Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application
Abstract
:1. Introduction
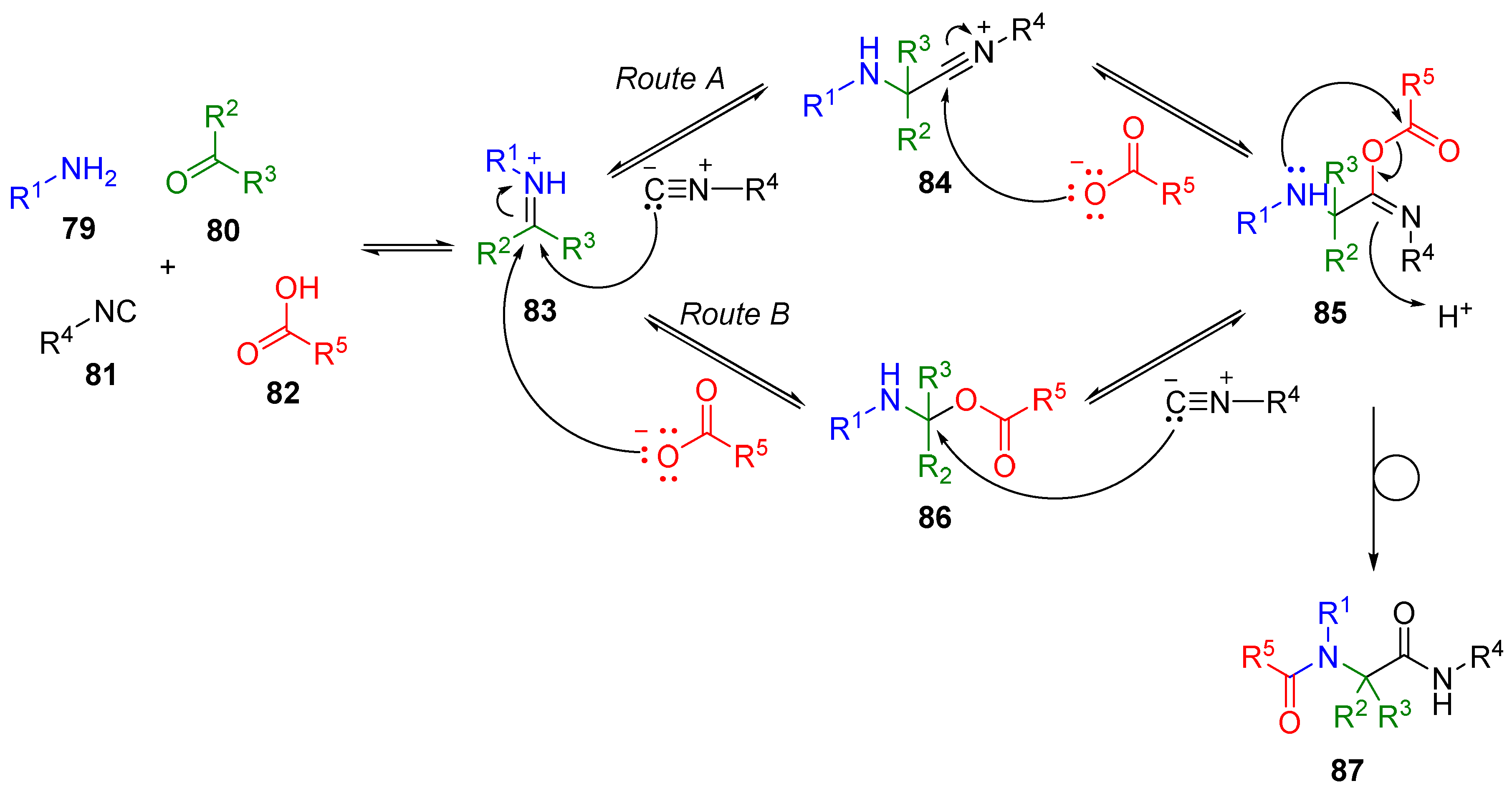
2. Isocyanide-Based Multicomponent Reactions (IMCRs)
2.1. Practical Assessments
2.2. Conventions
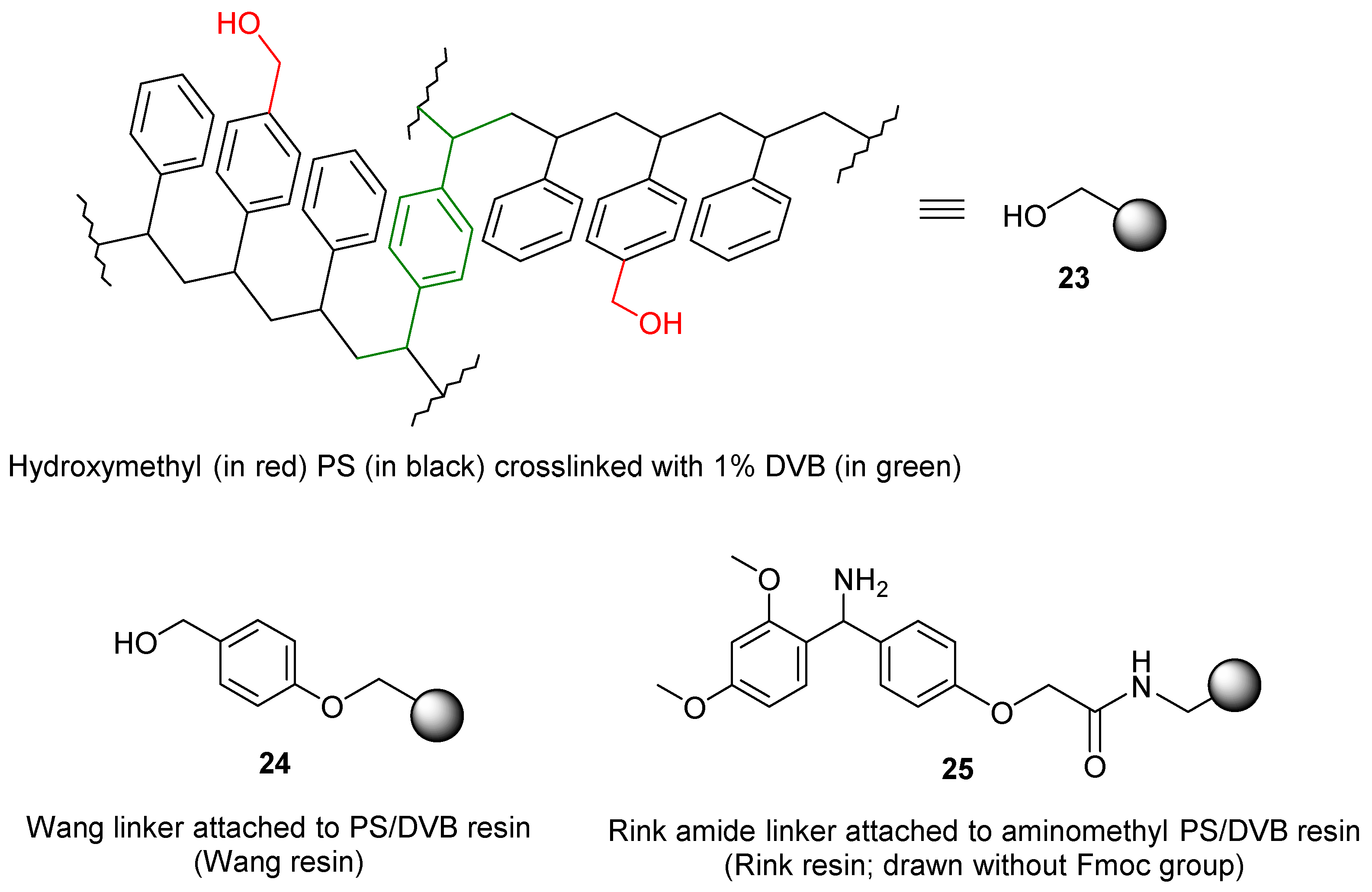
3. Resins
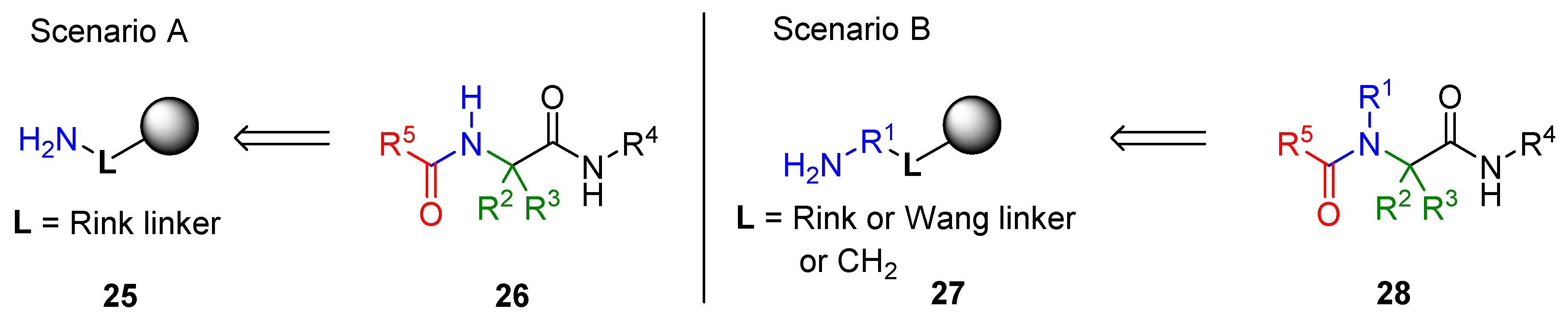
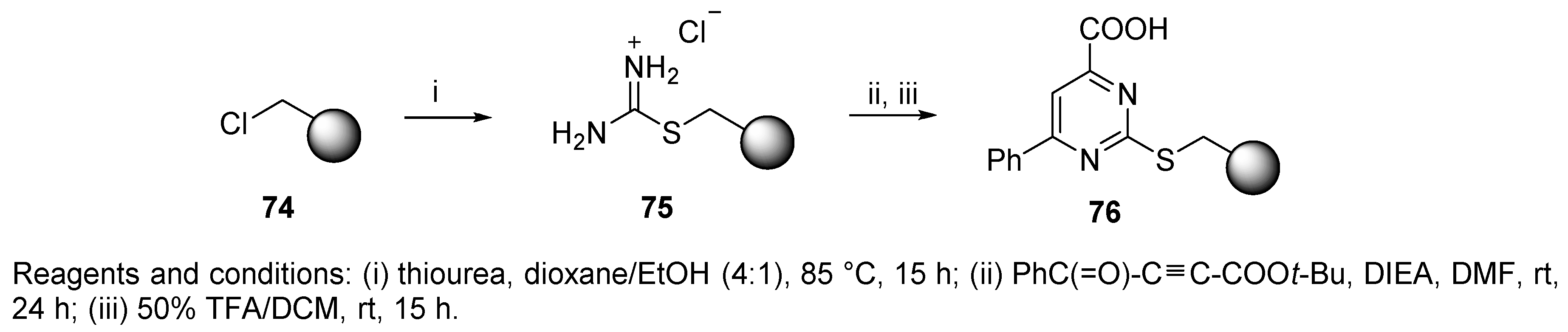
3.1. Amine Resin
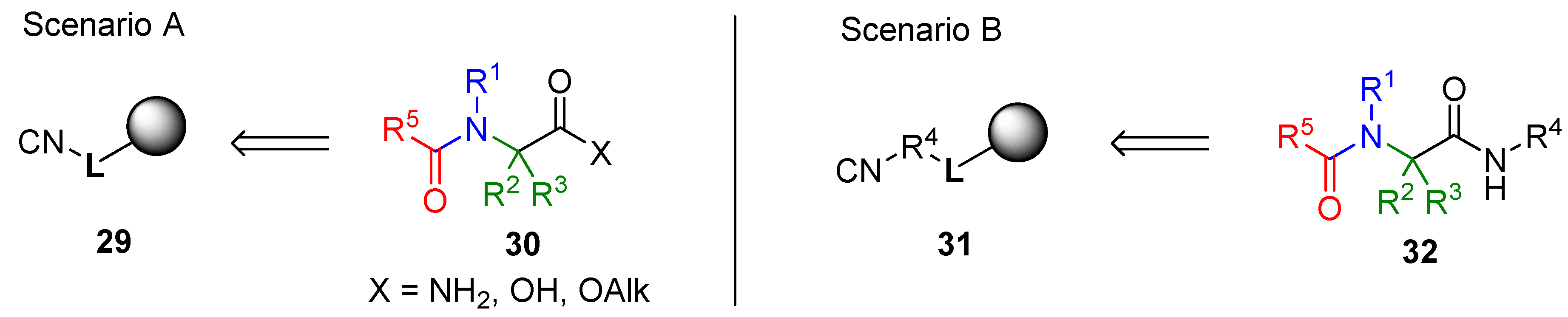
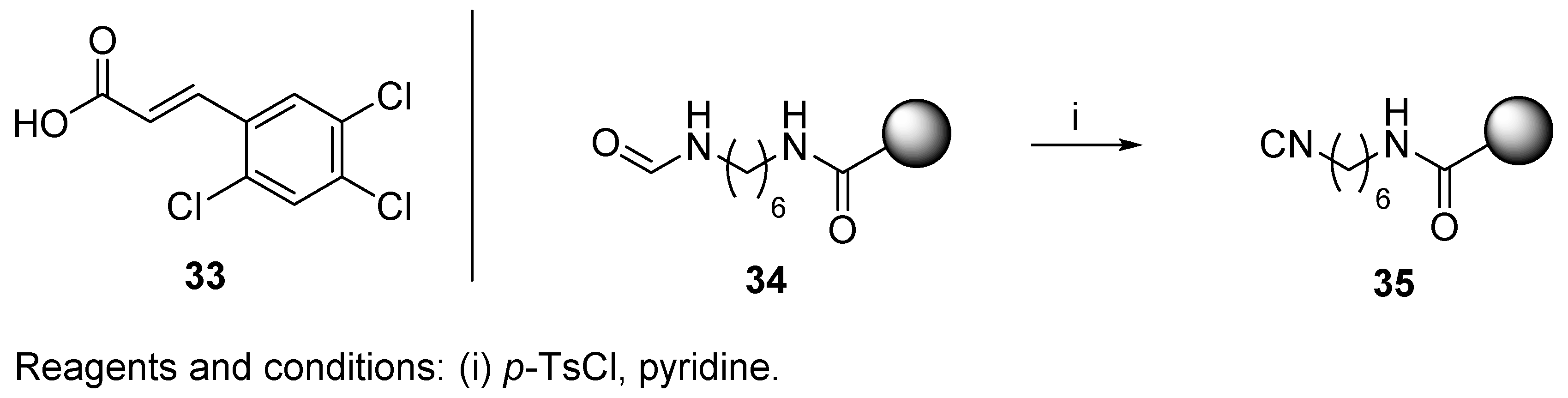
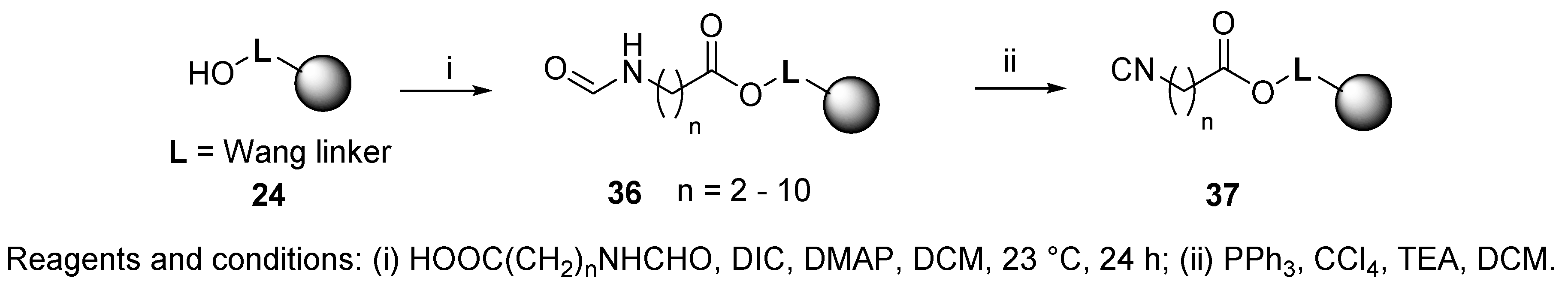
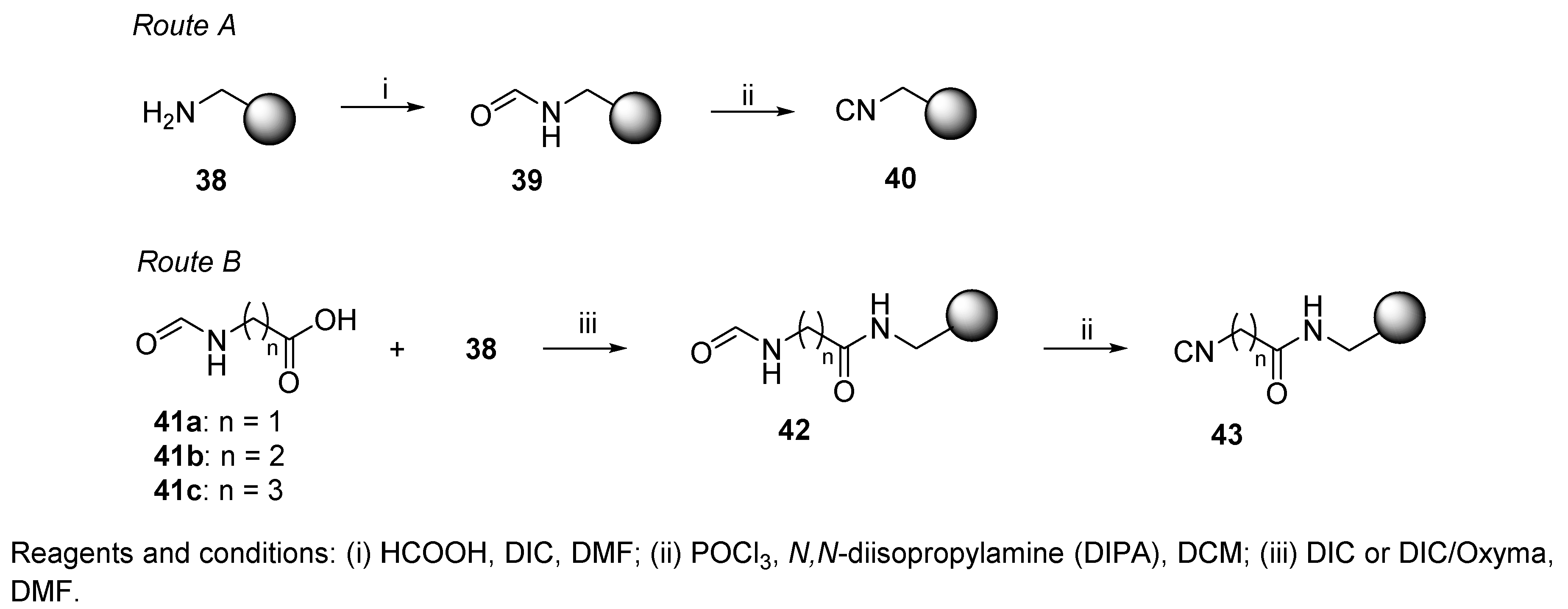
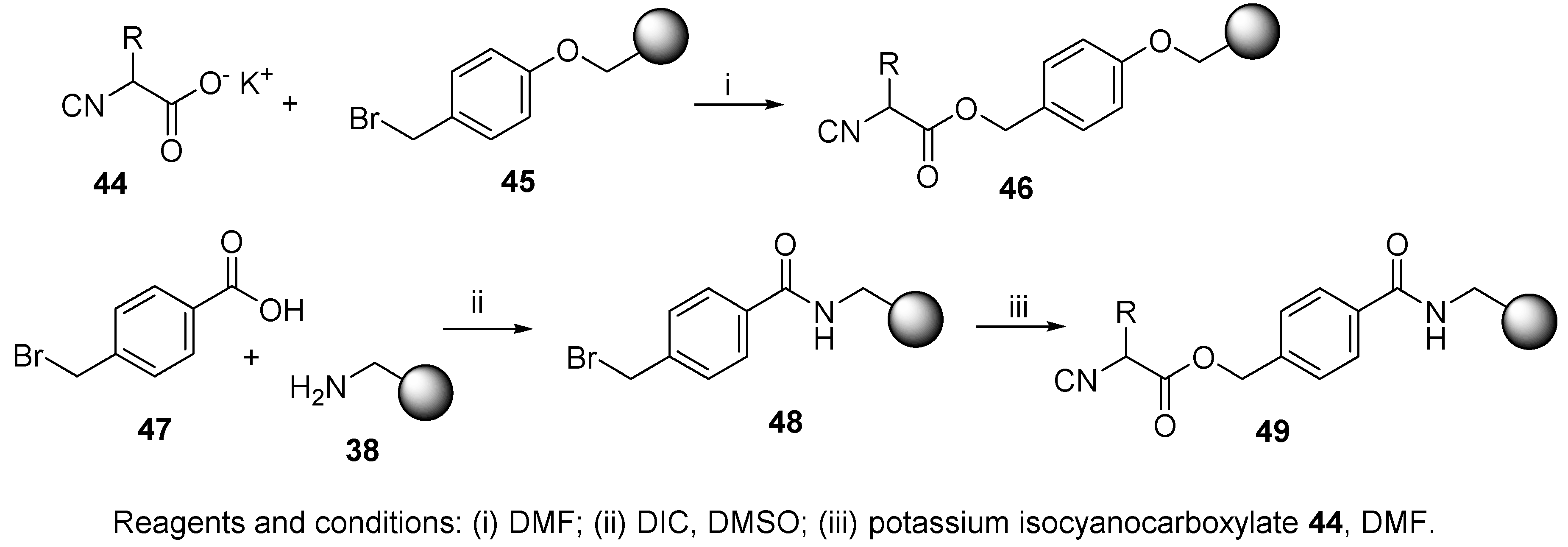
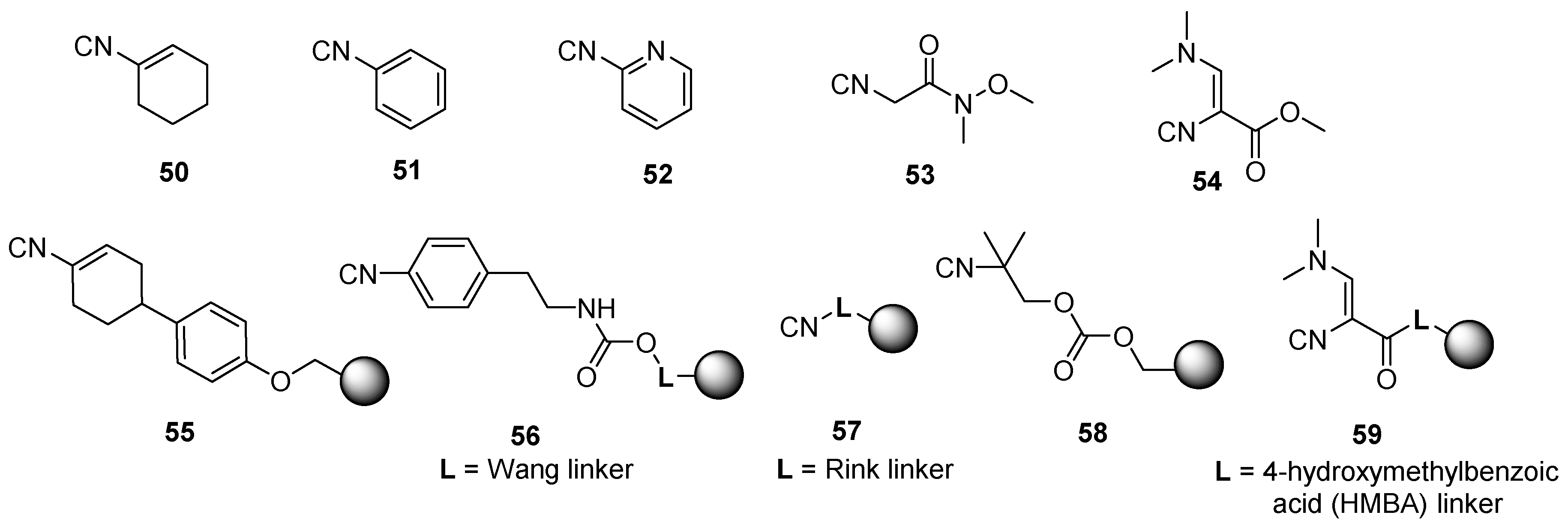
3.2. Isocyanide Resin
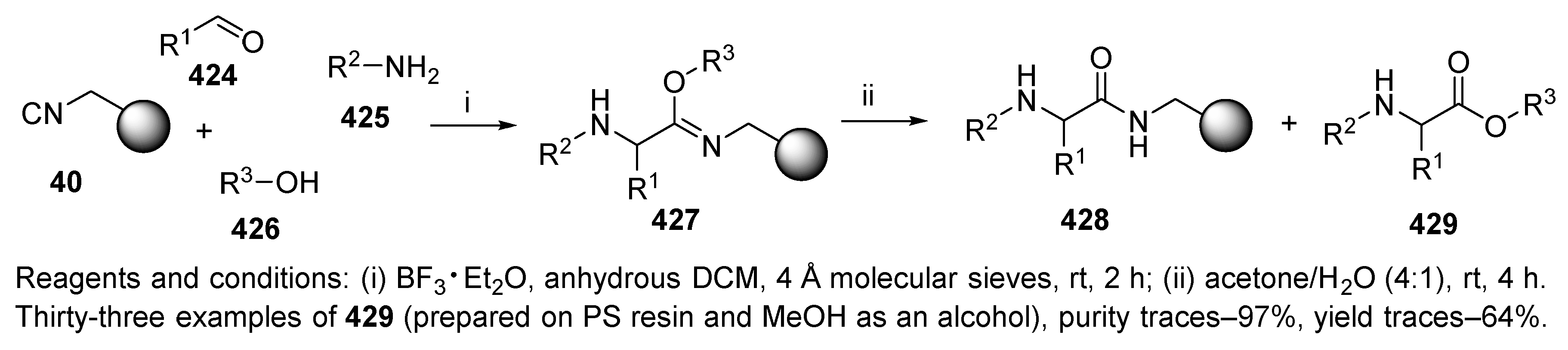
3.3. Aldehyde Resin
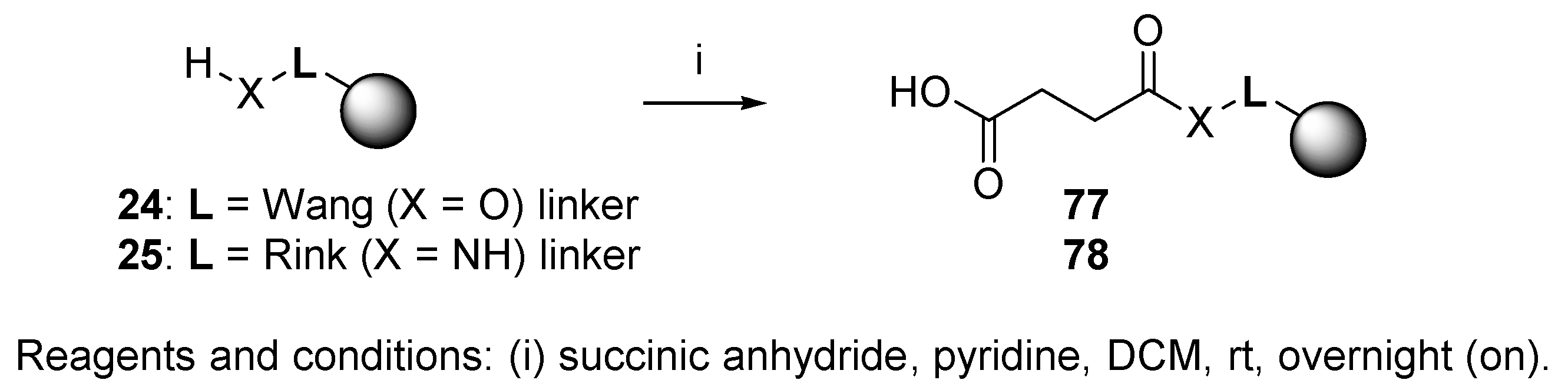
3.4. Carboxylic Acid Resin
4. Ugi Four-Component Reaction (U-4CR)
4.1. α-Acylamido Carboxamides with Three Diversity Positions
4.1.1. Amine Resin
4.1.2. Isocyanide Resin
4.2. α-Acylamido Carboxamides with Four Diversity Positions
4.2.1. Amine Resin
4.2.2. Isocyanide Resin
4.2.3. Aldehyde Resin
4.2.4. Carboxylic Acid Resin
5. U-4CR Followed by Cyclative Cleavage
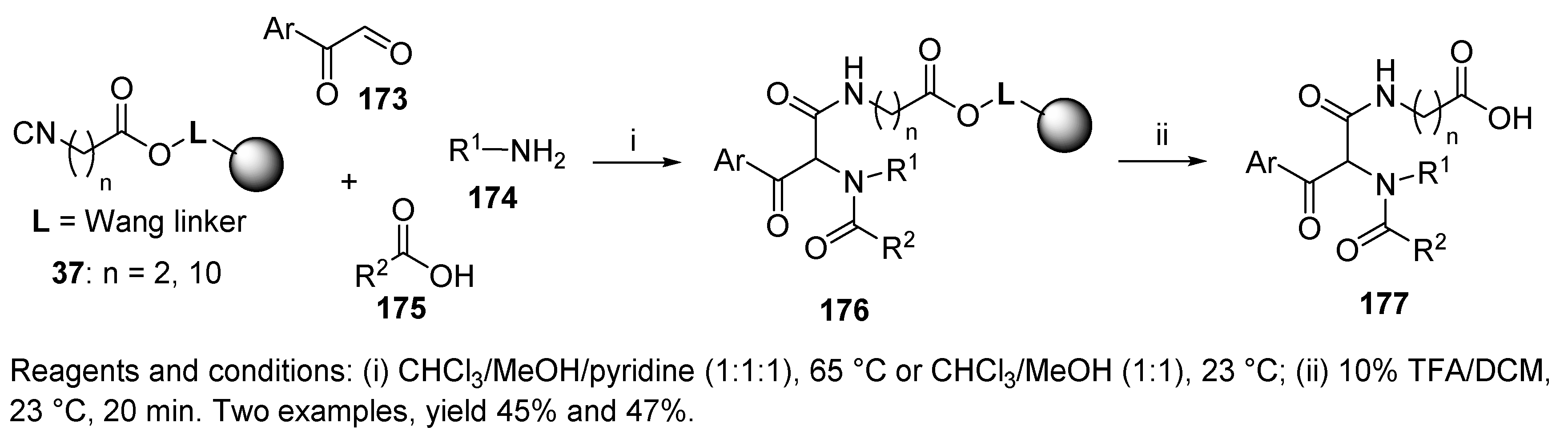
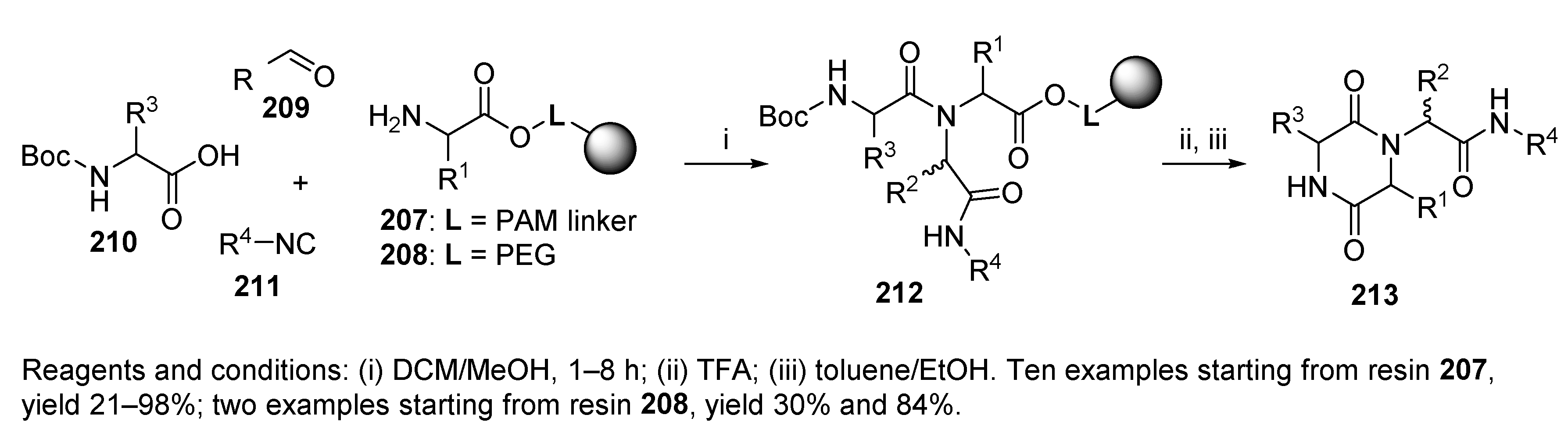
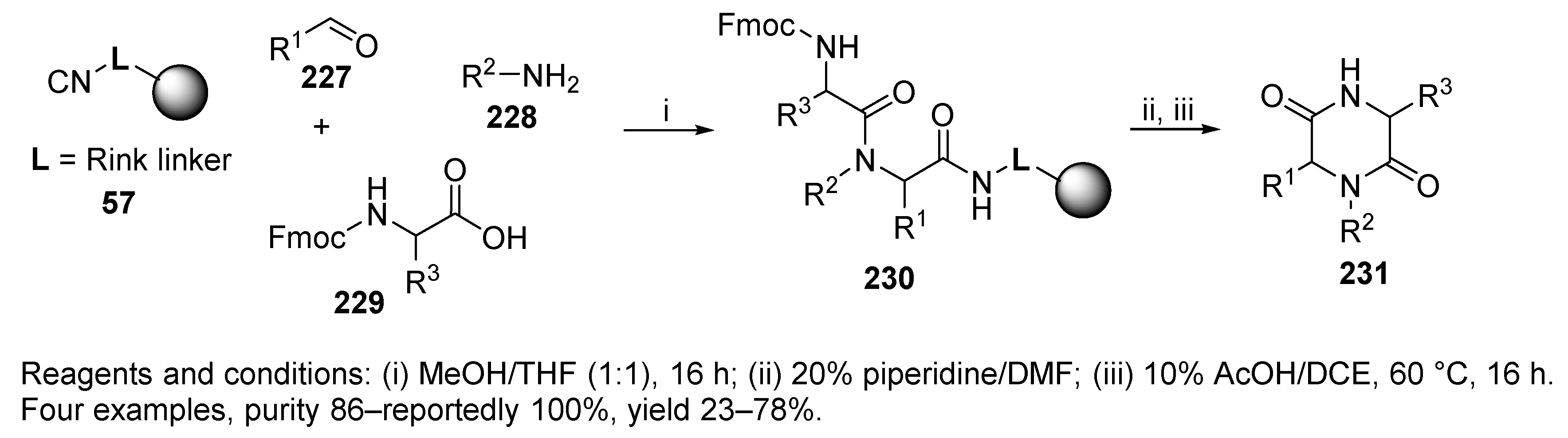
5.1. Diketopiperazines
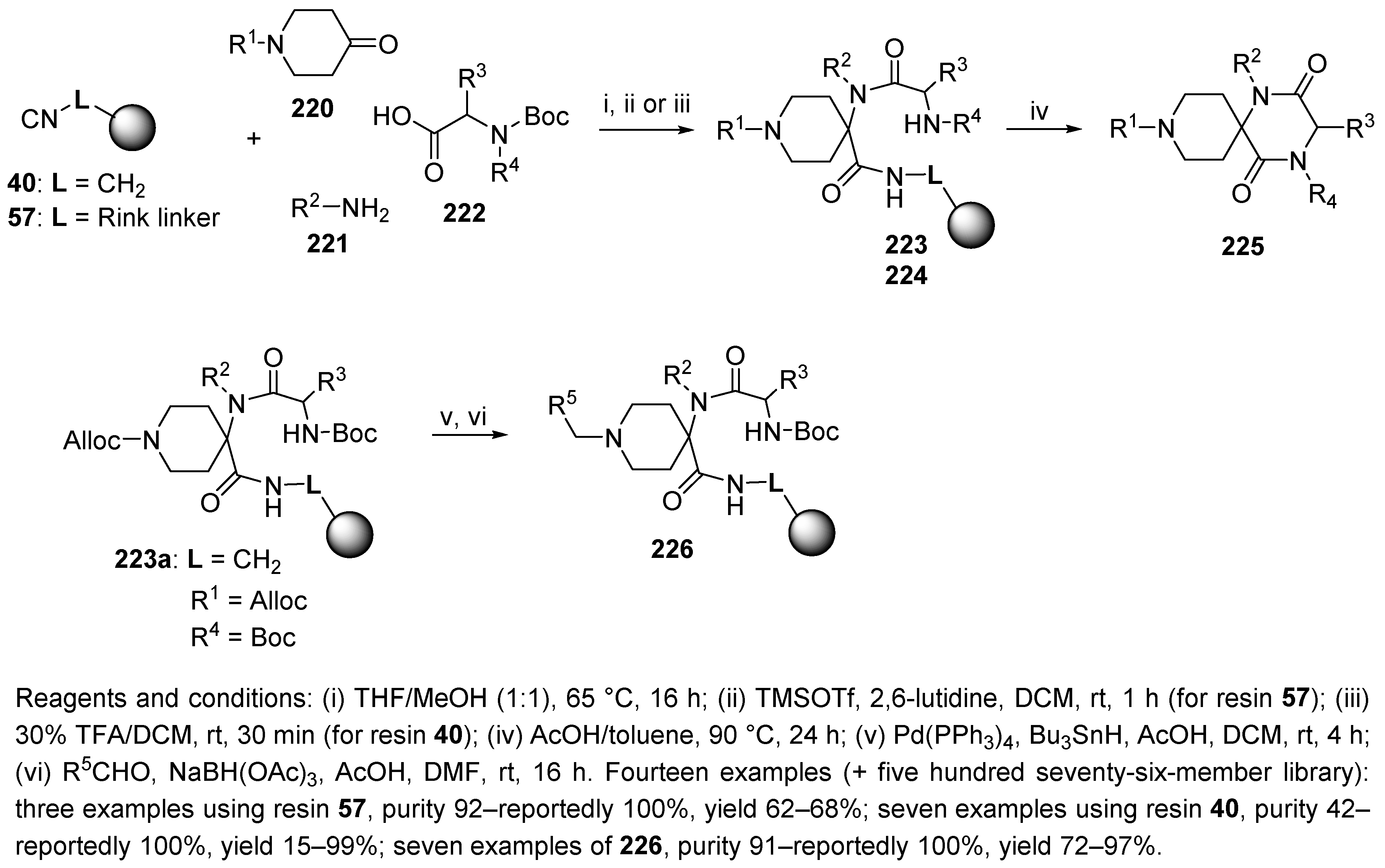
5.2. Ketopiperazines
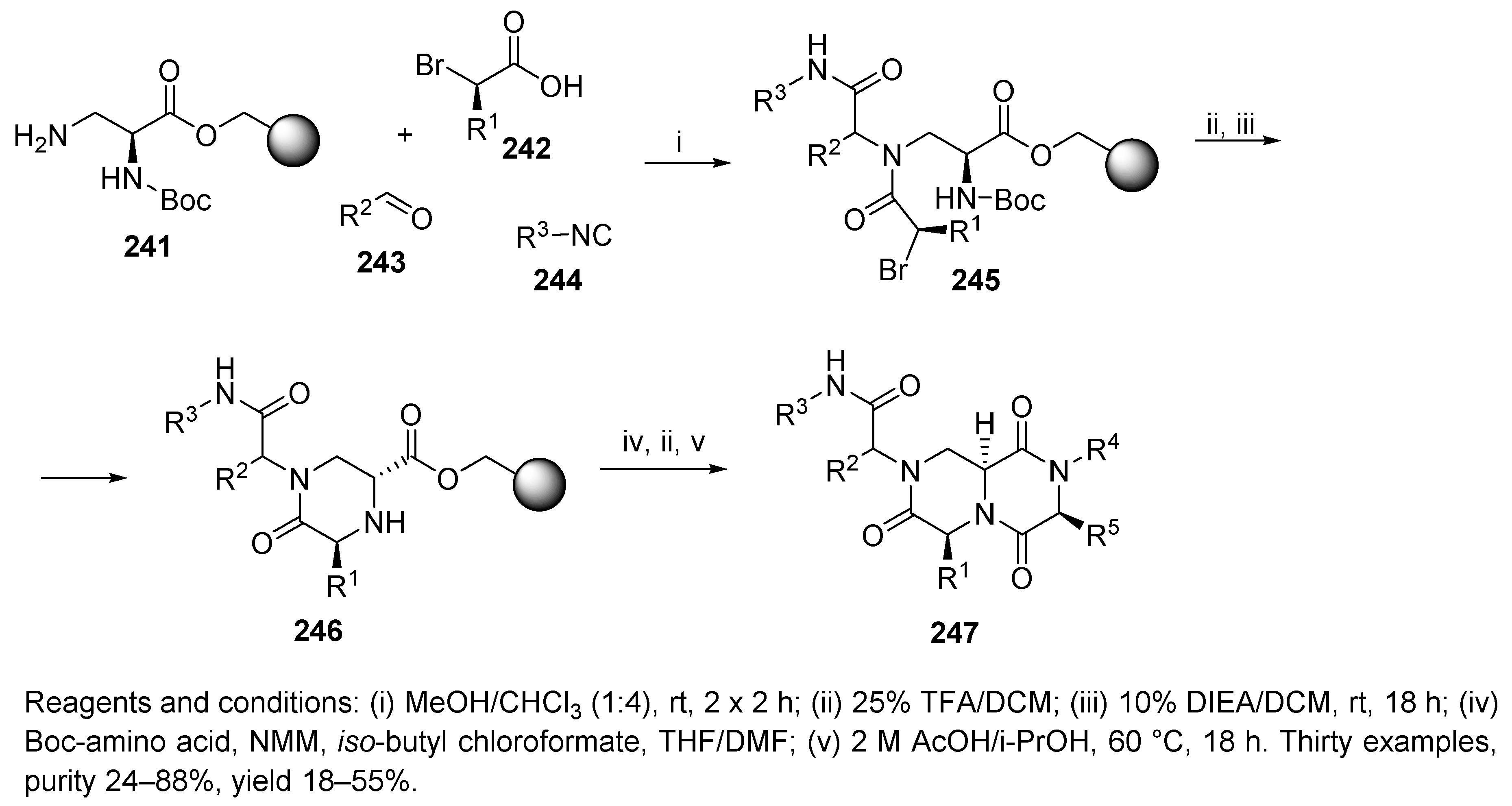
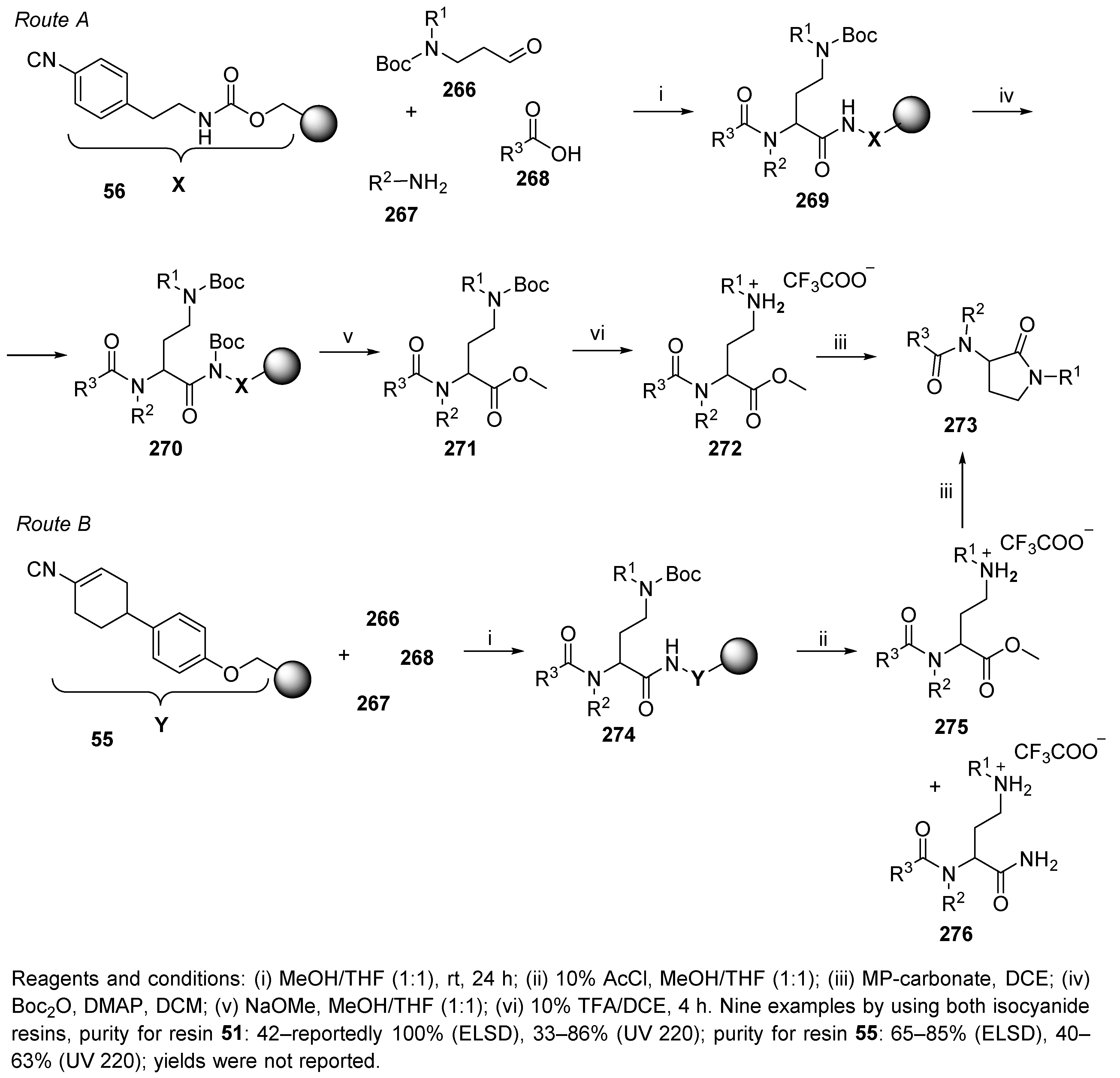
5.3. Bicyclic Diketopiperazines
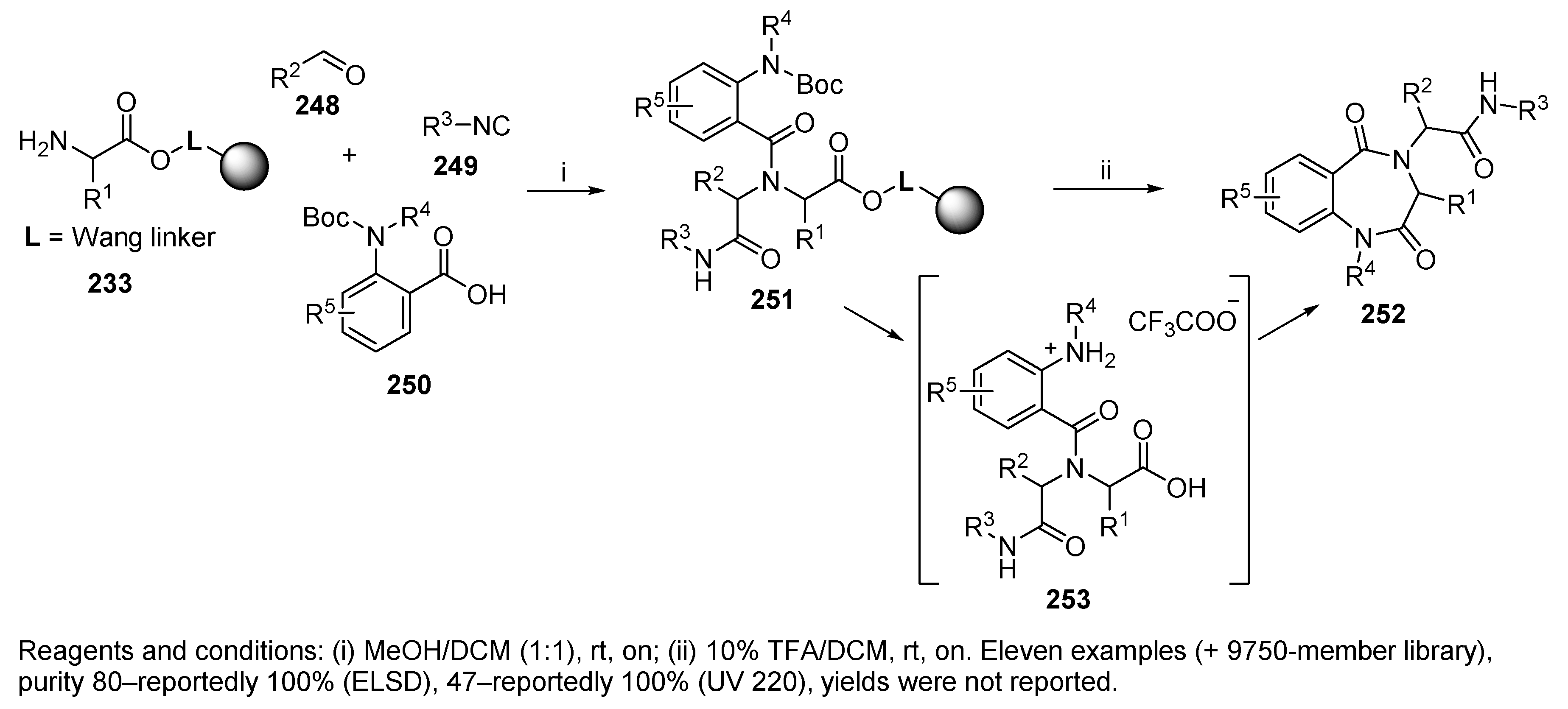
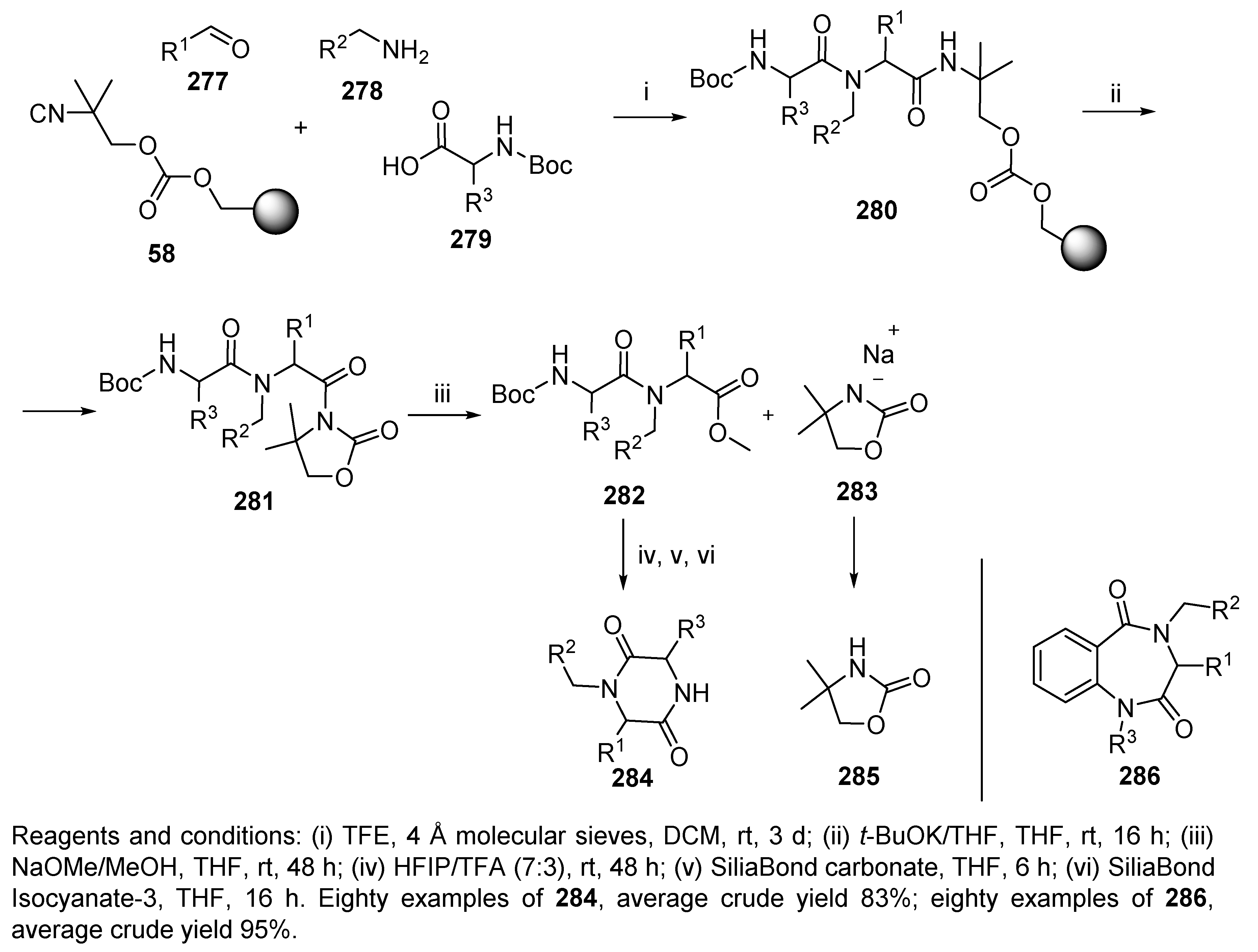
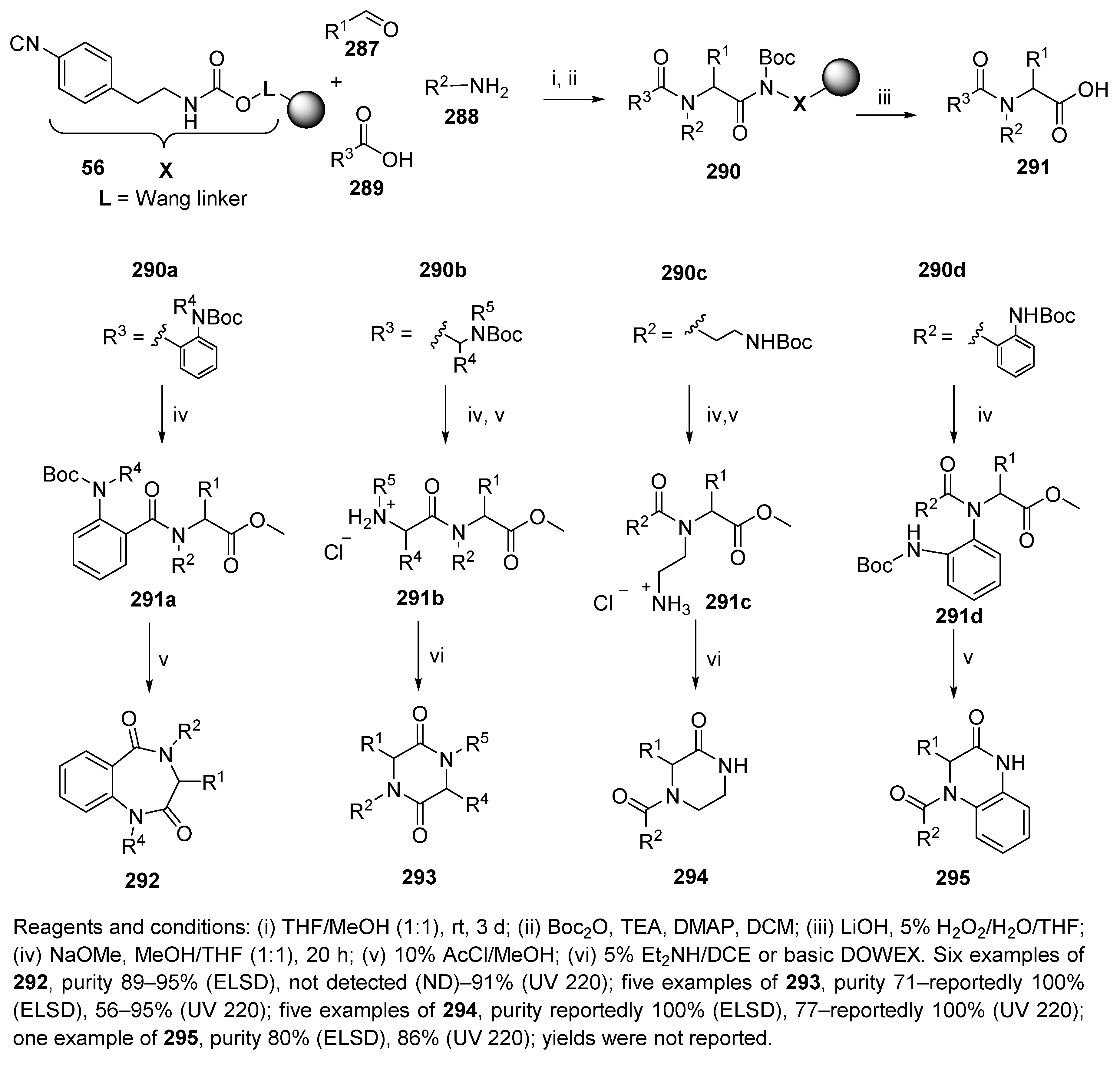
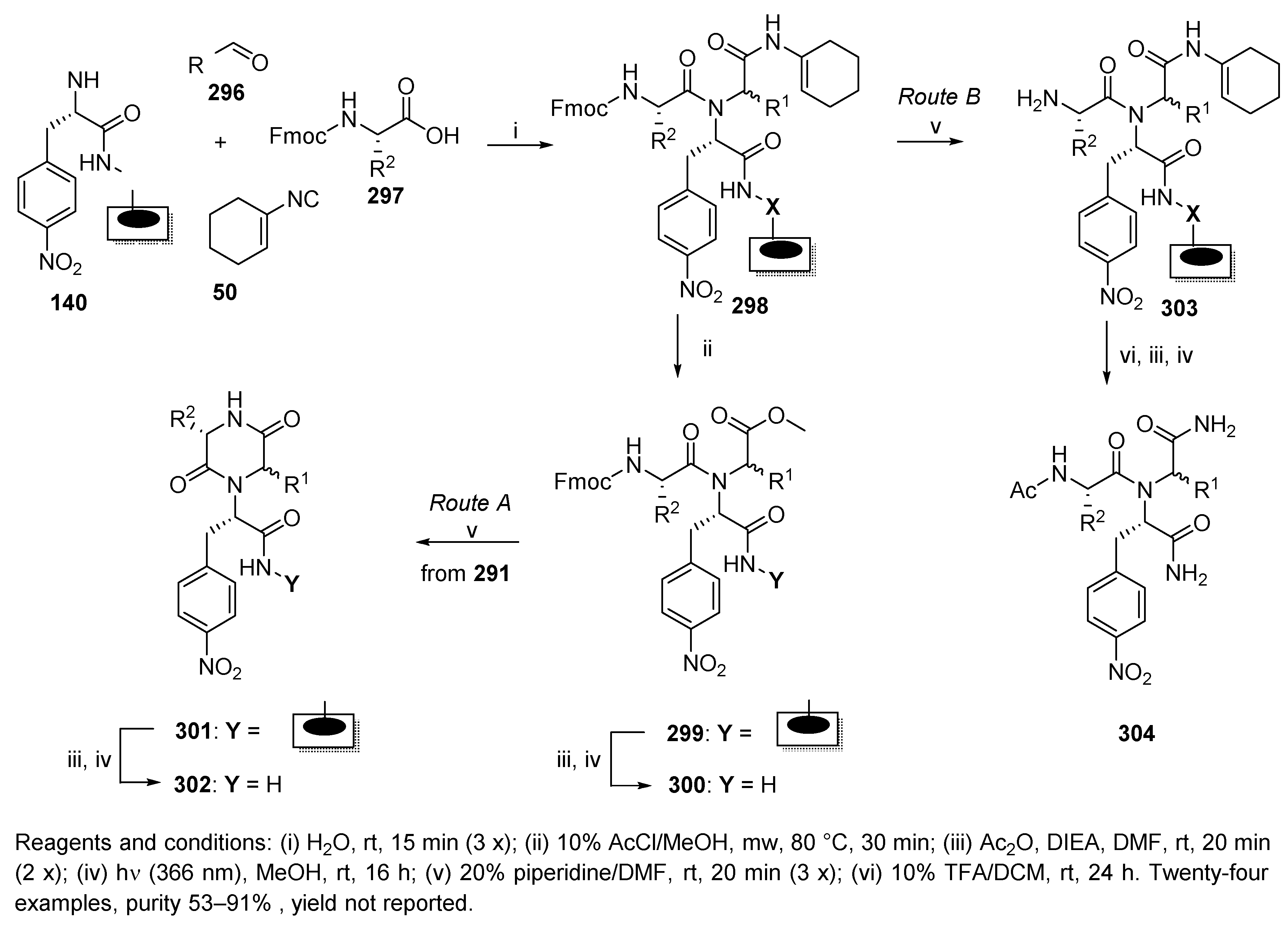
5.4. Benzodiazepines
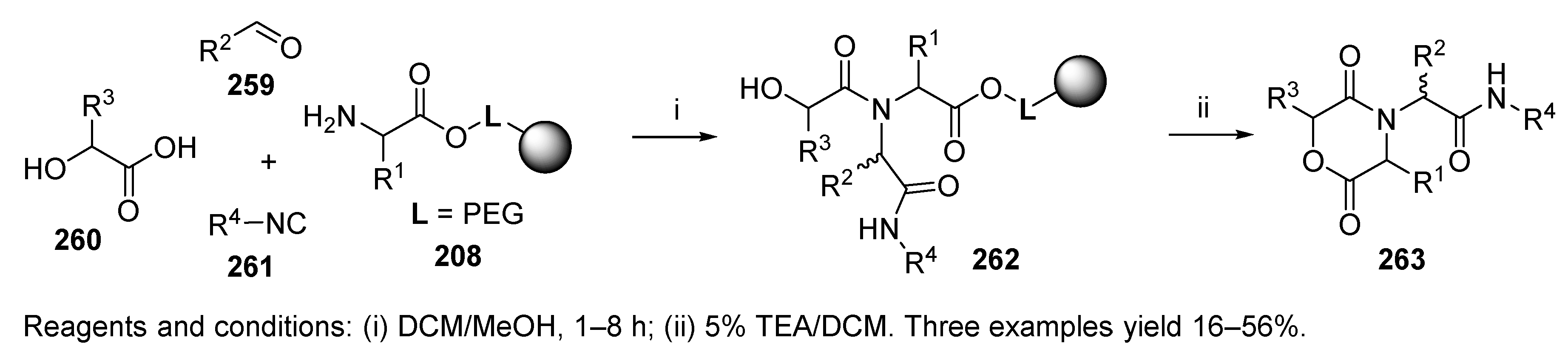
5.5. Diketomorpholines
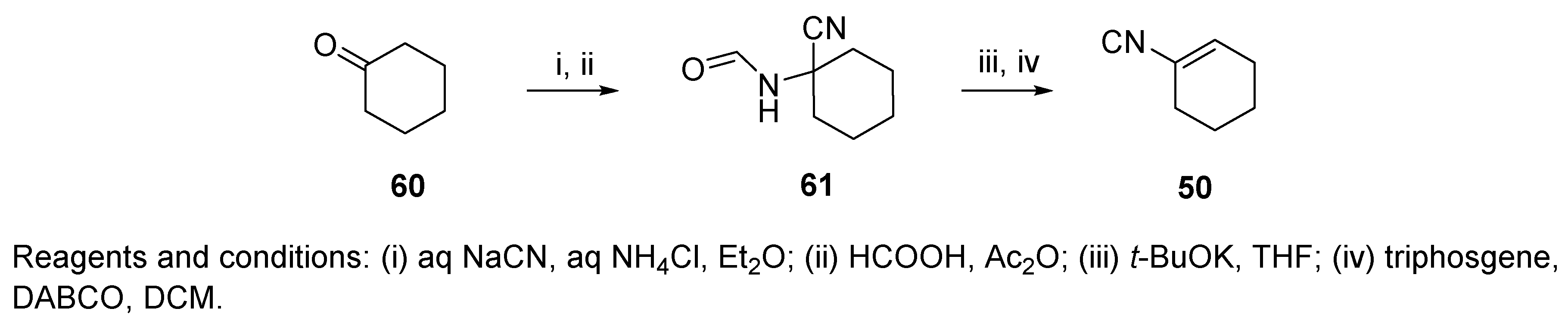
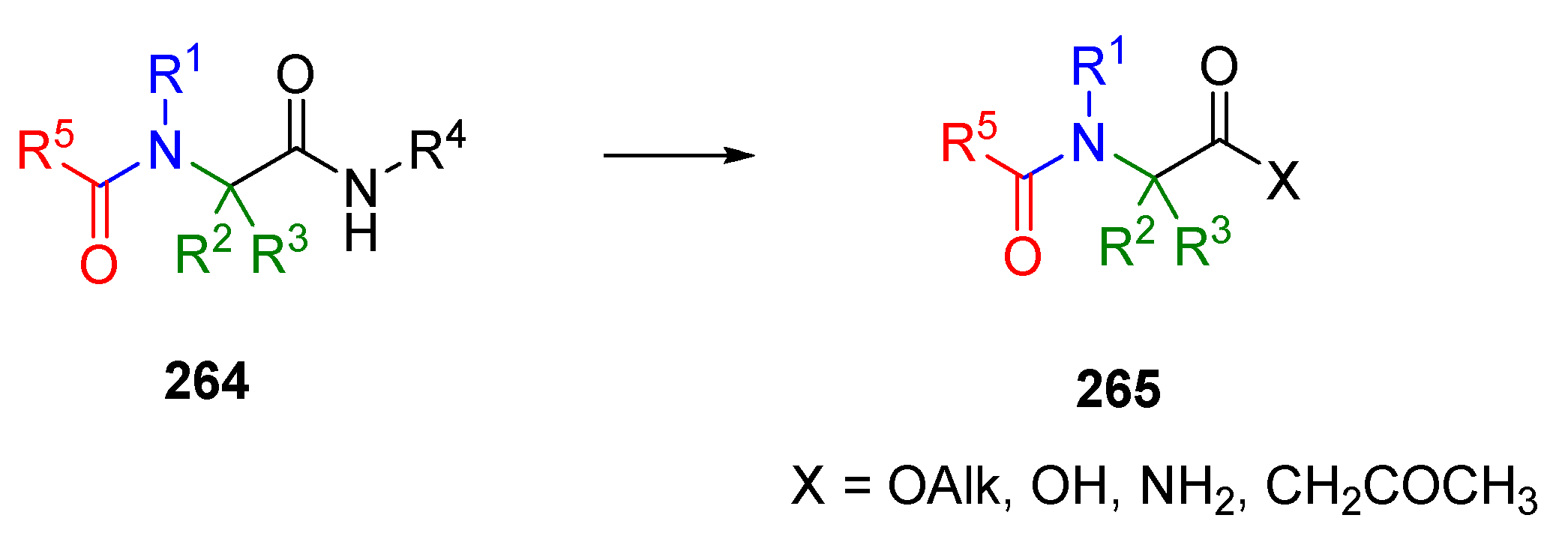
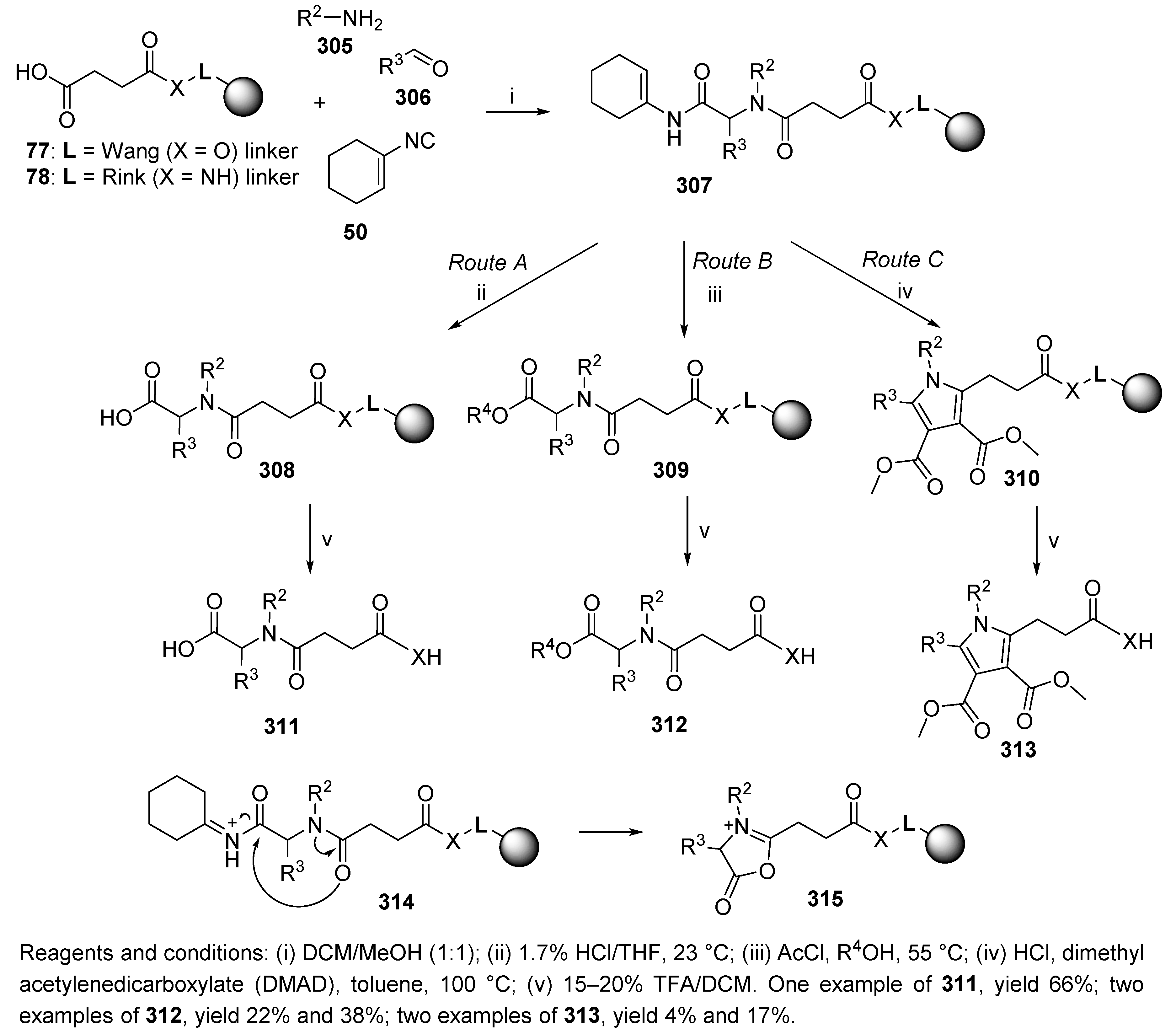
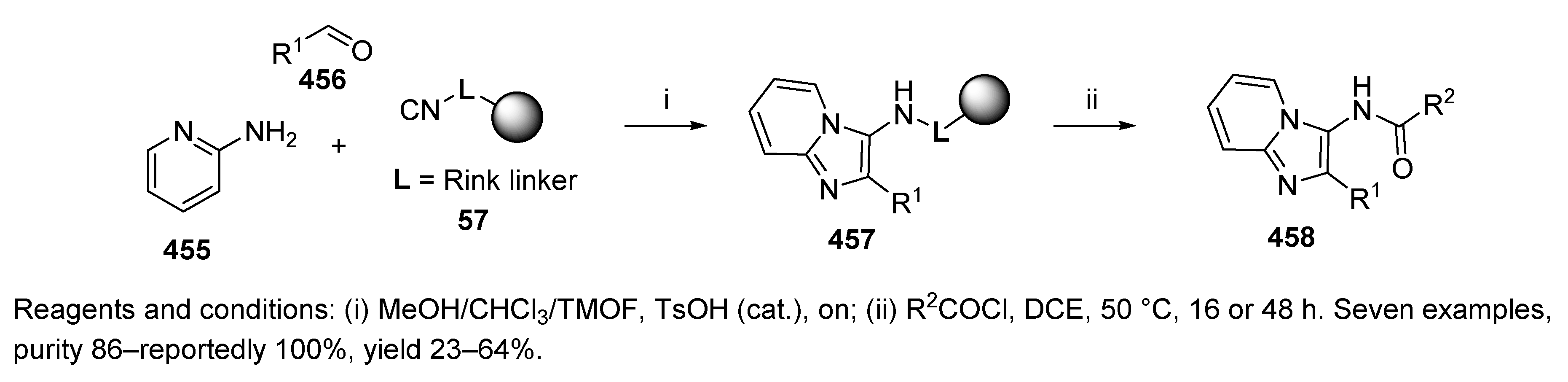
6. Post-Ugi Transformations of Convertible Isocyanides
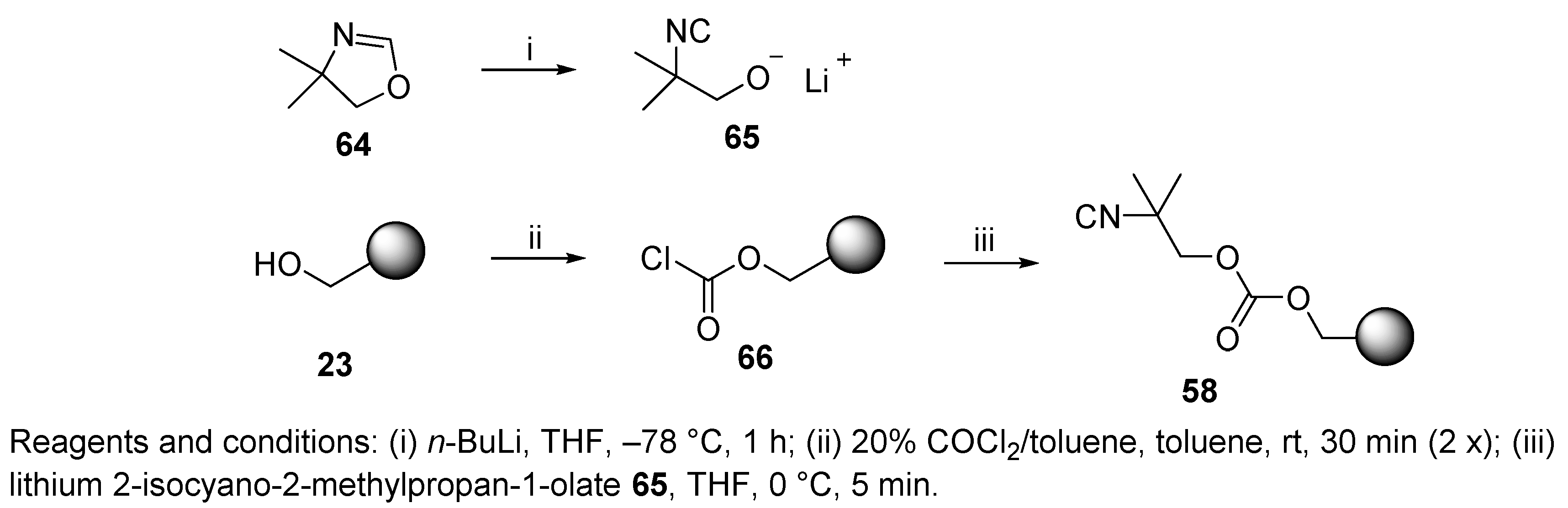
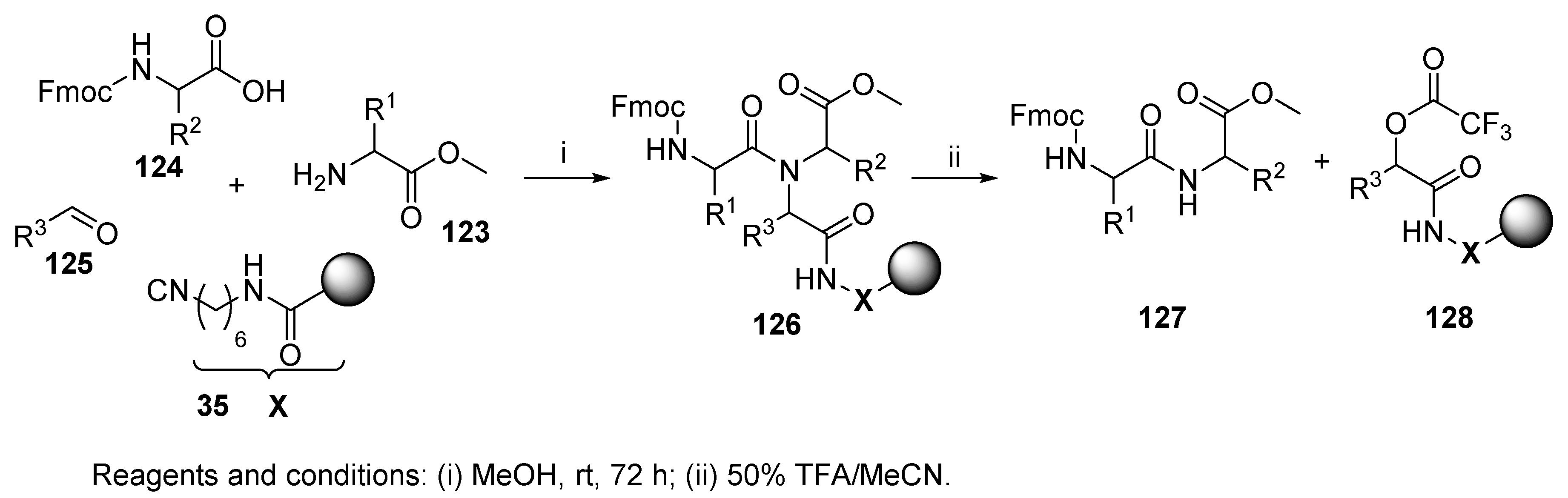
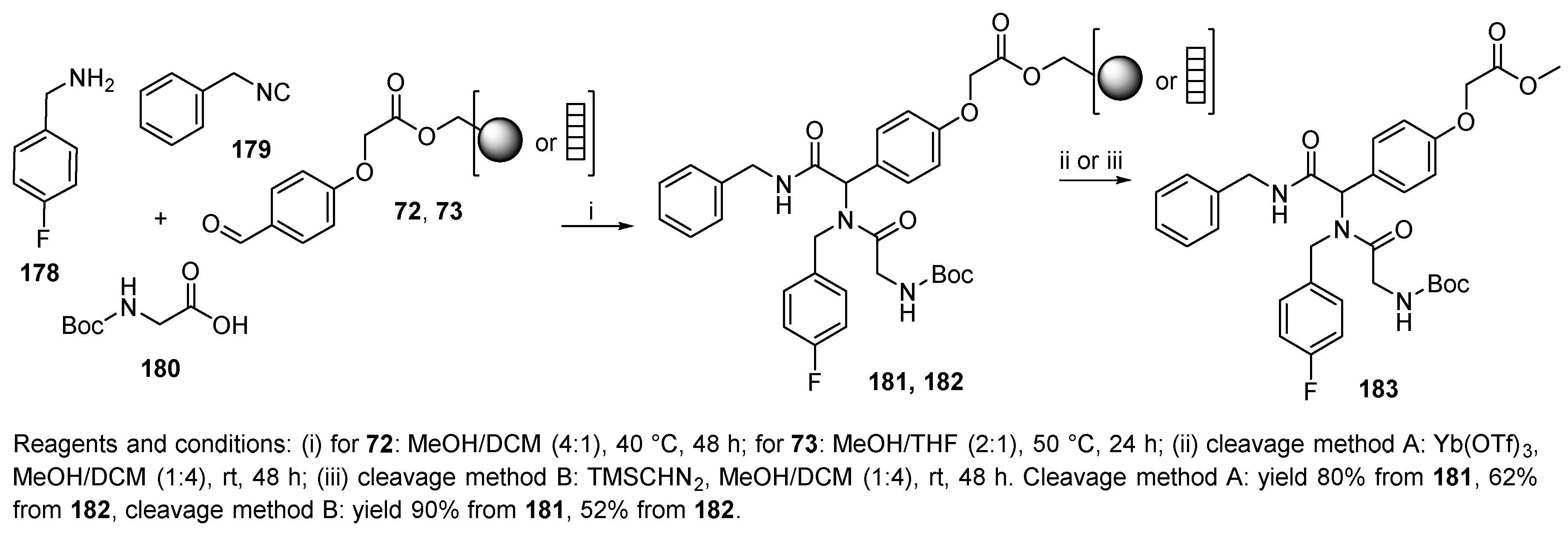
6.1. Polymer-Supported Convertible Isocyanides
6.2. Convertible Isocyanides in Solution
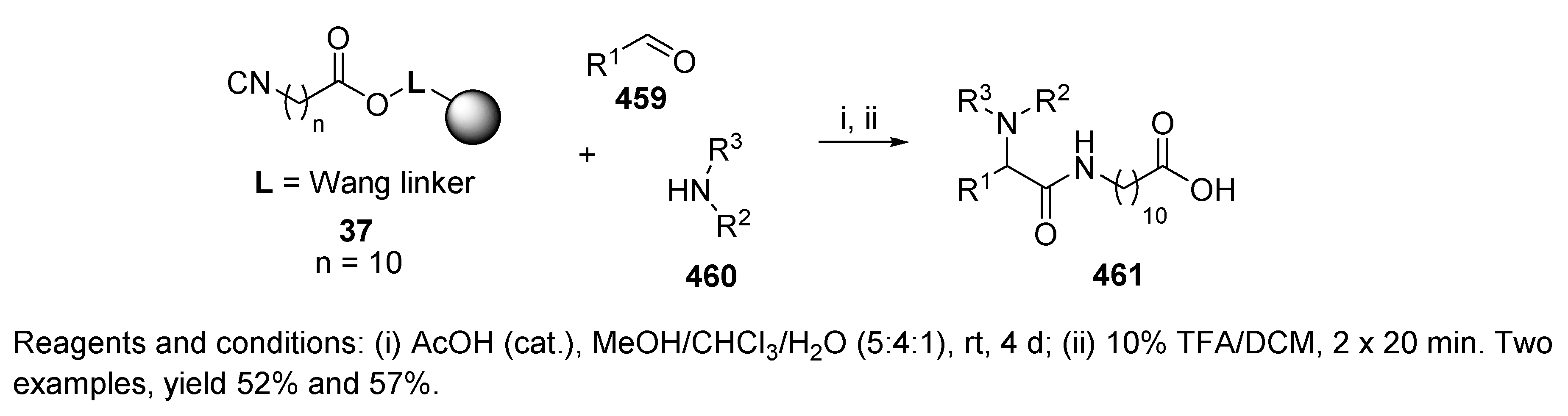
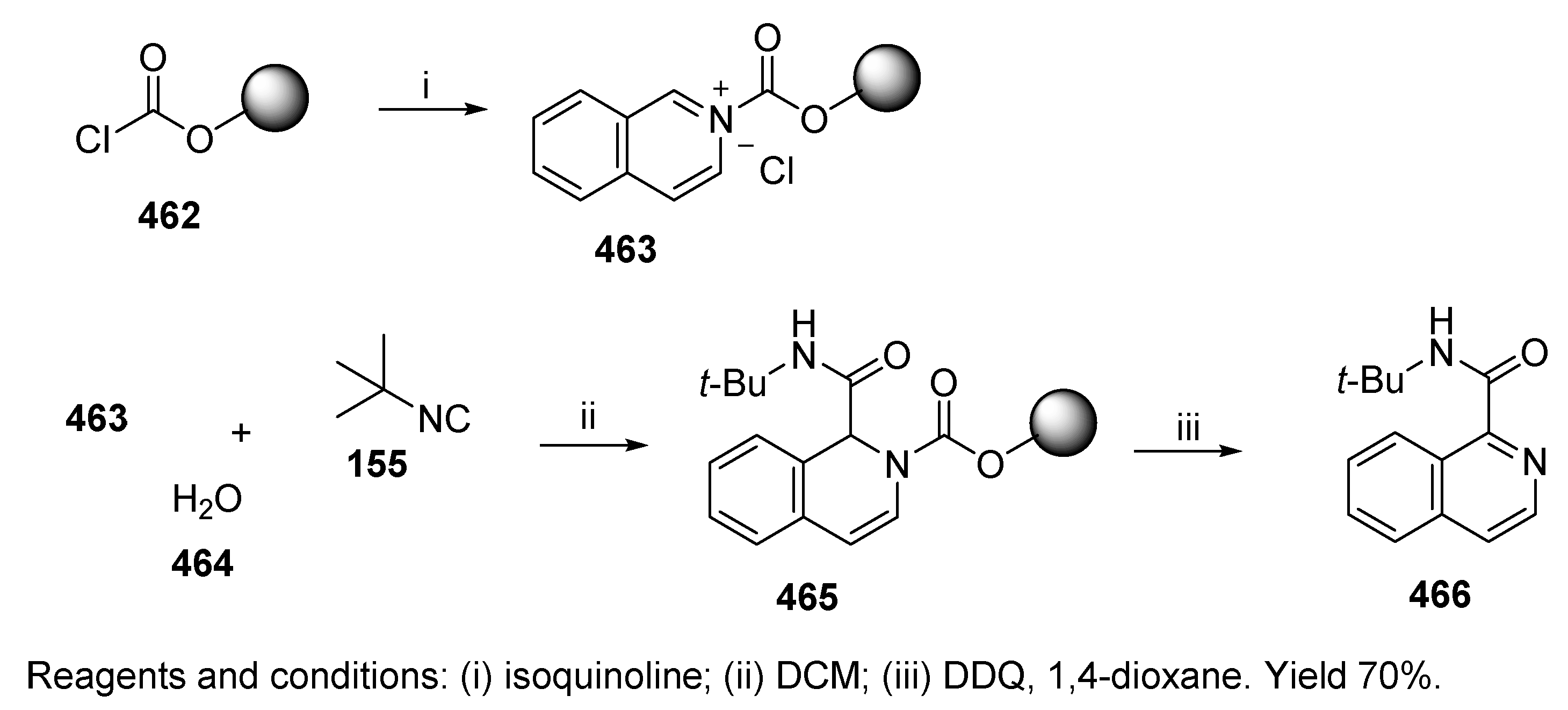
7. Other Post-Ugi Transformations
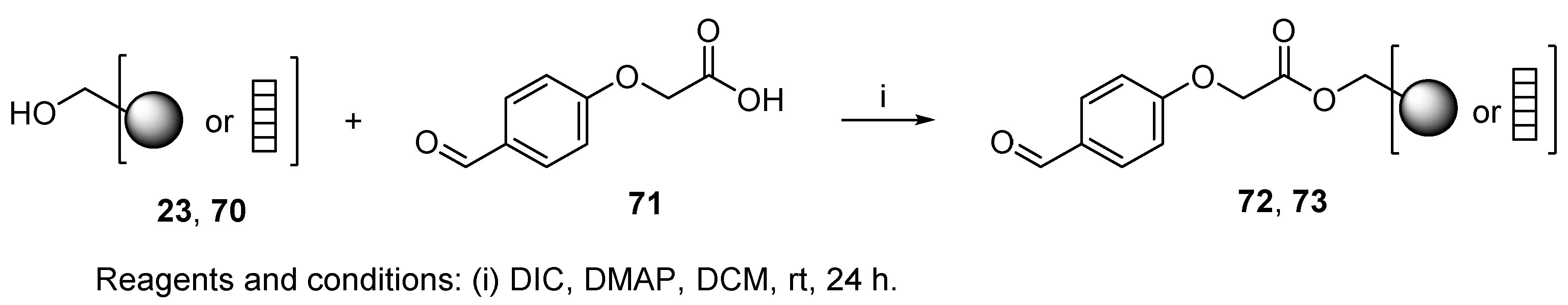
7.1. Spontaneous Transformations
7.2. Mediated Transformations
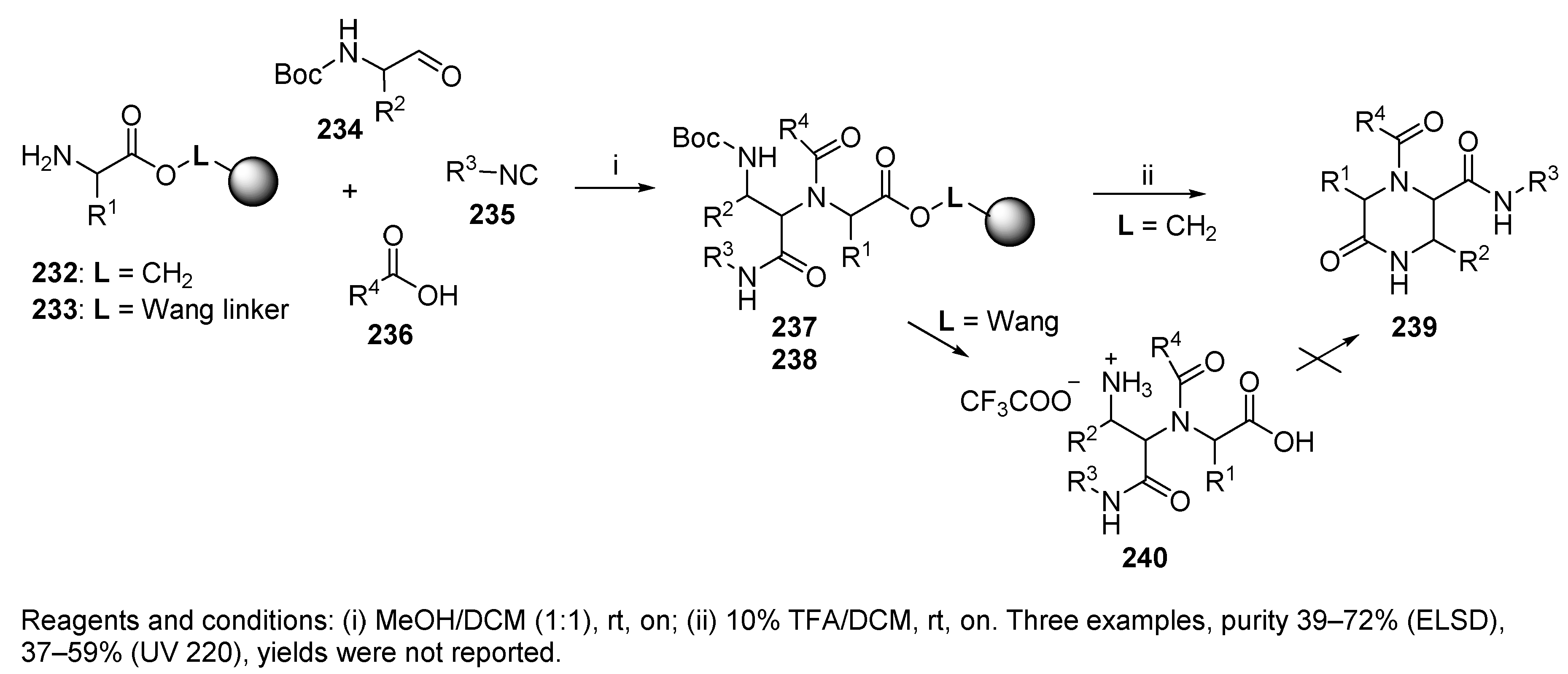
8. Ugi Four-Center Three-Component Reaction (U-4C-3CR)
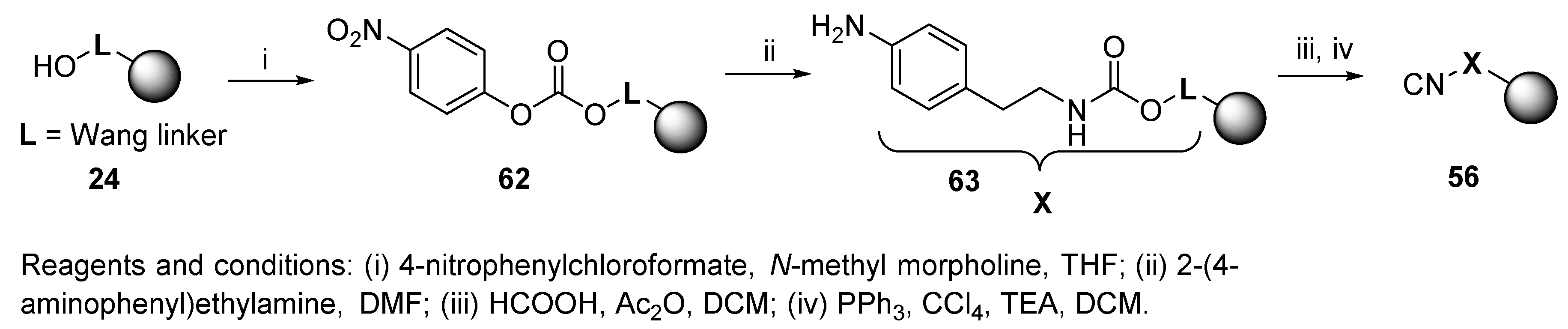
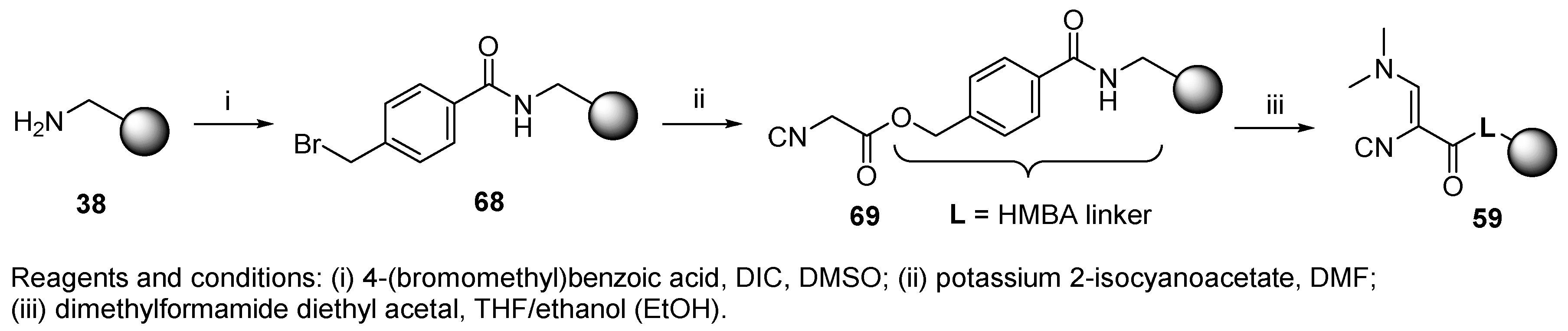
8.1. Resin-Bound Bifunctional Reagents
8.2. Bifunctional Reagents in Solutions
9. Non-Traditional Ugi Reaction
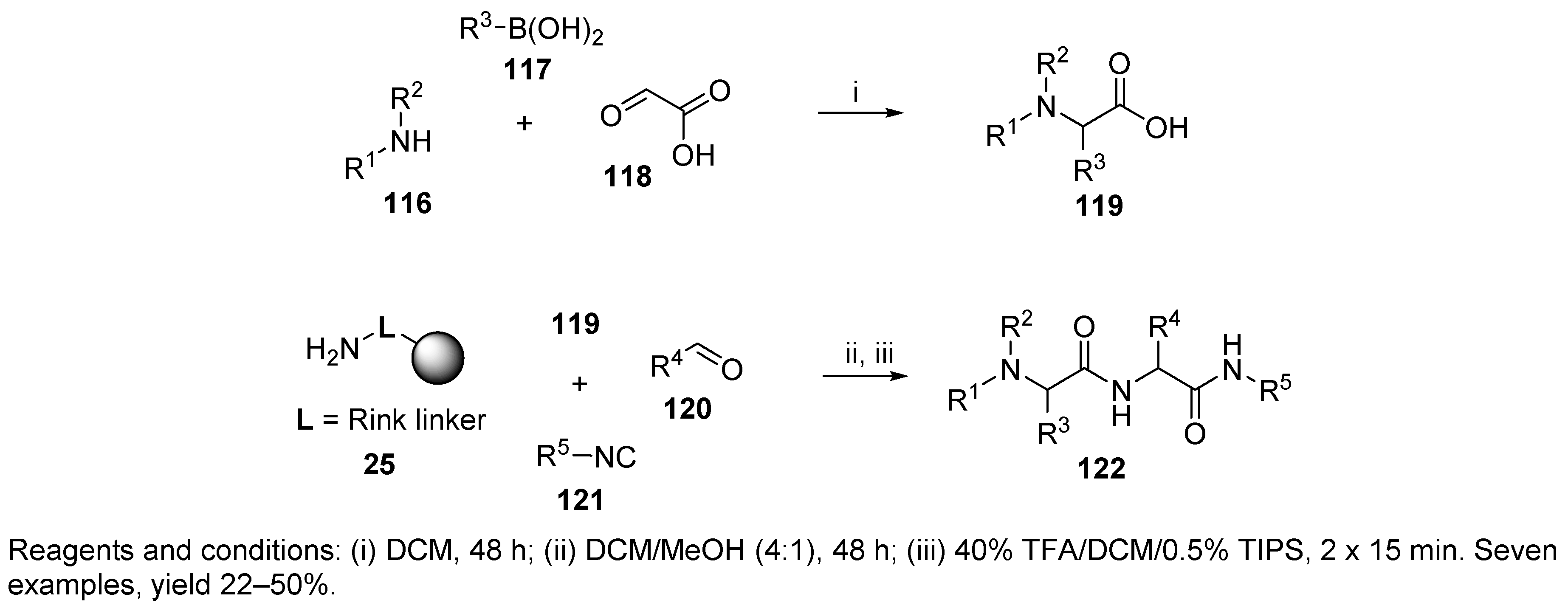
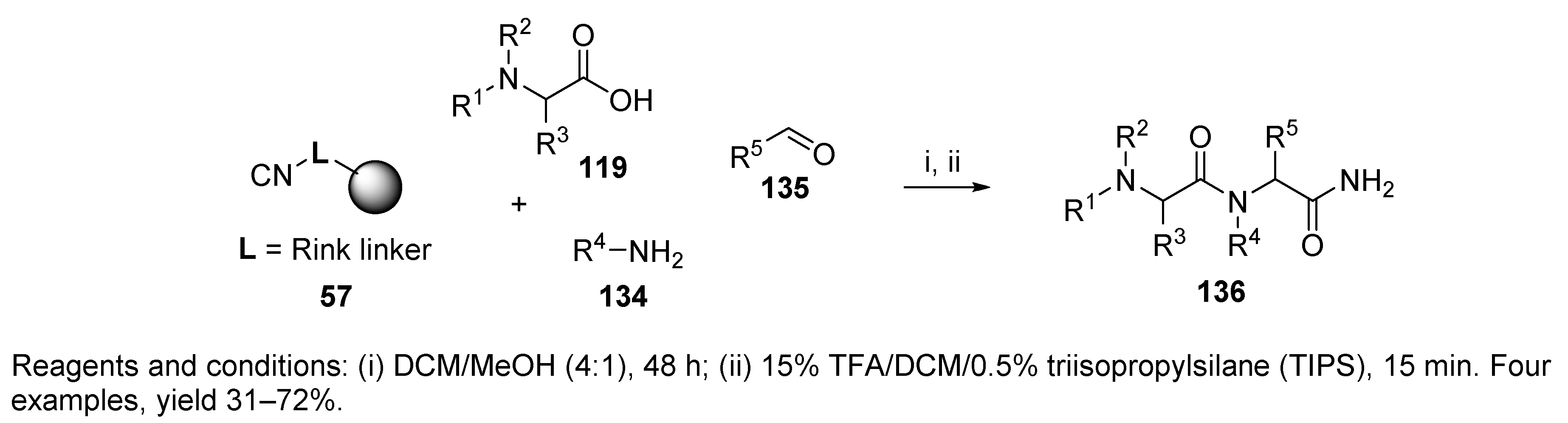
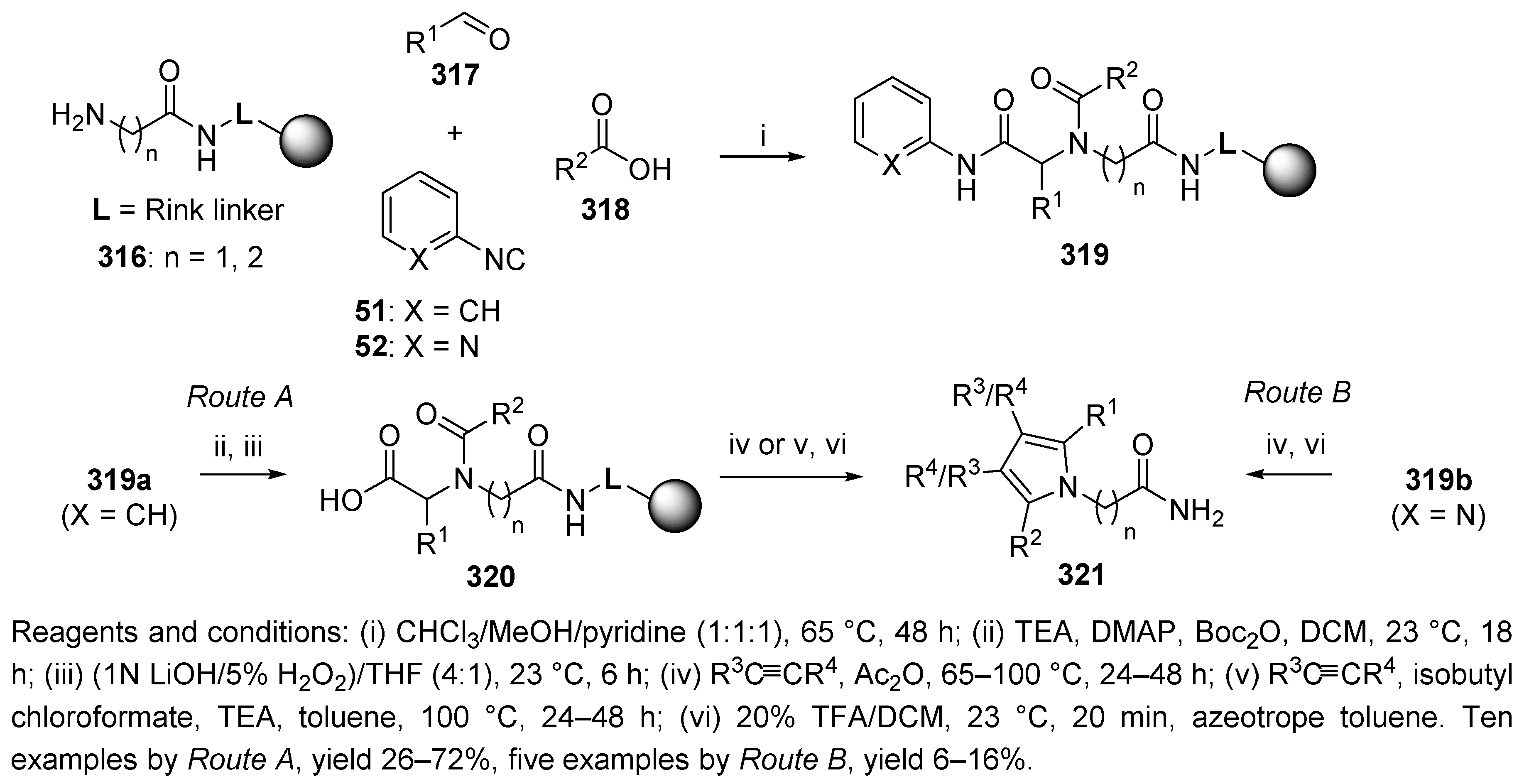
9.1. Alternative Amine Input
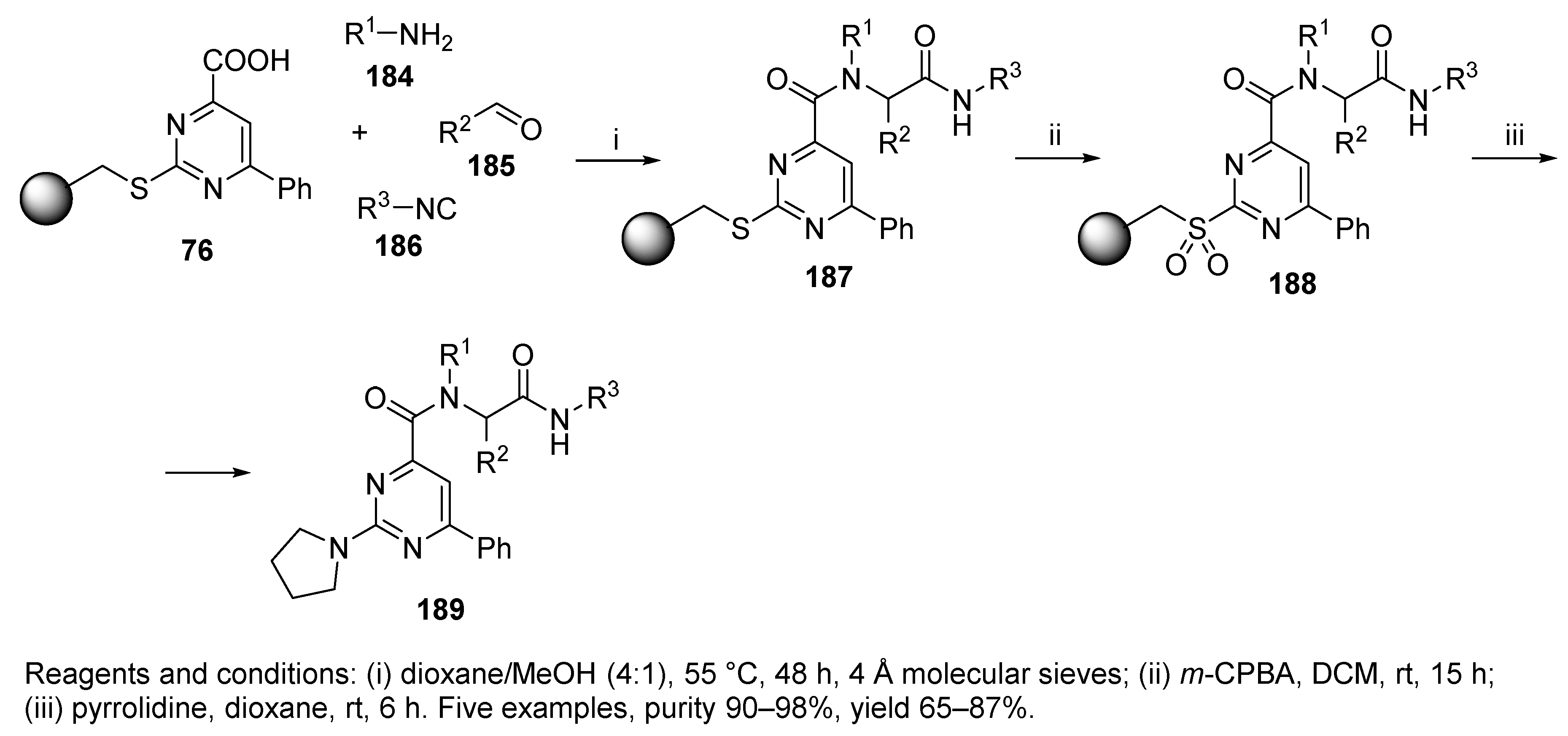
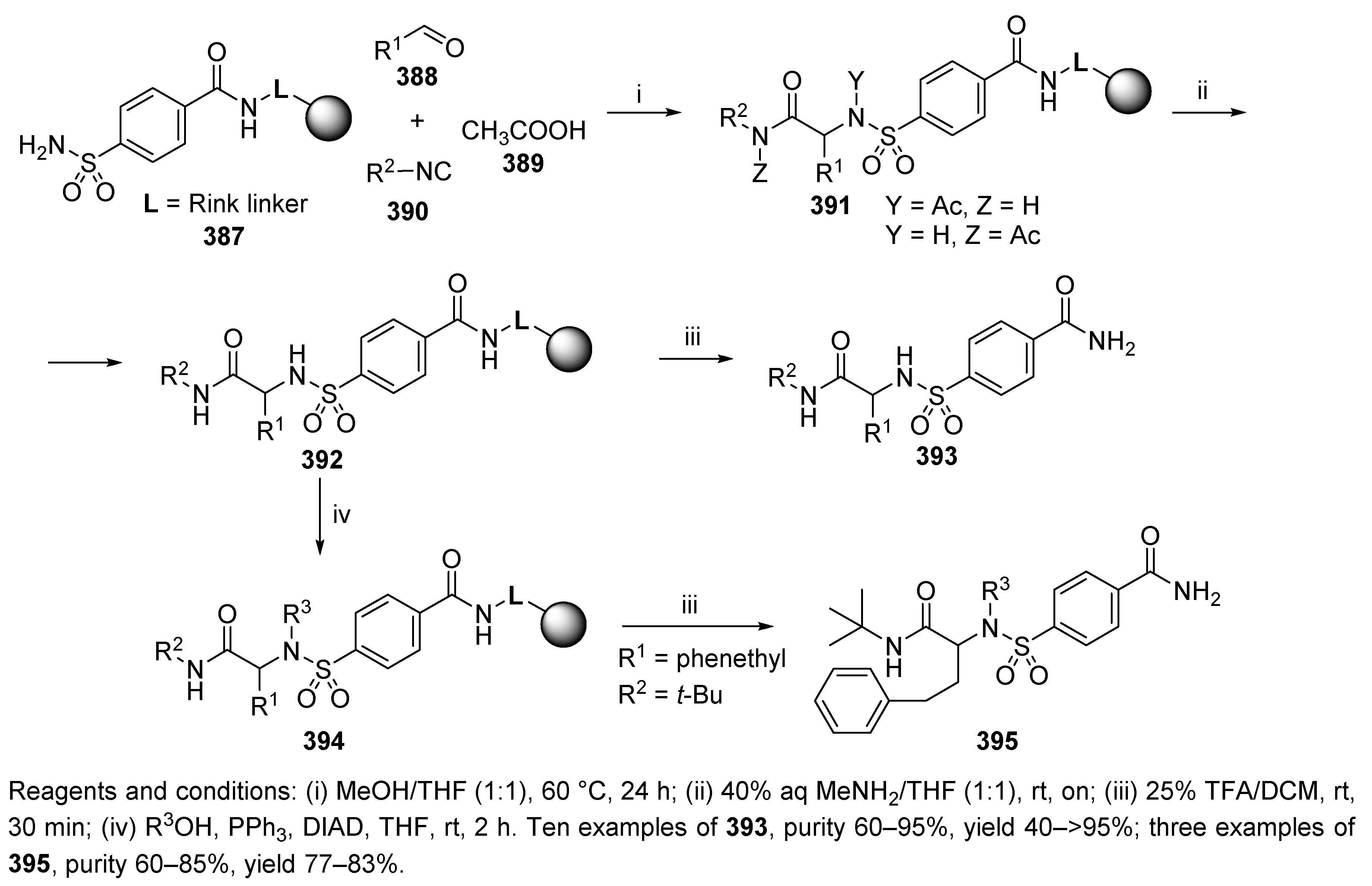
Sulfonamide
9.2. Alternative Acid Input
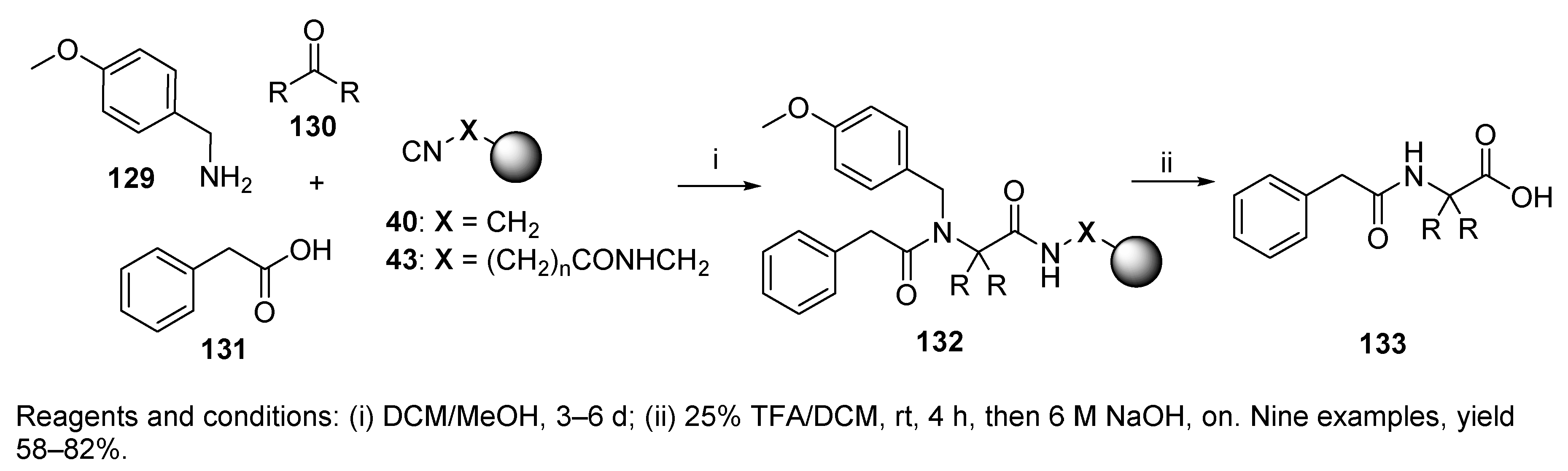
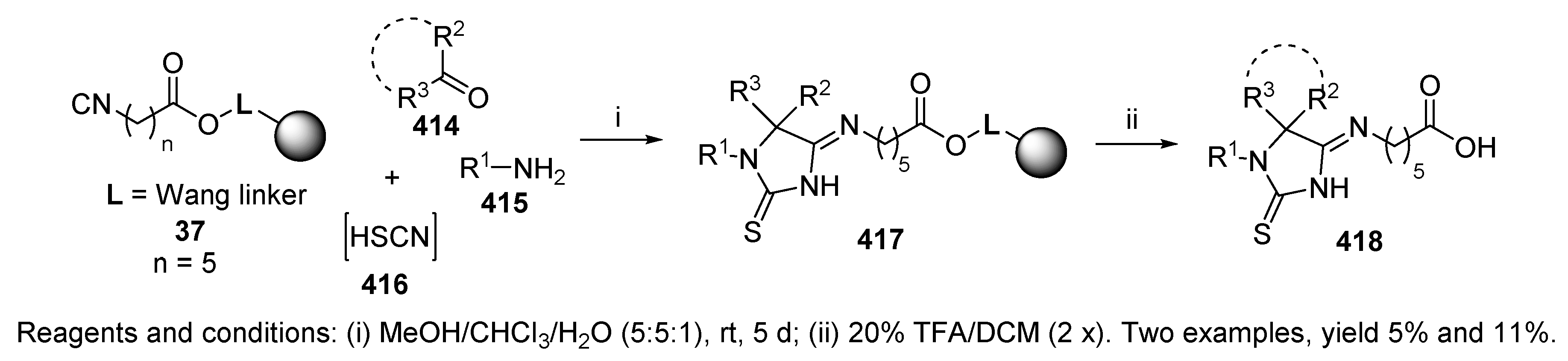
9.2.1. Thiocarboxylic Acid
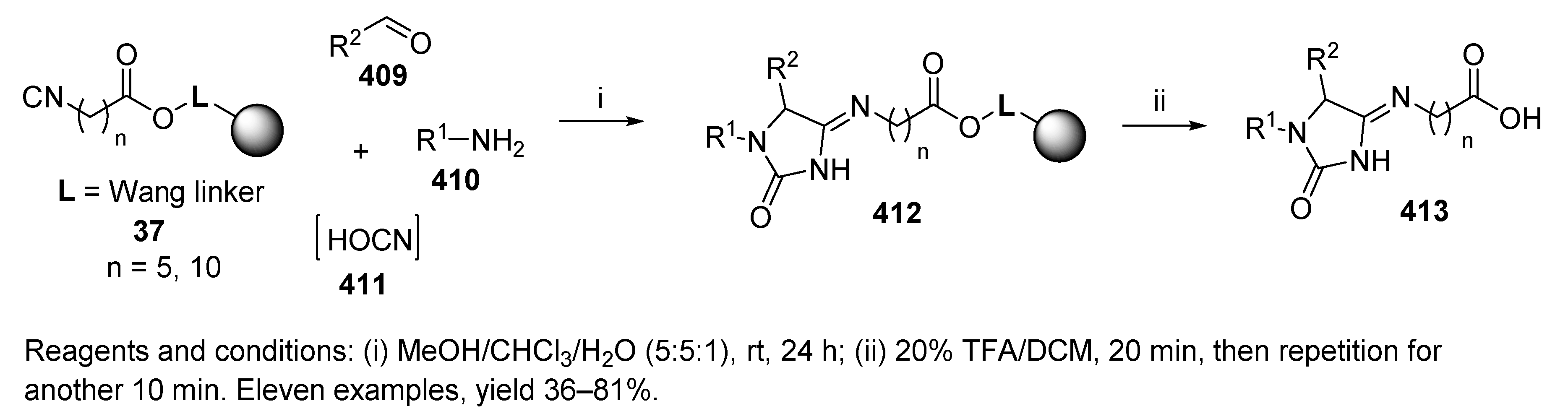
9.2.2. (Thio)cyanic Acids and Hydrogen Azide
9.2.3. Alcohol
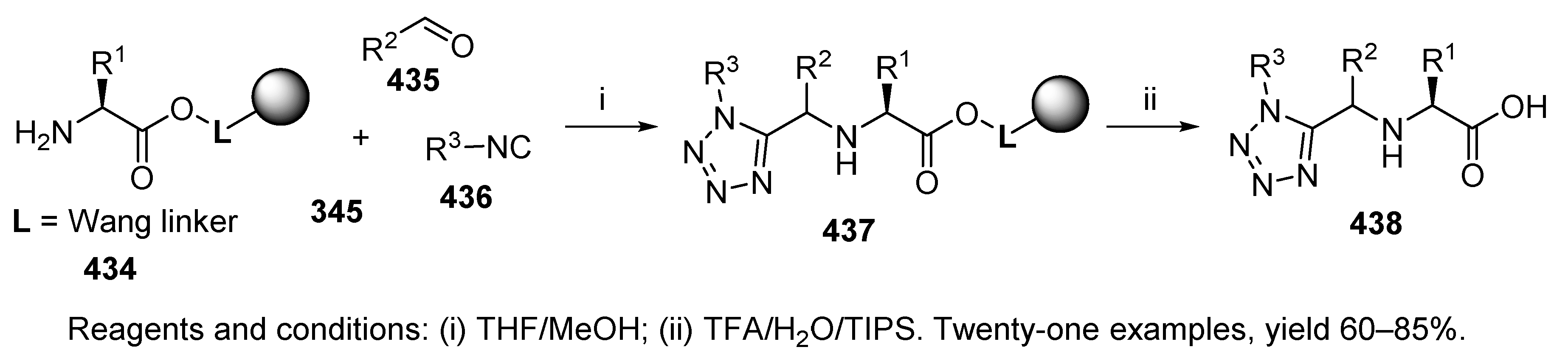
