correlation between PD-L1 Expression and CD8/NKP46/PD-1+ TILs, BRAFV600E and NRASQ61R Mutations
2.5. Association to Clinicopathological Parameters and Patient Outcome
3. Discussion
4. Materials and Methods
4.1. Patient Cohort
4.2. Immunohistochemistry
4.3. Staining Evaluation
4.4. BRAF and NRAS Molecular Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isager, P.; Engholm, G.; Overgaard, J.; Storm, H. Uveal and conjunctival malignant melanoma in Denmark 1943–1997: Observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006, 13, 85–96. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Yu, G.P.; Hu, D.N.; McCormick, S.; Finger, P.T. Conjunctival melanoma: Is it increasing in the United States? Am. J. Ophthalmol. 2003, 135, 800–806. [Google Scholar] [CrossRef]
- Gear, H.; Williams, H.; Kemp, E.G.; Roberts, F. BRAF mutations in conjunctival melanoma. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2484–2488. [Google Scholar] [CrossRef]
- Spendlove, H.E.; Damato, B.E.; Humphreys, J.; Barker, K.T.; Hiscott, P.S.; Houlston, R.S. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma Res. 2004, 14, 449–452. [Google Scholar] [CrossRef]
- Goldenberg-Cohen, N.; Cohen, Y.; Rosenbaum, E.; Herscovici, Z.; Chowers, I.; Weinberger, D.; Pe’er, J.; Sidransky, D. T1799A BRAF mutations in conjunctival melanocytic lesions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3027–3030. [Google Scholar] [CrossRef]
- Triay, E.; Bergman, L.; Nilsson, B.; All-Ericsson, C.; Seregard, S. Time trends in the incidence of conjunctival melanoma in Sweden. Br. J. Ophthalmol. 2009, 93, 1524–1528. [Google Scholar] [CrossRef]
- Griewank, K.G.; Westekemper, H.; Murali, R.; Mach, M.; Schilling, B.; Wiesner, T.; Schimming, T.; Livingstone, E.; Sucker, A.; Grabellus, F.; et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin. Cancer Res. 2013, 19, 3143–3152. [Google Scholar] [CrossRef]
- Sheng, X.; Li, S.; Chi, Z.; Si, L.; Cui, C.; Mao, L.; Lian, B.; Tang, B.; Wang, X.; Yan, X.; et al. Prognostic factors for conjunctival melanoma: A study in ethnic Chinese patients. Br. J. Ophthalmol. 2015, 99, 990–996. [Google Scholar] [CrossRef]
- Larsen, A.C.; Dahl, C.; Dahmcke, C.M.; Lade-Keller, J.; Siersma, V.D.; Toft, P.B.; Coupland, S.E.; Prause, J.U.; Guldberg, P.; Heegaard, S. BRAF mutations in conjunctival melanoma: Investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016, 94, 463–470. [Google Scholar] [CrossRef]
- Rossi, E.; Schinzari, G.; Maiorano, B.A.; Pagliara, M.M.; Di Stefani, A.; Bria, E.; Peris, K.; Blasi, M.A.; Tortora, G. Conjunctival melanoma: Genetic and epigenetic insights of a distinct type of melanoma. Int. J. Mol. Sci. 2019, 20, 5447. [Google Scholar] [CrossRef]
- Royer-Bertrand, B.; Torsello, M.; Rimoldi, D.; El Zaoui, I.; Cisarova, K.; Pescini-Gobert, R.; Raynaud, F.; Zografos, L.; Schalenbourg, A.; Speiser, D.; et al. Comprehensive genetic landscape of uveal melanoma by whole-genome sequencing. Am. J. Hum. Genet. 2016, 99, 1190–1198. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Livingstone, A.; Agarwal, A.; Stockler, M.R.; Menzies, A.M.; Howard, K.; Morton, R.L. Preferences for Immunotherapy in melanoma: A systematic review. Ann. Surg. Oncol. 2020, 27, 571–584. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Atkinson, V. Recent advances in malignant melanoma. Intern. Med. J. 2017, 47, 1114–1121. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III Melanoma: Updated results from the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: Three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef]
- You, W.; Shang, B.; Sun, J.; Liu, X.; Su, L.; Jiang, S. Mechanistic insight of predictive biomarkers for antitumor PD1/PDL1 blockade: A paradigm shift towards immunome evaluation (Review). Oncol. Rep. 2020, 44, 424–437. [Google Scholar] [CrossRef]
- Kini, A.; Fu, R.; Compton, C.; Miller, D.M.; Ramasubramanian, A. Pembrolizumab for recurrent conjunctival melanoma. JAMA Ophthalmol. 2017, 135, 891–892. [Google Scholar] [CrossRef]
- Sagiv, O.; Thakar, S.D.; Kandl, T.J.; Ford, J.; Sniegowski, M.C.; Hwu, W.J.; Esmaeli, B. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018, 136, 1236–1241. [Google Scholar] [CrossRef]
- Ford, J.; Thuro, B.A.; Thakar, S.; Hwu, W.J.; Richani, K.; Esmaeli, B. Immune checkpoint inhibitors for treatment of metastatic melanoma of the orbit and ocular adnexa. Ophthal. Plast. Reconstr. Surg. 2017, 33, e82–e85. [Google Scholar] [CrossRef]
- Chang, M.; Lally, S.E.; Dalvin, L.A.; Orloff, M.M.; Shields, C.L. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J. Ophthalmol. 2019, 67, 2071–2073. [Google Scholar]
- Thierauf, J.; Veit, J.A.; Affolter, A.; Bergmann, C.; Grunow, J.; Laban, S.; Lennerz, J.K.; Grunmuller, L.; Mauch, C.; Plinkert, P.K.; et al. Identification and clinical relevance of PD-L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res. 2015, 25, 503–509. [Google Scholar] [CrossRef]
- Cao, J.; Brouwer, N.J.; Richards, K.E.; Marinkovic, M.; van Duinen, S.; Hurkmans, D.; Verdegaal, E.M.E.; Jordanova, E.S.; Jager, M.J. PD-L1/PD-1 expression and tumor-infiltrating lymphocytes in conjunctival melanoma. Oncotarget 2017, 8, 54722–54734. [Google Scholar] [CrossRef]
- Iacono, D.; Cinausero, M.; Gerratana, L.; Angione, V.; Scott, C.A.; De Maglio, G.; Pizzolitto, S.; Di Loreto, C.; Puglisi, F.; Fasola, G.; et al. Tumour-infiltrating lymphocytes programmed death ligand 1 and cyclooxygenase-2 expression in skin melanoma of elderly patients: Clinicopathological correlations. Melanoma Res. 2018, 28, 547–554. [Google Scholar] [CrossRef]
- Joseph, R.W.; Elassaiss-Schaap, J.; Kefford, R.; Hwu, W.J.; Wolchok, J.D.; Joshua, A.M.; Ribas, A.; Hodi, F.S.; Hamid, O.; Robert, C.; et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin. Cancer Res. 2018, 24, 4960–4967. [Google Scholar] [CrossRef]
- Hino, R.; Kabashima, K.; Kato, Y.; Yagi, H.; Nakamura, M.; Honjo, T.; Okazaki, T.; Tokura, Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010, 116, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.C.; Nguyen, P.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Esandrio, J.; Xu, H.; Ogurtsova, A.; Bleich, K.B.; Cornish, T.C.; et al. PD-L1 expression in melanoma: A quantitative immunohistochemical antibody comparison. Clin. Cancer Res. 2017, 23, 4938–4944. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Hofman, V.; Dietel, M.; Soria, J.C.; Hofman, P. Assessment of the PD-L1 status by immunohistochemistry: Challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016, 468, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Zito, C.R.; Turcu, G.; Baine, M.K.; Zhang, H.; Adeniran, A.; Sznol, M.; Rimm, D.L.; Kluger, Y.; Chen, L.; et al. PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin. Cancer Res. 2017, 23, 4270–4279. [Google Scholar] [CrossRef]