9.2.4. Azide
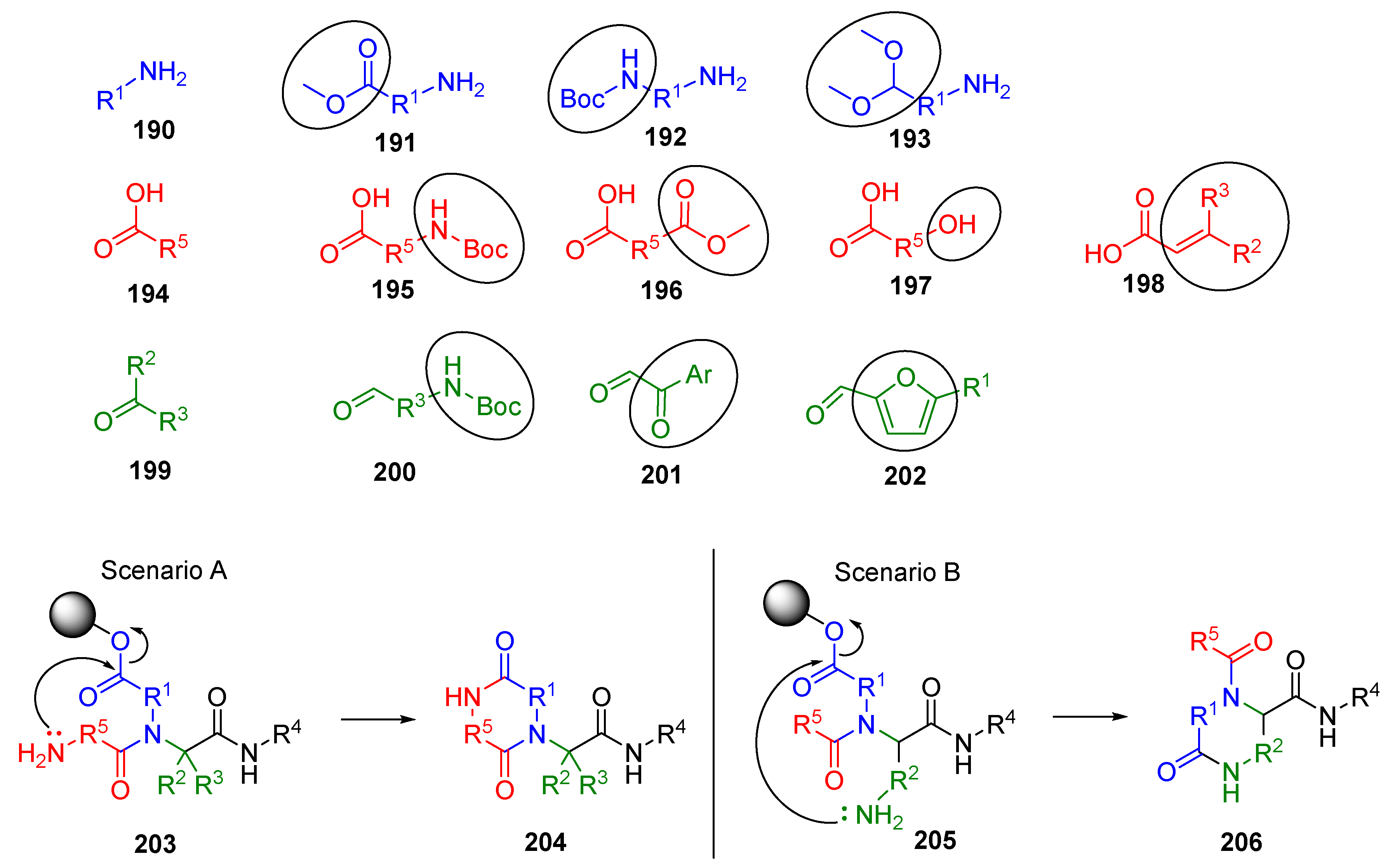
9.3. U-4CR Using Preformed Schiff Base
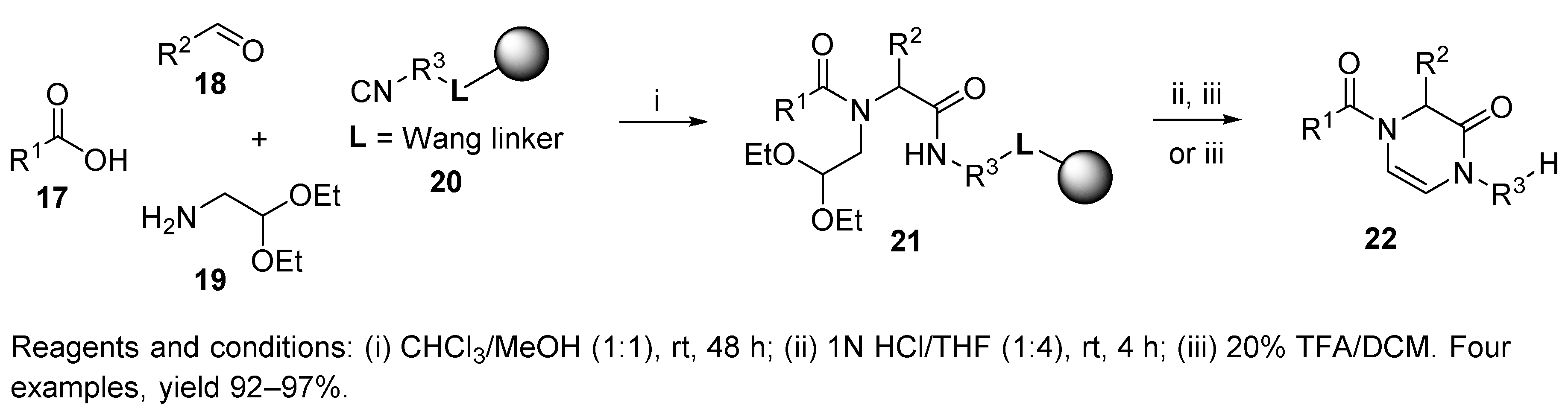
10. Other IMCRs
10.1. Passerini Three-Component Reaction (P-3CR)
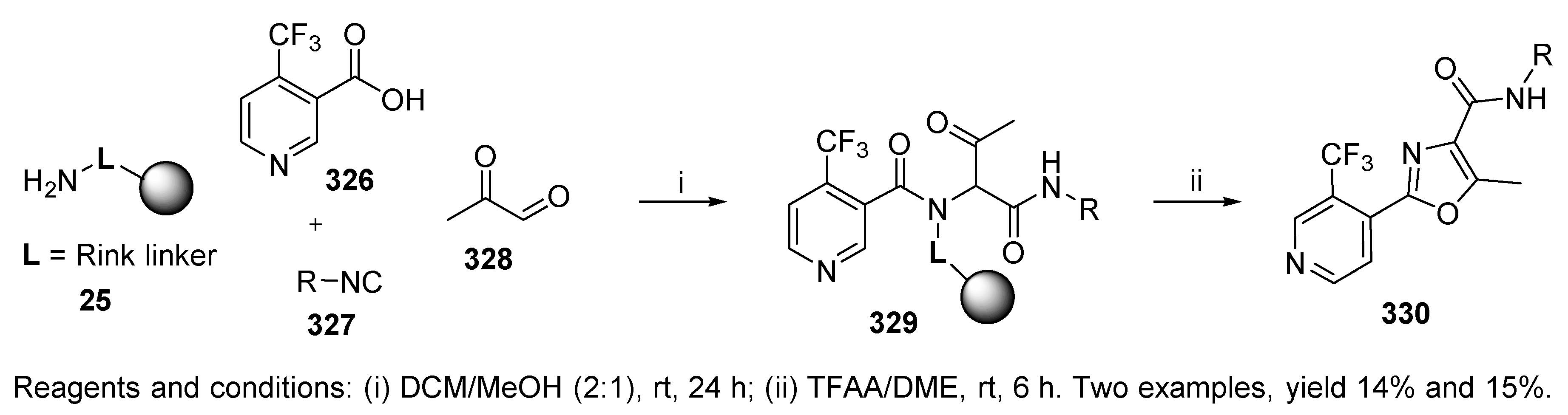
10.2. Groebke-Blackburn-Bienaymé Three-Component Reaction (GBB-3CR)
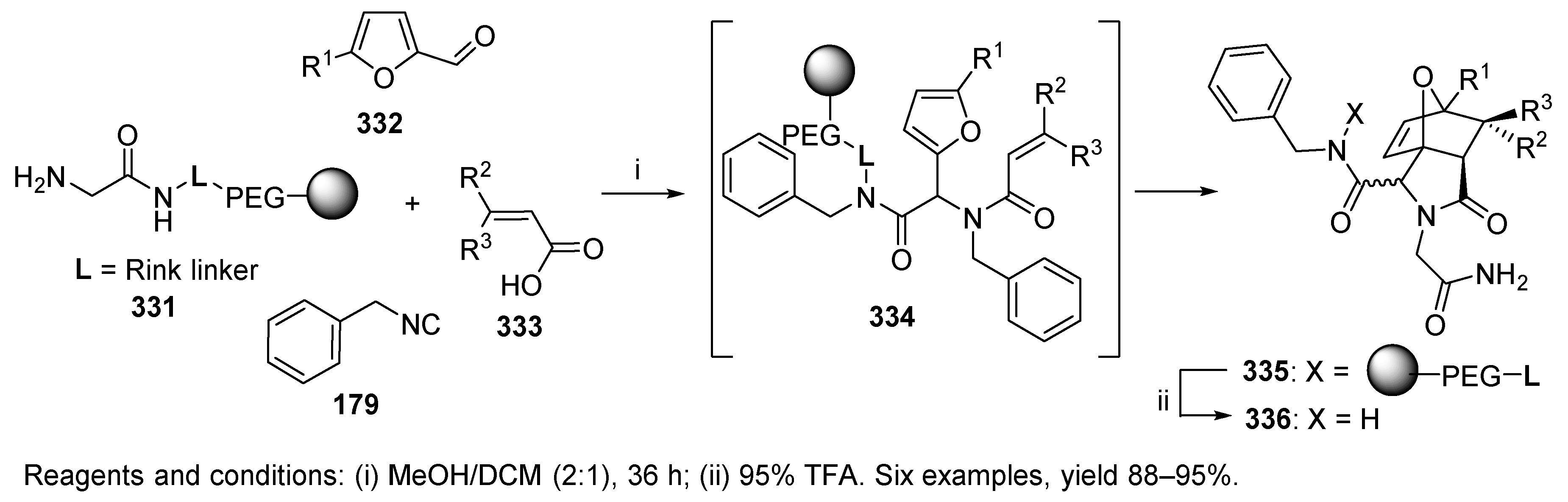
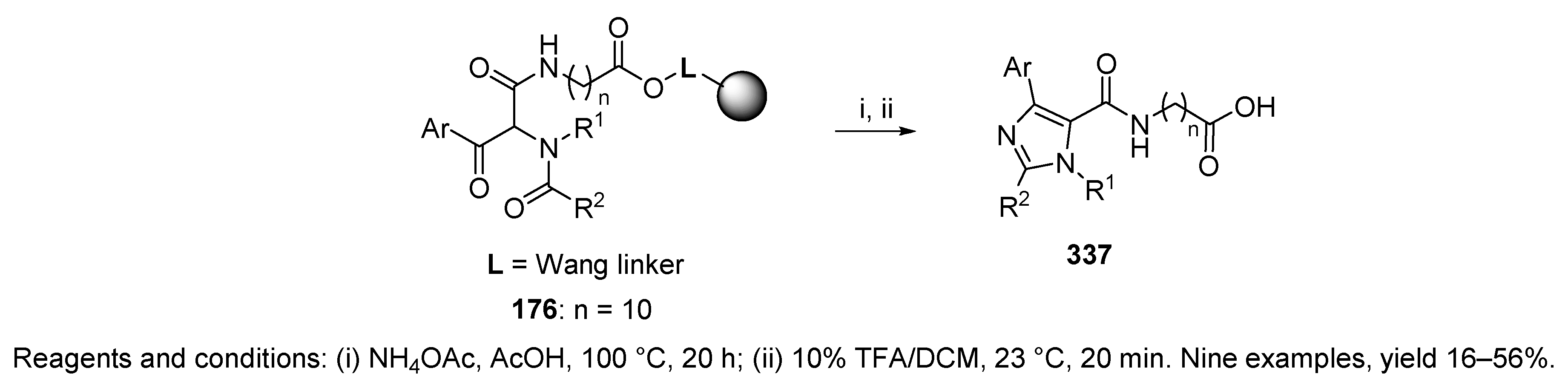
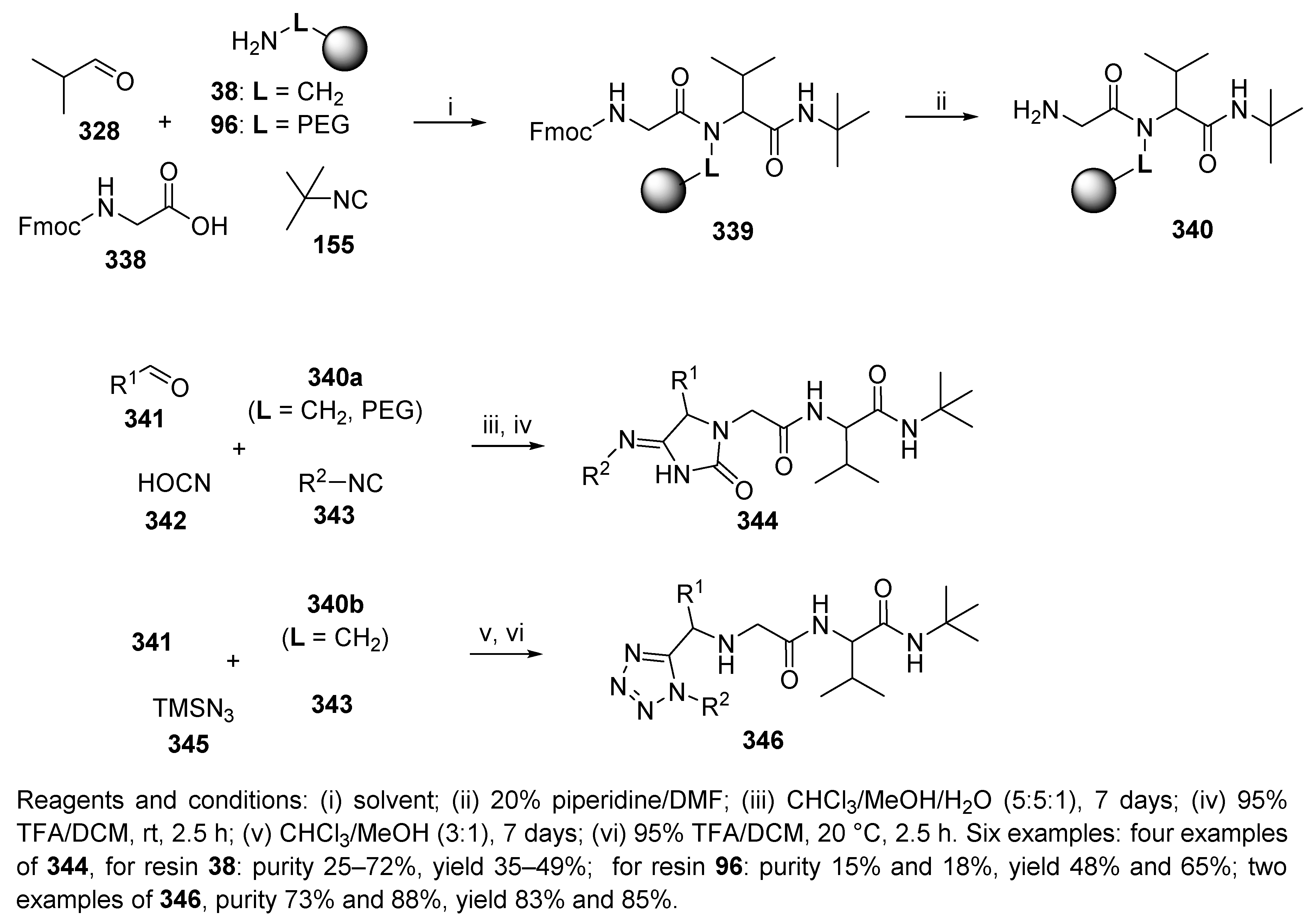
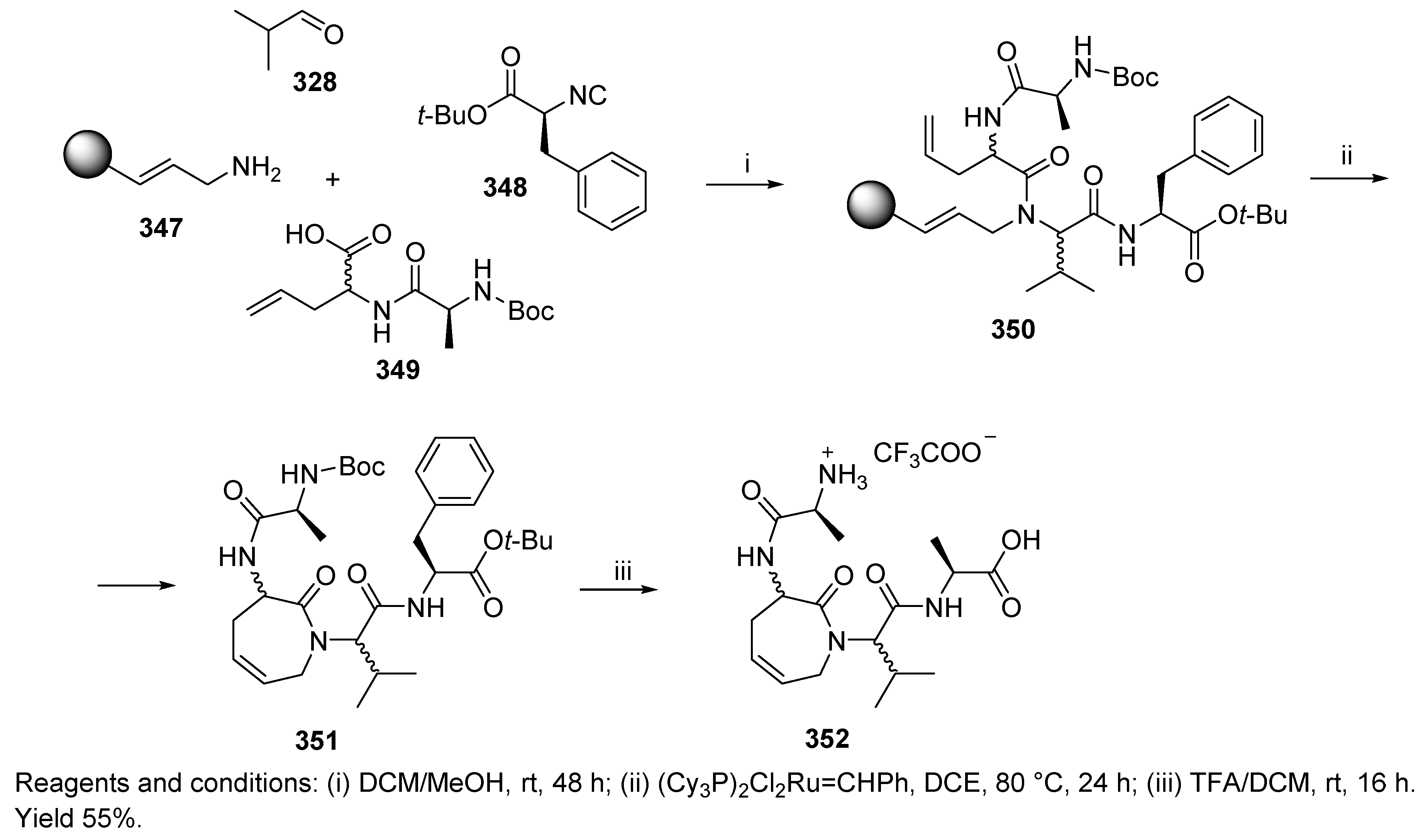
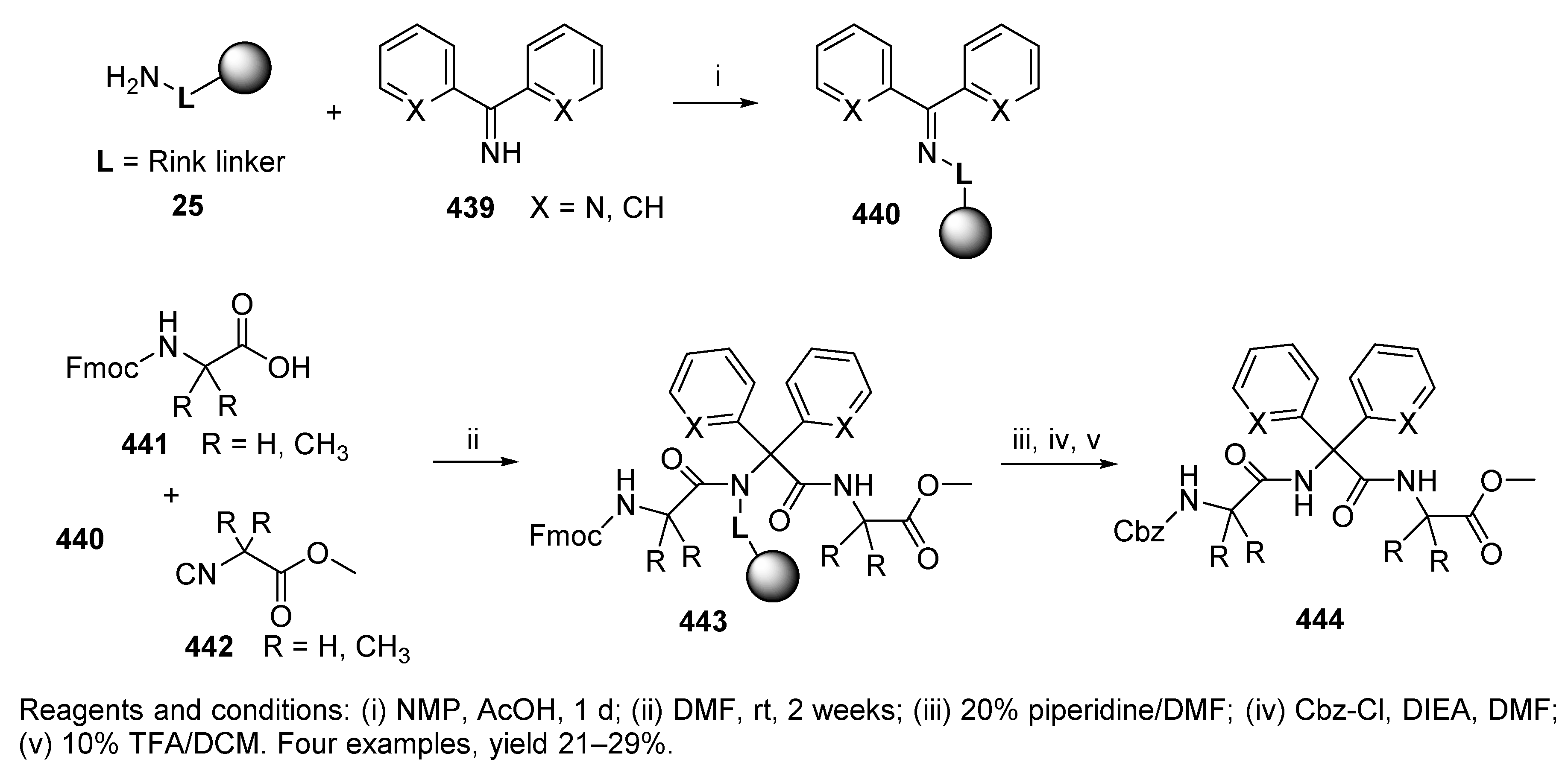
10.3. Miscellaneous
11. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulme, C.; Ma, L.; Cherrier, M.P.; Romano, J.J.; Morton, G.; Duquenne, C.; Salvino, J.; Labaudiniere, R. Novel applications of convertible isonitriles for the synthesis of mono and bicyclic γ-lactams via a UDC strategy. Tetrahedron Lett. 2000, 41, 1883–1887. [Google Scholar] [CrossRef]
- Kim, S.W.; Bauer, S.M.; Armstrong, R.W. Construction of combinatorial chemical libraries using a rapid and efficient solid phase synthesis based on a multicomponent condensation reaction. Tetrahedron Lett. 1998, 39, 6993–6996. [Google Scholar] [CrossRef]
- Lee, D.; Sello, J.K.; Schreiber, S.L. Pairwise Use of Complexity-Generating Reactions in Diversity-Oriented Organic Synthesis. Org. Lett. 2000, 2, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.; Ma, L.; Kumar, N.V.; Krolikowski, P.H.; Allen, A.C.; Labaudiniere, R. Novel applications of resin bound α-amino acids for the synthesis of benzodiazepines (via Wang resin) and ketopiperazines (via hydroxymethyl resin). Tetrahedron Lett. 2000, 41, 1509–1514. [Google Scholar] [CrossRef]
- Méndez, Y.; De Armas, G.; Pérez, I.; Rojas, T.; Valdés-Tresanco, M.E.; Izquierdo, M.; Alonso del Rivero, M.; Álvarez-Ginarte, Y.M.; Valiente, P.A.; Soto, C.; et al. Discovery of potent and selective inhibitors of the Escherichia coli M1-aminopeptidase via multicomponent solid-phase synthesis of tetrazole-peptidomimetics. Eur. J. Med. Chem. 2019, 163, 481–499. [Google Scholar] [CrossRef]
- Chen, J.J.; Golebiowski, A.; Klopfenstein, S.R.; West, L. The universal Rink-isonitrile resin: Applications in Ugi reactions. Tetrahedron Lett. 2002, 43, 4083–4085. [Google Scholar] [CrossRef]
- Ugi, I. Neuere Methoden der präparativen organischen Chemie IV: Mit Sekundär-Reaktionen gekoppelte α-Additionen von Immonium-Ionen und Anionen an Isonitrile. Angew. Chem. 1962, 74, 9–22. [Google Scholar] [CrossRef]
- Chen, J.J.; Golebiowski, A.; McClenaghan, J.; Klopfenstein, S.R.; West, L. Universal Rink-isonitrile resin: Application for the traceless synthesis of 3-acylamino imidazo[1,2-a]pyridines. Tetrahedron Lett. 2001, 42, 2269–2271. [Google Scholar] [CrossRef]
- Díaz, J.L.; Miguel, M.; Lavilla, R. N-Acylazinium Salts: A New Source of Iminium Ions for Ugi-Type Processes. J. Org. Chem. 2004, 69, 3550–3553. [Google Scholar] [CrossRef]
- Banfi, L.; Basso, A.; Guanti, G.; Riva, R. Passerini reaction—Amine Deprotection—Acyl Migration (PADAM): A convenient strategy for the solid-phase preparation of peptidomimetic compounds. Mol. Divers. 2003, 6, 227–235. [Google Scholar] [CrossRef]