- Buttner, R.; Gosney, J.R.; Skov, B.G.; Adam, J.; Motoi, N.; Bloom, K.J.; Dietel, M.; Longshore, J.W.; Lopez-Rios, F.; Penault-Llorca, F.; et al. Programmed death-ligand 1 immunohistochemistry testing: A review of analytical assays and clinical implementation in non-small-cell lung cancer. J. Clin. Oncol. 2017, 35, 3867–3876. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef]
- Morrison, C.; Pabla, S.; Conroy, J.M.; Nesline, M.K.; Glenn, S.T.; Dressman, D.; Papanicolau-Sengos, A.; Burgher, B.; Andreas, J.; Giamo, V.; et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J. Immunother. Cancer 2018, 6, 32. [Google Scholar] [CrossRef]
- Ladanyi, A.; Papp, E.; Mohos, A.; Balatoni, T.; Liszkay, G.; Olah, J.; Varga, A.; Lengyel, Z.; Emri, G.; Ferrone, S. Role of the anatomic site in the association of HLA class I antigen expression level in metastases with clinical response to ipilimumab therapy in patients with melanoma. J. Immunother. Cancer 2020, 8, e000209. [Google Scholar] [CrossRef]
- Danilova, L.; Wang, H.; Sunshine, J.; Kaunitz, G.J.; Cottrell, T.R.; Xu, H.; Esandrio, J.; Anders, R.A.; Cope, L.; Pardoll, D.M.; et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc. Natl. Acad. Sci. USA 2016, 113, E7769–E7777. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Carbognin, L.; Pilotto, S.; Milella, M.; Vaccaro, V.; Brunelli, M.; Calio, A.; Cuppone, F.; Sperduti, I.; Giannarelli, D.; Chilosi, M.; et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE 2015, 10, e0130142. [Google Scholar] [CrossRef]
- Frydenlund, N.; Leone, D.; Yang, S.; Hoang, M.P.; Deng, A.; Hernandez-Perez, M.; Singh, R.; Biswas, A.; Yaar, R.; Mahalingam, M. Tumoral PD-L1 expression in desmoplastic melanoma is associated with depth of invasion, tumor-infiltrating CD8 cytotoxic lymphocytes and the mixed cytomorphological variant. Mod. Pathol. 2017, 30, 357–369. [Google Scholar] [CrossRef]
- Ilie, M.; Long-Mira, E.; Bence, C.; Butori, C.; Lassalle, S.; Bouhlel, L.; Fazzalari, L.; Zahaf, K.; Lalvee, S.; Washetine, K.; et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD-L1 therapeutic strategies. Ann. Oncol. 2016, 27, 147–153. [Google Scholar] [CrossRef]
- Arnon, T.I.; Markel, G.; Mandelboim, O. Tumor and viral recognition by natural killer cells receptors. Semin. Cancer Biol. 2006, 16, 348–358. [Google Scholar] [CrossRef]
- Steiniche, T.; Vestergaard Danielsen, A.; Wang, Z.; Feng, Y.; Switten Nielsen, P.; Bastholt, L.; Schmidt, H.; Svane, I.M.; Dolled-Filhart, M.; Emancipator, K.; et al. PD-L1 expression and survival among melanoma patients treated with standard immunotherapy or chemotherapy. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e319–e321. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Liu, L. Prognostic significance of PD-L1 in solid tumor: An updated meta-analysis. Medicine 2017, 96, e6369. [Google Scholar] [CrossRef]