- Basso, A.; Banfi, L.; Riva, R.; Piaggio, P.; Guanti, G. Solid-phase synthesis of modified oligopeptides via Passerini multicomponent reaction. Tetrahedron Lett. 2003, 44, 2367–2370. [Google Scholar] [CrossRef]
- Touré, B.B.; Hall, D.G. Natural Product Synthesis Using Multicomponent Reaction Strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Lawrenson, S.; North, M.; Peigneguy, F.; Routledge, A. Greener solvents for solid-phase synthesis. Green Chem. 2017, 19, 952–962. [Google Scholar] [CrossRef] [Green Version]
- Reguera, L.; Méndez, Y.; Humpierre, A.R.; Valdés, O.; Rivera, D.G. Multicomponent Reactions in Ligation and Bioconjugation Chemistry. Acc. Chem. Res. 2018, 51, 1475–1486. [Google Scholar] [CrossRef]
- Reguera, L.; Rivera, D.G. Multicomponent Reaction Toolbox for Peptide Macrocyclization and Stapling. Chem. Rev. 2019, 119, 9836–9860. [Google Scholar] [CrossRef]
- Kunig, V.B.K.; Ehrt, C.; Dömling, A.; Brunschweiger, A. Isocyanide Multicomponent Reactions on Solid-Phase-Coupled DNA Oligonucleotides for Encoded Library Synthesis. Org. Lett. 2019, 21, 7238–7243. [Google Scholar] [CrossRef]
- Oertel, K.; Zech, G.; Kunz, H. Stereoselective Combinatorial Ugi-Multicomponent Synthesis on Solid Phase. Angew. Chem. Int. Ed. 2000, 39, 1431–1433. [Google Scholar] [CrossRef]
- Zech, G.; Kunz, H. Synthesis of a Polymer-Bound Galactosylamine and Its Application as an Immobilized Chiral Auxiliary in Stereoselective Syntheses of Piperidine and Amino Acid Derivatives. Chem. Eur. J. 2004, 10, 4136–4149. [Google Scholar] [CrossRef]
- Arcadia, C.E.; Kennedy, E.; Geiser, J.; Dombroski, A.; Oakley, K.; Chen, S.L.; Sprague, L.; Ozmen, M.; Sello, J.; Weber, P.M.; et al. Multicomponent molecular memory. Nat. Commun. 2020, 11, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugi, I. Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 2001, 73, 187–191. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Lieke, W. Ueber das Cyanallyl. Justus Liebigs Ann. Chem. 1859, 112, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Gautier, A. Ueber die Einwirkung des Chlorwasserstoffs u. a. auf das Aethyl- und Methylcyanur. Justus Liebigs Ann. Chem. 1867, 142, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Ugi, I.; Dömling, A.; Gruber, B.; Almstetter, M. Multicomponent reactions and their libraries—A New approach to preparative organic chemistry. Croat. Chem. Acta 1997, 70, 631–647. [Google Scholar]
- Ugi, I. The α-Addition of Immonium Ions and Anions to Isonitriles Accompanied by Secondary Reactions. Angew. Chem. Int. Ed. Engl. 1962, 1, 8–21. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versuche mit Isonitrilen. Angew. Chem. 1959, 71, 386. [Google Scholar]
- Arshady, R.; Ugi, I. Solid Phase Peptide Synthesis by Four Component Condensation: Peptide Formation on an Isocyano Polymer Support. Z. Für Nat. B 1981, 36, 1202–1203. [Google Scholar] [CrossRef] [Green Version]
- Bienaymé, H.; Bouzid, K. A New Heterocyclic Multicomponent Reaction for the Combinatorial Synthesis of Fused 3-Aminoimidazoles. Angew. Chem. Int. Ed. 1998, 37, 2234–2237. [Google Scholar] [CrossRef]
- Blackburn, C.; Guan, B.; Fleming, P.; Shiosaki, K.; Tsai, S. Parallel synthesis of 3-aminoimidazo[1,2–a]pyridines and pyrazines by a new three-component condensation. Tetrahedron Lett. 1998, 39, 3635–3638. [Google Scholar] [CrossRef]
- Groebke, K.; Weber, L.; Mehlin, F. Synthesis of Imidazo[1,2-a] Annulated Pyridines, Pyrazines, and Pyrimidines by a Novel Three-Component Condensation. Synlett 1998, 6, 661–663. [Google Scholar] [CrossRef]
- La Venia, A.; Lemrová, B.; Krchňák, V. Regioselective Incorporation of Backbone Constraints Compatible with Traditional Solid-Phase Peptide Synthesis. ACS Comb. Sci. 2013, 15, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.F.; Chen, M.; Arrhenius, T.; Nadzan, A. A convenient solution and solid-phase synthesis of Δ5-2-oxopiperazines via N-acyliminium ions cyclization. Tetrahedron Lett. 2002, 43, 6293–6295. [Google Scholar] [CrossRef]
- Tempest, P.A.; Brown, S.D.; Armstrong, R.W. Solid-Phase, Parallel Syntheses by Ugi Multicomponent Condensation. Angew. Chem. Int. Ed. Engl. 1996, 35, 640–642. [Google Scholar] [CrossRef]
- Golebiowski, A.; Jozwik, J.; Klopfenstein, S.R.; Colson, A.O.; Grieb, A.L.; Russell, A.F.; Rastogi, V.L.; Diven, C.F.; Portlock, D.E.; Chen, J.J. Solid-Supported Synthesis of Putative Peptide β-Turn Mimetics via Ugi Reaction for Diketopiperazine Formation. J. Comb. Chem. 2002, 4, 584–590. [Google Scholar] [CrossRef]
- Hoel, A.M.L.; Nielsen, J. Microwave-assisted solid-phase Ugi four-component condensations. Tetrahedron Lett. 1999, 40, 3941–3944. [Google Scholar] [CrossRef]
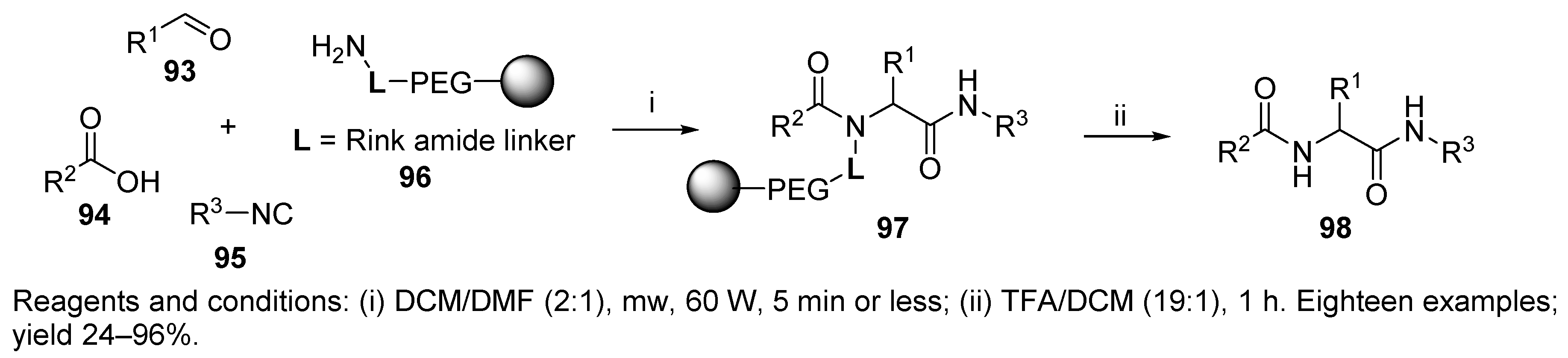
- Lin, Q.; O’Neil, J.C.; Blackwell, H.E. Small Molecule Macroarray Construction via Ugi Four-Component Reactions. Org. Lett. 2005, 7, 4455–4458. [Google Scholar] [CrossRef]
- Henkel, B.; Weber, L. A Novel Four-Component Synthesis of N-Substituted Amino Acid Esters. Synlett 2002, 1877–1879. [Google Scholar] [CrossRef]
- Obrecht, D.; Abrecht, C.; Grieder, A.; Villalgordo, J.M. A Novel and Efficient Approach for the Combinatorial Synthesis of Structurally Diverse Pyrimidines on solid support. Helv. Chim. Acta 1997, 80, 65–72. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.; Song, Q. Synthesis of β-Aminoenones via Cross-Coupling of In-Situ-Generated Isocyanides with 1,3-Dicarbonyl Compounds. Org. Lett. 2018, 20, 4777–4781. [Google Scholar] [CrossRef] [PubMed]
- El Kaim, L.; Grimaud, L.; Schiltz, A. “Isocyanide-free” Ugi reactions. Org. Biomol. Chem. 2009, 7, 3024–3026. [Google Scholar] [CrossRef]
- Campian, E.; Lou, B.; Saneii, H. Solid-phase synthesis of α-sulfonylamino amide derivatives based on Ugi-type condensation reaction using sulfonamides as amine input. Tetrahedron Lett. 2002, 43, 8467–8470. [Google Scholar] [CrossRef]
- Hanyu, M.; Murashima, T.; Miyazawa, T.; Yamada, T. Synthesis of tripeptides containing a very crowded α-,α-disubstituted glycine with pyridine rings by solid-phase Ugi reaction. Tetrahedron Lett. 2004, 45, 8871–8874. [Google Scholar] [CrossRef]