- Skuciova, V.; Drahosova, S.; Vybohova, D.; Cigerova, V.; Adamkov, M. The relationships between PD-L1 expression, CD8+ TILs and clinico-histomorphological parameters in malignant melanomas. Pathol. Res. Pract. 2020, 216, 153071. [Google Scholar] [CrossRef]
- Massi, D.; Brusa, D.; Merelli, B.; Falcone, C.; Xue, G.; Carobbio, A.; Nassini, R.; Baroni, G.; Tamborini, E.; Cattaneo, L.; et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann. Oncol. 2015, 26, 1980–1987. [Google Scholar] [CrossRef]
- Oba, J.; Nakahara, T.; Abe, T.; Hagihara, A.; Moroi, Y.; Furue, M. Expression of programmed death receptor ligand 1 in melanoma may indicate tumor progression and poor patient survival. J. Am. Acad. Dermatol. 2014, 70, 954–956. [Google Scholar] [CrossRef]
- Anastassiou, G.; Heiligenhaus, A.; Bechrakis, N.; Bader, E.; Bornfeld, N.; Steuhl, K.P. Prognostic value of clinical and histopathological parameters in conjunctival melanomas: A retrospective study. Br. J. Ophthalmol. 2002, 86, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Missotten, G.S.; Keijser, S.; De Keizer, R.J.; De Wolff-Rouendaal, D. Conjunctival melanoma in The Netherlands: A nationwide study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Tuomaala, S.; Toivonen, P.; Al-Jamal, R.; Kivela, T. Prognostic significance of histopathology of primary conjunctival melanoma in Caucasians. Curr. Eye Res. 2007, 32, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Berta-Antalics, A.I.; Kruse, F.E.; Holbach, L. Pathology and prognostic factors of conjunctival melanoma. Ophthalmologe 2015, 112, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.L.; Ozgur, O.K.; Myers, J.N.; Peng, A.; Ning, J.; Zafereo, M.E.; Thakar, S.; Thuro, B.; Prieto, V.G.; Ross, M.I.; et al. Sentinel lymph node biopsy for ocular adnexal melanoma. Acta Ophthalmol. 2017, 95, e323–e328. [Google Scholar] [CrossRef]
- Tuomaala, S.; Kivela, T. Metastatic pattern and survival in disseminated conjunctival melanoma: Implications for sentinel lymph node biopsy. Ophthalmology 2004, 111, 816–821. [Google Scholar] [CrossRef]
- Mor, J.M.; Heindl, L.M. Systemic BRAF/MEK Inhibitors as a potential treatment option in metastatic conjunctival melanoma. Ocul. Oncol. Pathol. 2017, 3, 133–141. [Google Scholar] [CrossRef]
- Dummer, R.; Lebbe, C.; Atkinson, V.; Mandala, M.; Nathan, P.D.; Arance, A.; Richtig, E.; Yamazaki, N.; Robert, C.; Schadendorf, D.; et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: Safety run-in and biomarker cohorts of COMBI-i. Nat. Med. 2020, 26, 1557–1563. [Google Scholar] [CrossRef]
- Jakob, J.A.; Bassett, R.L., Jr.; Ng, C.S.; Curry, J.L.; Joseph, R.W.; Alvarado, G.C.; Rohlfs, M.L.; Richard, J.; Gershenwald, J.E.; Kim, K.B.; et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012, 118, 4014–4023. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomized dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Robert, L.; Homet Moreno, B.; Ribas, A. Combining targeted therapy with immunotherapy in BRAF-mutant melanoma: Promise and challenges. J. Clin. Oncol. 2014, 32, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Lin, S.-F.; Chiu, C.-H.; Wu, Y.-C.; Hsieh, W.; Ho, H.; Chou, T. PD-L1 status in Taiwanese lung adenocarcinoma patients: Comparison of PD-L1 immunohistochemical assays using antibody clone 22C3, SP142 and SP263 with clinicopathological correlation. Ann. Oncol. 2016, 27 (Suppl. 9), ix123–ix125. [Google Scholar] [CrossRef]