- Habashita, H.; Kokubo, M.; Hamano, S.I.; Hamanaka, N.; Toda, M.; Shibayama, S.; Tada, H.; Sagawa, K.; Fukushima, D.; Maeda, K.; et al. Design, Synthesis, and Biological Evaluation of the Combinatorial Library with a New Spirodiketopiperazine Scaffold. Discovery of Novel Potent and Selective Low-Molecular-Weight CCR5 Antagonists. J. Med. Chem. 2006, 49, 4140–4152. [Google Scholar] [CrossRef]
- Szardenings, A.K.; Burkoth, T.S.; Lu, H.H.; Tien, D.W.; Campbell, D.A. A simple procedure for the solid phase synthesis of diketopiperazine and diketomorpholine derivatives. Tetrahedron 1997, 53, 6573–6593. [Google Scholar] [CrossRef]
- Liu, Z.; Nefzi, A. Solid-Phase Synthesis of N-Substituted Pyrrolidinone-Tethered N-Substituted Piperidines via Ugi Reaction. J. Comb. Chem. 2010, 12, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Nefzi, A. Ugi four-center three-component reaction for the parallel solid-phase synthesis of N-substituted thiomopholinones. Tetrahedron Lett. 2012, 53, 1013–1014. [Google Scholar] [CrossRef]
- Aguiam, N.R.; Castro, V.I.; Ribeiro, A.I.F.; Fernandes, R.D.V.; Carvalho, C.M.; Costa, S.P.G.; Pereira-Lima, S.M.M.A. α-,α-Dialkylglycines obtained by solid phase Ugi reaction performed over isocyanide functionalized resins. Tetrahedron 2013, 69, 9161–9165. [Google Scholar] [CrossRef]
- Zhang, C.; Moran, E.J.; Woiwode, T.F.; Short, K.M.; Mjalli, A.M.M. Synthesis of tetrasubstituted imidazoles via α-(N-acyl-N-alkylamino)-β-ketoamides on Wang resin. Tetrahedron Lett. 1996, 37, 751–754. [Google Scholar] [CrossRef]
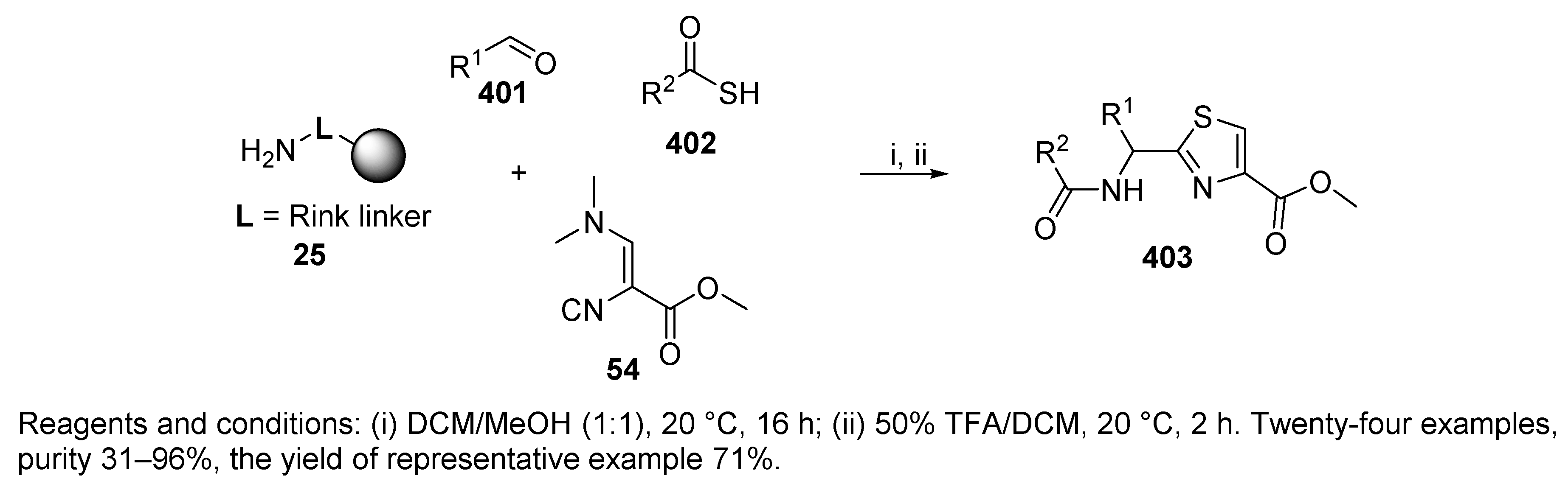
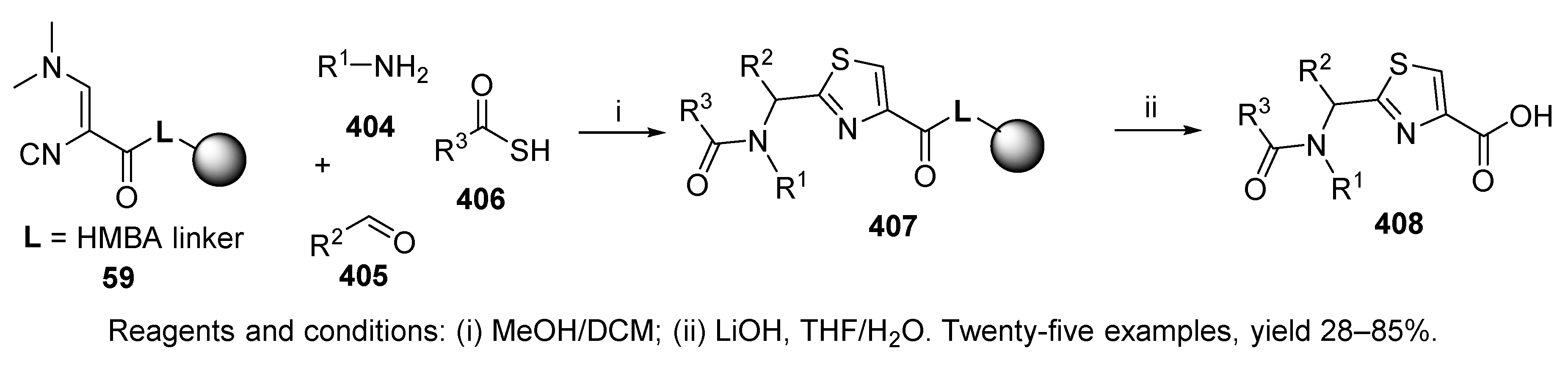
- Henkel, B.; Sax, M.; Dömling, A. A new and efficient multicomponent solid-phase synthesis of 2-acylaminomethylthiazoles. Tetrahedron Lett. 2003, 44, 3679–3682. [Google Scholar] [CrossRef]
- Henkel, B.; Westner, B.; Dömling, A. Polymer-Bound 3-N,N-(Dimethylamino)-2-isocyanoacrylate for the Synthesis of Thiazoles via a Multicomponent Reaction. Synlett 2003, 2410–2412. [Google Scholar] [CrossRef]
- Short, K.M.; Ching, B.W.; Mjalli, A.M.M. The synthesis of hydantoin 4-imides on solid support. Tetrahedron Lett. 1996, 37, 7489–7492. [Google Scholar] [CrossRef]
- Short, K.M.; Ching, B.W.; Mjalli, A.M.M. Exploitation of the Ugi 4CC reaction: Preparation of small molecule combinatorial libraries via solid phase. Tetrahedron 1997, 53, 6653–6679. [Google Scholar] [CrossRef]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Piscopio, A.D.; Miller, J.F.; Koch, K. Ring closing metathesis in organic synthesis: Evolution of a high speed, solid phase method for the preparation of β-turn mimetics. Tetrahedron 1999, 55, 8189–8198. [Google Scholar] [CrossRef]
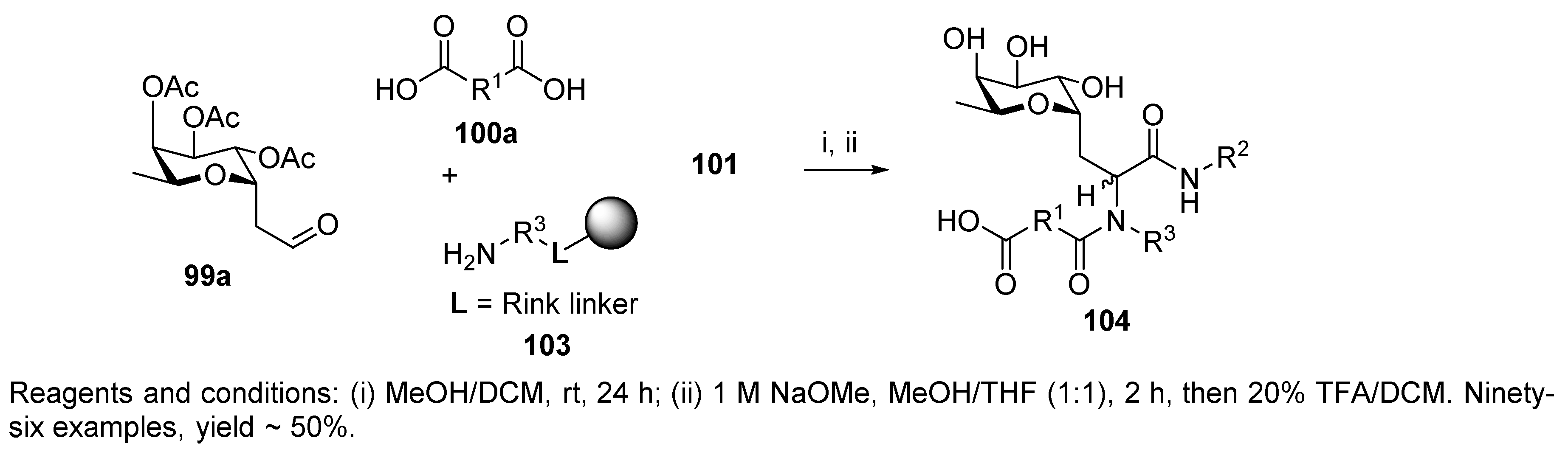
- Sutherlin, D.P.; Stark, T.M.; Hughes, R.; Armstrong, R.W. Generation of C-Glycoside Peptide Ligands for Cell Surface Carbohydrate Receptors Using a Four-Component Condensation on Solid Support. J. Org. Chem. 1996, 61, 8350–8354. [Google Scholar] [CrossRef]
- Portlock, D.E.; Naskar, D.; West, L.; Ostaszewski, R.; Chen, J.J. Solid-phase synthesis of five-dimensional libraries via a tandem Petasis-Ugi multi-component condensation reaction. Tetrahedron Lett. 2003, 44, 5121–5124. [Google Scholar] [CrossRef]
- Golebiowski, A.; Klopfenstein, S.R.; Shao, X.; Chen, J.J.; Colson, A.O.; Grieb, A.L.; Russell, A.F. Solid-Supported Synthesis of a Peptide β-Turn Mimetic. Org. Lett. 2000, 2, 2615–2617. [Google Scholar] [CrossRef]
- Constabel, F.; Ugi, I. Repetitive Ugi reactions. Tetrahedron 2001, 57, 5785–5789. [Google Scholar] [CrossRef]
- Lin, Q.; Blackwell, H.E. Rapid synthesis of diketopiperazine macroarrays via Ugi four-component reactions on planar solid supports. Chem. Commun. 2006, 2884–2886. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Blackwell, H.E. Efficient Construction of Diketopiperazine Macroarrays through a Cyclative-Cleavage Strategy and Their Evaluation as Luminescence Inhibitors in the Bacterial Symbiont Vibrio fischeri. J. Comb. Chem. 2009, 11, 1094–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcaccini, S.; Torroba, T. The use of the Ugi four-component condensation. Nat. Protoc. 2007, 2, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Banfi, L.; Guanti, G.; Riva, R.; Basso, A. Multicomponent Reactions in Solid-Phase Synthesis. Curr. Opin. Drug Discov. Dev. 2007, 10, 704–714. [Google Scholar]
- Ramón, D.J.; Yus, M. Asymmetric Multicomponent Reactions (AMCRs): The New Frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar] [CrossRef]
- Hlaváč, J.; Soural, M.; Krchňák, V. Practical Aspects of Combinatorial Solid-Phase Synthesis. In Solid-Phase Organic Synthesis; Wiley: Hoboken, NJ, USA, 2011; pp. 95–130. [Google Scholar] [CrossRef]
- Wang, S.-S. p-Alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J. Am. Chem. Soc. 1973, 95, 1328–1333. [Google Scholar] [CrossRef]
- Rink, H. Solid-phase synthesis of protected peptide fragments using a trialkoxy-diphenyl-methylester resin. Tetrahedron Lett. 1987, 28, 3787–3790. [Google Scholar] [CrossRef]
- Xiaodong, C.; Moran, E.J.; Siev, D.; Lio, A.; Ohashi, C.; Mjalli, A.M.M. Synthesis of NH-acyl-α-aminoamides on Rink resin: Inhibitors of the hematopoietic protein tyrosine phosphatase (HePTP). Bioorg. Med. Chem. Lett. 1995, 5, 2953–2958. [Google Scholar] [CrossRef]
- Pulici, M.; Quartieri, F.; Felder, E.R. Trifluoroacetic Anhydride-Mediated Solid-Phase Version of the Robinson-Gabriel Synthesis of Oxazoles. J. Comb. Chem. 2005, 7, 463–473. [Google Scholar] [CrossRef]
- Li, Z.; Yeo, S.L.; Pallen, C.J.; Ganesan, A. Solid-phase synthesis of potential protein tyrosine phosphatase inhibitors via the Ugi four-component condensation. Bioorg. Med. Chem. Lett. 1998, 8, 2443–2446. [Google Scholar] [CrossRef]
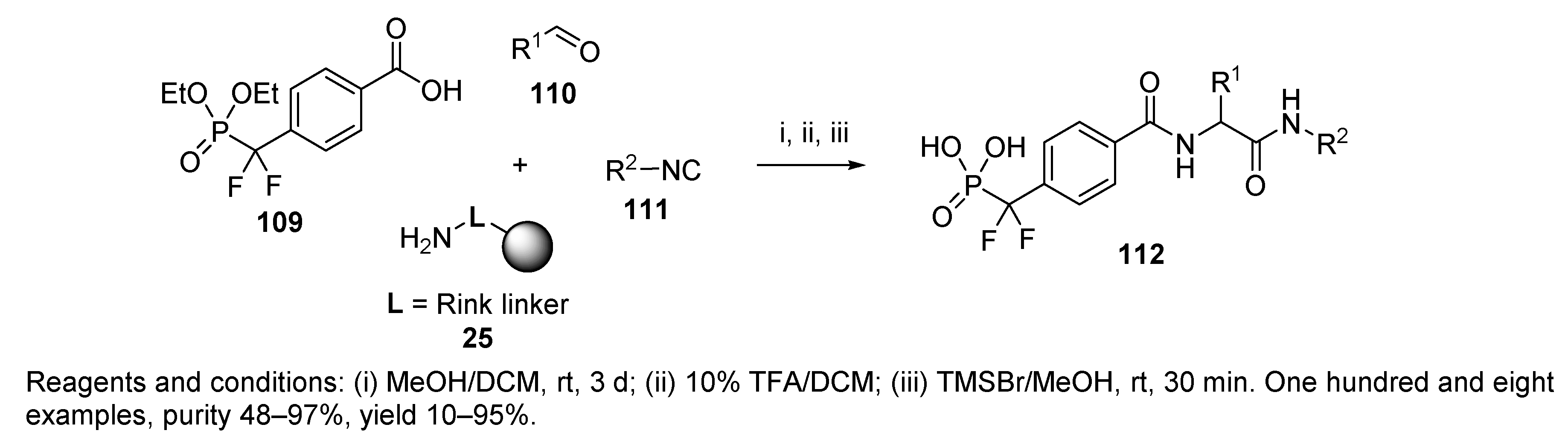
- Sun, D.; Lee, R.E. Solid-Phase Synthesis Development of a Thymidinyl and 2′-Deoxyuridinyl Ugi Library for Anti-bacterial Agent Screening. Tetrahedron Lett. 2005, 46, 8497–8501. [Google Scholar] [CrossRef]
- Henkel, B.; Sax, M.; Dömling, A. New method for the preparation of solid-phase bound isocyanocarboxylic acids and Ugi reactions therewith. Tetrahedron Lett. 2003, 44, 7015–7018. [Google Scholar] [CrossRef]
- Arshady, R.; Ugi, I. Synthesis and characterization of polymer supports carrying isocyano groups. Polymer 1990, 31, 1164–1169. [Google Scholar] [CrossRef]
- Ugi, I.; Rosendahl, F.K.; Isonitrile, X.V. Δ1-Cyclohexenyl-isocyanid. Justus Liebigs Ann. Chem. 1963, 666, 65–67. [Google Scholar] [CrossRef]