- Adam, J.; Le Stang, N.; Rouquette, I.; Cazes, A.; Badoual, C.; Pinot-Roussel, H.; Tixier, L.; Danel, C.; Damiola, F.; Damotte, D.; et al. Multicenter French harmonization study for PD-L1 IHC testing in non-small cell lung cancer. Ann. Oncol. 2018, 29, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Long-Mira, E.; Funck-Brentano, E.; Lassalle, S.; Butori, C.; Lespinet-Fabre, V.; Bordone, O.; Gay, A.; Zahaf, K.; Poissonnet, G.; et al. Immunohistochemistry as a potential tool for routine detection of the NRAS Q61R mutation in patients with metastatic melanoma. J. Am. Acad. Dermatol. 2015, 72, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Ilie, M.; Lassalle, S.; Butori, C.; Poissonnet, G.; Washetine, K.; Mouroux, J.; Lespinet, V.; Lacour, J.P.; Taly, V.; et al. Why and how immunohistochemistry should now be used to screen for the BRAFV600E status in metastatic melanoma? The experience of a single institution (LCEP, Nice, France). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2436–2443. [Google Scholar] [CrossRef] [PubMed]



| PD-L1 IHC Assays | Not Determined (Insufficient Material, Melanin) | PD-L1 Expression in Tumour Cells Nb. of Cases (%) (Range) | PD-L1 Expression in Immune Cells IC<1 Nb. of Cases (%) | PD-L1 Expression in Immune Cells IC≥1% and <5% Nb. of Cases (%) | PD-L1 Expression in Immune Cells IC≥5% and <10% Nb. of Cases (%) | PD-L1 Expression in Immune Cells IC≥10% Nb. of Cases (%) |
|---|---|---|---|---|---|---|
| SP142 (n = 64) | 1 | 0 (0%) | 36 (56%) | 5 (8%) | 11 (17%) | 12 (19%) |
| SP263 (n = 60) | 5 | 6 (10%) (3–15% TC) | 25 (42%) | 5 (8%) | 9 (15%) | 21 (35%) |
| Clinical and Pathological Features | CD8 | PD-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | <5% | ≥5% and ≤50% | >50% | p Value | 0 | <5% | ≥5% and ≤50% | >50% | p Value | |
| PAM associated melanoma | ||||||||||
| Yes | 0 | 2 | 1 | 0 | 0.7 | 25 | 7 | 9 | 0 | 1 |
| No (de novo melanoma) | 2 | 13 | 20 | 3 | 2 | 0 | 1 | 0 | ||
| Presence of epithelioid tumour cells | ||||||||||
| Yes | 6 | 19 | 25 | 4 | 0.02 | 35 | 9 | 13 | 1 | 1 |
| No | 0 | 1 | 1 | 3 | 4 | 1 | 1 | 0 | ||
| Primary site | ||||||||||
| Limbal | 19 | 5 | 2 | 0 | 0.14 | 1 | 7 | 13 | 1 | 0.058 |
| Non-limbal | 10 | 2 | 7 | 1 | 2 | 7 | 6 | 5 | ||
| Bulbar | 6 | 16 | 23 | 2 | 0.007 | 34 | 9 | 8 | 0 | 0.3 |
| Non-bulbar | 0 | 4 | 3 | 5 | 5 | 1 | 5 | 1 | ||
| Thickness | ||||||||||
| <2mm | 2 | 10 | 19 | 4 | 0.21 | 24 | 6 | 8 | 0 | 0.79 |
| ≥2mm | 4 | 10 | 7 | 3 | 15 | 4 | 5 | 1 | ||
| Mitotic rate/mm2 | ||||||||||
| <1 | 1 | 10 | 12 | 2 | 0.4 | 15 | 5 | 7 | 0 | 0.7 |
| ≥1 | 5 | 10 | 14 | 5 | 24 | 5 | 6 | 1 | ||
| pTNM | ||||||||||
| pT1 | 3 | 15 | 22 | 2 | 0.014 | 30 | 8 | 8 | 0 | 0.06 |
| pT2-3-4 | 1 | 2 | 4 | 5 | 6 | 1 | 3 | 2 | ||