- Keating, T.A.; Armstrong, R.W. Molecular Diversity via a Convertible Isocyanide in the Ugi Four-Component Condensation. J. Am. Chem. Soc. 1995, 117, 7842–7843. [Google Scholar] [CrossRef]
- Hulme, C.; Morrissette, M.M.; Volz, F.A.; Burns, C.J. The solution phase synthesis of diketopiperazine libraries via the Ugi reaction: Novel application of Armstrong’s convertible isonitrile. Tetrahedron Lett. 1998, 39, 1113–1116. [Google Scholar] [CrossRef]
- Keating, T.A.; Armstrong, R.W. Postcondensation Modifications of Ugi Four-Component Condensation Products: 1-Isocyanocyclohexene as a Convertible Isocyanide. Mechanism of Conversion, Synthesis of Diverse Structures, and Demonstration of Resin Capture. J. Am. Chem. Soc. 1996, 118, 2574–2583. [Google Scholar] [CrossRef]
- Miller, J.F.; Koch, K.; Piscopio, A.D. Preparation and synthetic application of an immobilized, convertible isonitrile. Abstr. Pap. Am. Chem. Soc. 1997, 214, 232. [Google Scholar]
- Hulme, C.; Peng, J.; Morton, G.; Salvino, J.M.; Herpin, T.; Labaudiniere, R. Novel safety-catch linker and its application with a Ugi/De-BOC/Cyclization (UDC) strategy to access carboxylic acids, 1,4-benzodiazepines, diketopiperazines, ketopiperazines and dihydroquinoxalinones. Tetrahedron Lett. 1998, 39, 7227–7230. [Google Scholar] [CrossRef]
- Kennedy, A.L.; Fryer, A.M.; Josey, J.A. A New Resin-Bound Universal Isonitrile for the Ugi 4CC Reaction: Preparation and Applications to the Synthesis of 2,5-Diketopiperazines and 1,4-Benzodiazepine-2,5-diones. Org. Lett. 2002, 4, 1167–1170. [Google Scholar] [CrossRef]
- Oikawa, M.; Takeda, Y.; Sasaki, M. 2-Oxo-1,2-ethylenedioxy group as a linker for solution-, liquid-, and solid-phase syntheses to discover drug-like small molecules. Tetrahedron Lett. 2005, 46, 4667–4670. [Google Scholar] [CrossRef]
- Strocker, A.M.; Keating, T.A.; Tempest, P.A.; Armstrong, R.W. Use of a convertible isocyanide for generation of Ugi reaction derivatives on solid support: Synthesis of α-acylaminoesters and pyrroles. Tetrahedron Lett. 1996, 37, 1149–1152. [Google Scholar] [CrossRef]
- Chéron, N.; Ramozzi, R.; Kaim, L.E.; Grimaud, L.; Fleurat-Lessard, P. Challenging 50 Years of Established Views on Ugi Reaction: A Theoretical Approach. J. Org. Chem. 2012, 77, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Holden, C.M.; Greaney, M.F. Modern Aspects of the Smiles Rearrangement. Chem. Eur. J. 2017, 23, 8992–9008. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Ugi, I. The Seven-Component Reaction. Angew. Chem. Int. Ed. Engl. 1993, 32, 563–564. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Sarma, K.D. Multicomponent Reactions Are Accelerated in Water. J. Am. Chem. Soc. 2004, 126, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.; Brase, S. Traceless and multifunctional linkers for the generation of small molecules on solid supports. Curr. Opin. Chem. Biol. 2004, 8, 230–237. [Google Scholar] [CrossRef]
- Wiehn, M.S.; Jung, N.; Brase, S. Safety-catch and traceless linkers in solid phase organic synthesis. In Power Functional Resins in Organic Synthesis; Tulla-Puche, J., Albericio, F., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 437–465. [Google Scholar]