| Recurrence | ||||||||||
| Yes | 3 | 5 | 8 | 0 | 0.3 | 11 | 2 | 3 | 0 | 0.6 |
| No | 2 | 7 | 10 | 4 | 15 | 6 | 6 | 0 | ||
| Metastasis | ||||||||||
| Yes | 0 | 4 | 2 | 0 | 0.08 | 4 | 1 | 1 | 0 | 1 |
| No | 4 | 4 | 14 | 4 | 18 | 5 | 7 | 0 | ||
| Clinical and Pathological Features | Conjunctival Melanomas n = 65 (%) | Recurrence n (%) | Metastases n (%) | Melanoma-Related Death n (%) |
|---|---|---|---|---|
| Age at diagnosis, median (range), y | 69.4 (28–95) | 71.2 (47–90) | 64 (49–77) | 68.2 (56–77) |
| Gender | ||||
| Male | 25 (38) | 12 (70%) | 2 (29%) | 2 (50%) |
| Female | 40 (62) | 5 (30%) | 5 (71%) | 2 (50%) |
| Associated lesion | ||||
| PAM | 43 (66) | 11 (92%) | 7 (100%) | 4 (100%) |
| Nevi | 0 (0) | 0 | 0 | 0 |
| None (de novo) | 7 (11) | 1 (8%) | 0 | 0 |
| Recurrence | 6 (9) | 0 | 0 | 0 |
| Histotype | ||||
| Epithelioid | 44 (68) | 14 (82%) | 6 (86%) | 4 (100%) |
| Fusiform | 5 (8) | 0 | 0 | 0 |
| Mixed | 16 (24) | 3 (18%) | 1 (14%) | 0 |
| Primary site | ||||
| Bulbar | 53 (81) | 13 (76%) | 5 (71%) | 3 (75%) |
| Bulbar limbal | 28 (53) | 5 (38) | 2 | 1 (33) |
| Bulbar non limbal | 8 (15) | 4 (31) | 2 | 2 (67) |
| Bulbar unknown | 17 (32) | 4 (31) | 1 | 0 |
| Palpebral | 5 (8) | 2 (12%) | 1 (14%) | 0 |
| Forniceal | 5 (8) | 2 (12%) | 1 (14%) | 1 (25%) |
| Caruncular | 2 (3) | 0 | 0 | 0 |
| Thickness | ||||
| <2mm | 40 | 9 (53%) | 3 (43%) | 2 (50%) |
| ≥2mm | 25 | 8 (47%) | 4 (57%) | 2 (50%) |
| Mitotic rate/mm2 | ||||
| <1 | 29 (45) | 5 (29%) | 3 (43%) | 1 (25%) |
| ≥1 | 36 (55) | 12 (71%) | 4 (57%) | 3 (75%) |
| pTNM | ||||
| pT1a | 10 (15) | 1 (8%) | 0 | 1 (33%) |
| pT1b | 20 (31) | 3 (25%) | 1 (17%) | 2 (66%) |
| pT1c | 17 (26) | 5 (42%) | 3 (50%) | 0 |
| pT2a | 1 (2) | 0 | 0 | 0 |
| pT2b | 2 (3) | 1 (8%) | 0 | 0 |
| pT2c | 6 (9) | 1 (8%) | 1 (17%) | 0 |
| pT3 | 2 (3) | 1 (8%) | 1 (17%) | 0 |
| pT4 | 1 (1) | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lassalle, S.; Nahon-Esteve, S.; Frouin, E.; Boulagnon-Rombi, C.; Josselin, N.; Cassoux, N.; Barnhill, R.; Scheller, B.; Baillif, S.; Hofman, P. PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome. Int. J. Mol. Sci. 2020, 21, 9147. https://doi.org/10.3390/ijms21239147
Lassalle S, Nahon-Esteve S, Frouin E, Boulagnon-Rombi C, Josselin N, Cassoux N, Barnhill R, Scheller B, Baillif S, Hofman P. PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome. International Journal of Molecular Sciences. 2020; 21(23):9147. https://doi.org/10.3390/ijms21239147
Chicago/Turabian StyleLassalle, Sandra, Sacha Nahon-Esteve, Eric Frouin, Camille Boulagnon-Rombi, Nicolas Josselin, Nathalie Cassoux, Raymond Barnhill, Boris Scheller, Stéphanie Baillif, and Paul Hofman. 2020. "PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome" International Journal of Molecular Sciences 21, no. 23: 9147. https://doi.org/10.3390/ijms21239147
APA StyleLassalle, S., Nahon-Esteve, S., Frouin, E., Boulagnon-Rombi, C., Josselin, N., Cassoux, N., Barnhill, R., Scheller, B., Baillif, S., & Hofman, P. (2020). PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome. International Journal of Molecular Sciences, 21(23), 9147. https://doi.org/10.3390/ijms21239147