- Cankařová, N.; Schütznerová, E.; Krchňák, V. Traceless Solid-Phase Organic Synthesis. Chem. Rev. 2019, 119, 12089–12207. [Google Scholar] [CrossRef]
- Nixey, T.; Tempest, P.; Hulme, C. Two-step solution-phase synthesis of novel quinoxalinones utilizing a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett. 2002, 43, 1637–1639. [Google Scholar] [CrossRef]
- Scott, B.O.; Siegmund, A.C.; Marlowe, C.K.; Pei, Y.; Spear, K.L. Solid phase organic synthesis (SPOS): A novel route to diketopiperazines and diketomorpholines. Mol. Divers. 1996, 1, 125–134. [Google Scholar] [CrossRef]
- Mjalli, A.M.M.; Sarshar, S.; Baiga, T.J. Solid phase synthesis of pyrroles derived from a four component condensation. Tetrahedron Lett. 1996, 37, 2943–2946. [Google Scholar] [CrossRef]
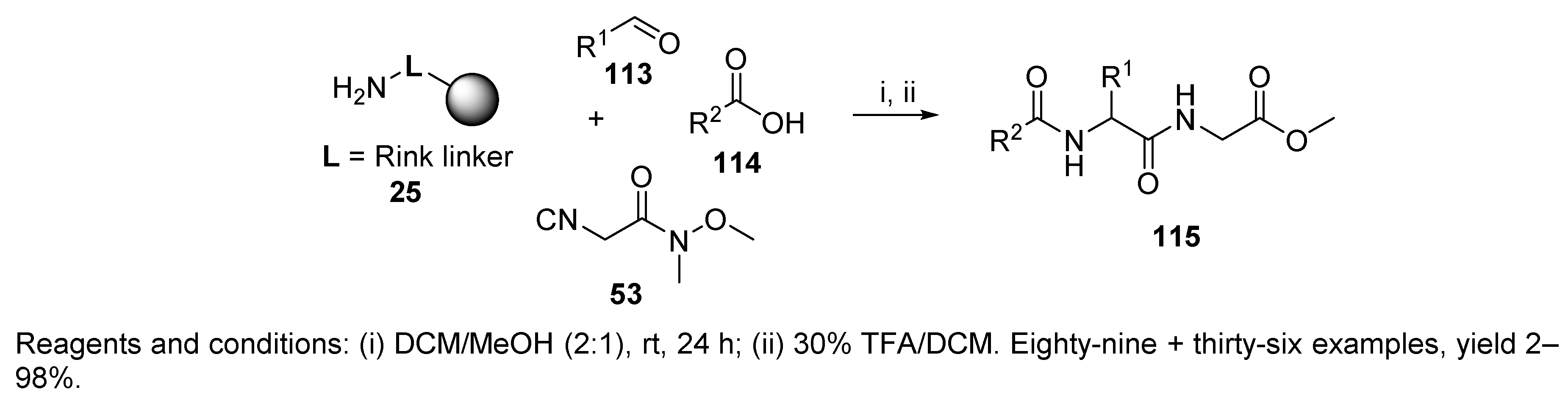
- Nahm, S.; Weinreb, S.M. N-methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 1981, 22, 3815–3818. [Google Scholar] [CrossRef]
- Dinh, T.Q.; Armstrong, R.W. Synthesis of ketones and aldehydes via reactions of Weinreb-type amides on solid support. Tetrahedron Lett. 1996, 37, 1161–1164. [Google Scholar] [CrossRef]
- Paulvannan, K. Preparation of tricyclic nitrogen heterocycles via tandem four-component condensation/intramolecular Diels-Alder reaction. Tetrahedron Lett. 1999, 40, 1851–1854. [Google Scholar] [CrossRef]
- Hulme, C.; Peng, J.; Louridas, B.; Menard, P.; Krolikowski, P.; Kumar, N.V. Applications of N-BOC-diamines for the solution phase synthesis of ketopiperazine libraries utilizing a Ugi/De-BOC/Cyclization (UDC) strategy. Tetrahedron Lett. 1998, 39, 8047–8050. [Google Scholar] [CrossRef]
- Kolb, J.; Beck, B.; Almstetter, M.; Heck, S.; Herdtweck, E.; Dömling, A. New MCRs: The first 4-component reaction leading to 2,4-disubstituted thiazoles. Mol. Divers. 2003, 6, 297–313. [Google Scholar] [CrossRef]
- Heck, S.; Domling, A. A Versatile Multi-Component One-Pot Thiazole Synthesis. Synlett 2000, 424–426. [Google Scholar] [CrossRef]
- Schöllkopf, U.; Porsch, P.H.; Lau, H.H. Synthesen mit α-metallierten Isocyaniden, XLIV. Notiz über β-Dimethylamino-α-isocyanacrylsäureester und ihre Verwendung in der Heterocyclenchemie. Liebigs Ann. Chem 1979, 1444–1446. [Google Scholar] [CrossRef]
- Mckervey, M.A.; O’Sullivan, M.B.; Myers, P.L.; Green, R.H. Reductive acylation of α-keto azides derived from L-amino acids using N-protected L-aminothiocarboxylic S-acids. J. Chem. Soc. Chem. Commun. 1993, 94–96. [Google Scholar] [CrossRef]
- Venkatraman, J.; Shankaramma, S.C.; Balaram, P. Design of Folded Peptides. Chem. Rev. 2001, 101, 3131–3152. [Google Scholar] [CrossRef]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 7007–7049. [Google Scholar] [CrossRef]
- Gunawan, S.; Hulme, C. Construction of functionalized tricyclic dihydropyrazino-quinazolinedione chemotypes via an Ugi/N-acyliminium ion cyclization cascade. Tetrahedron Lett. 2013, 54, 4467–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]






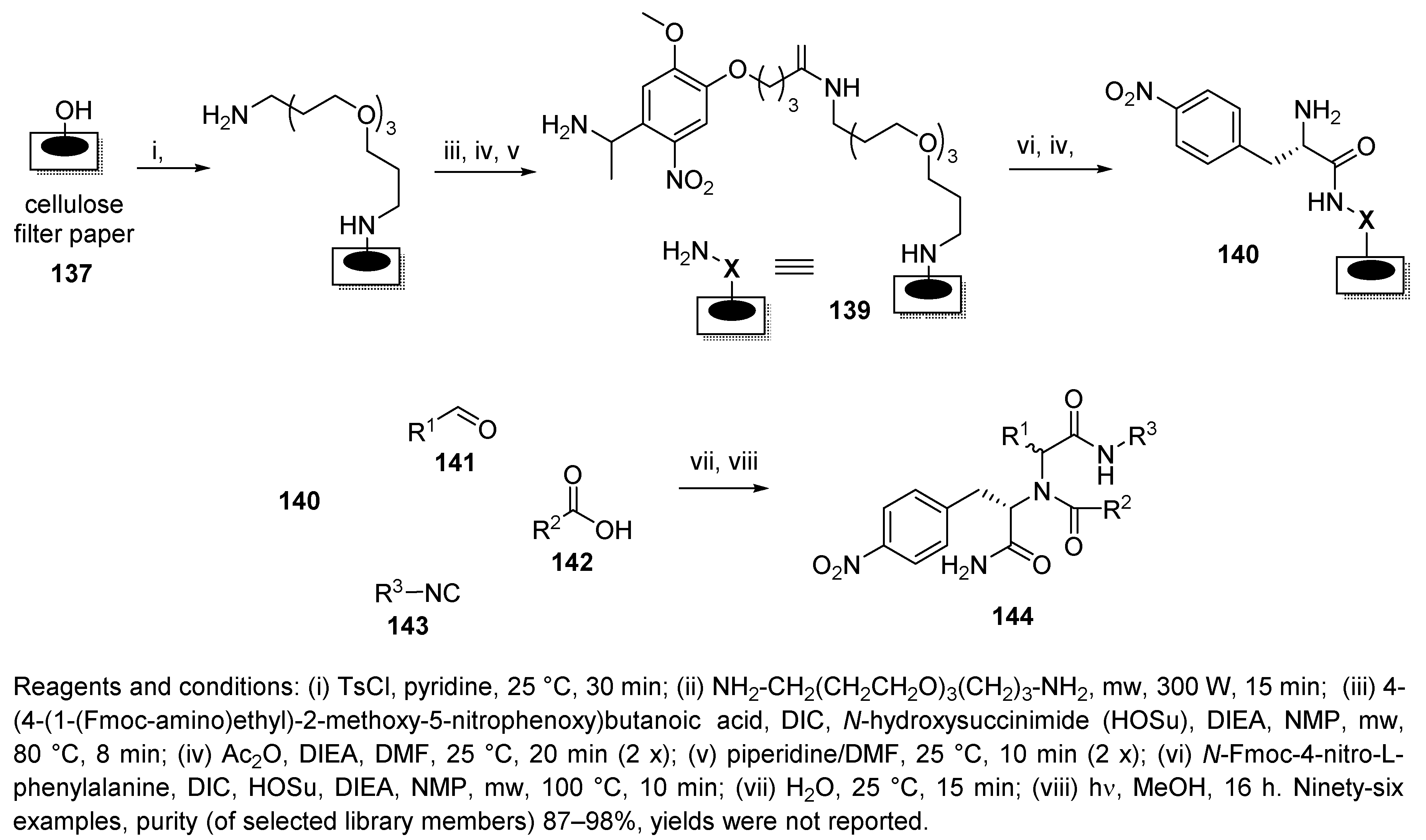
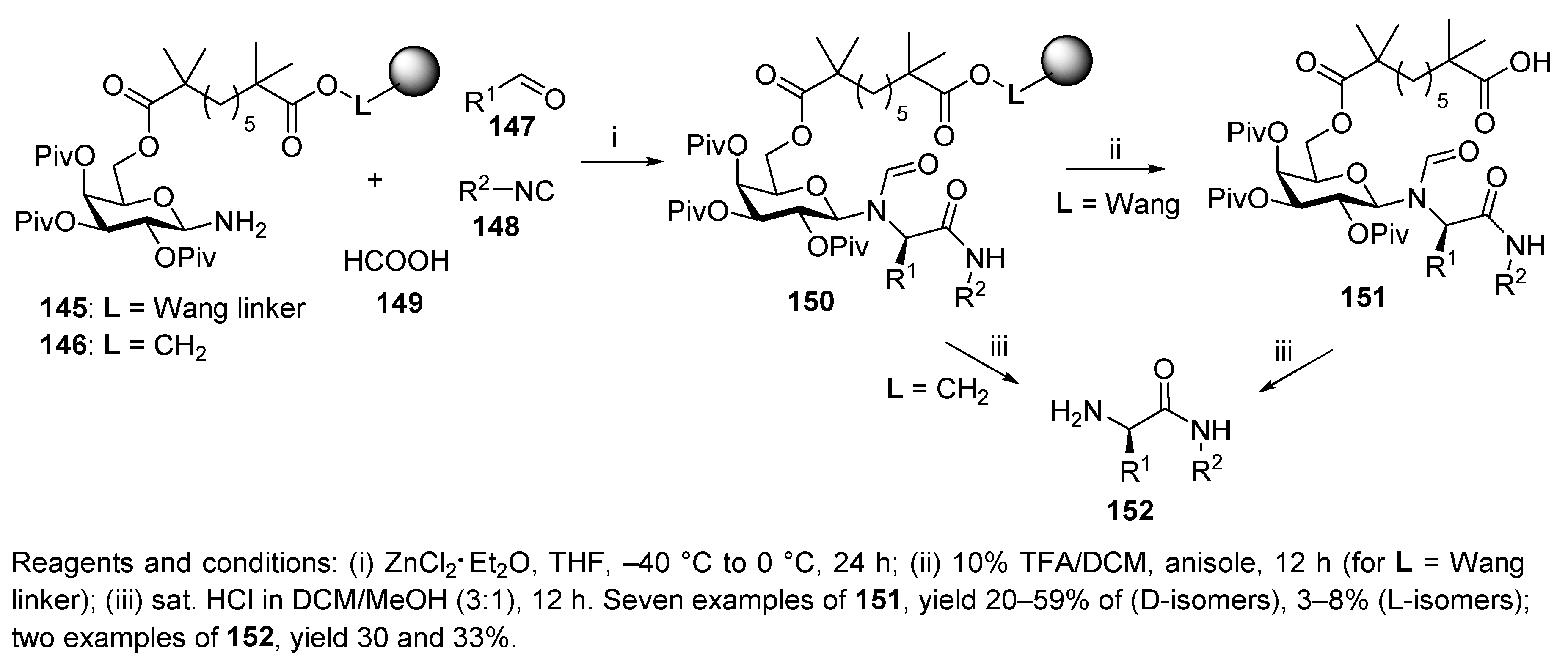
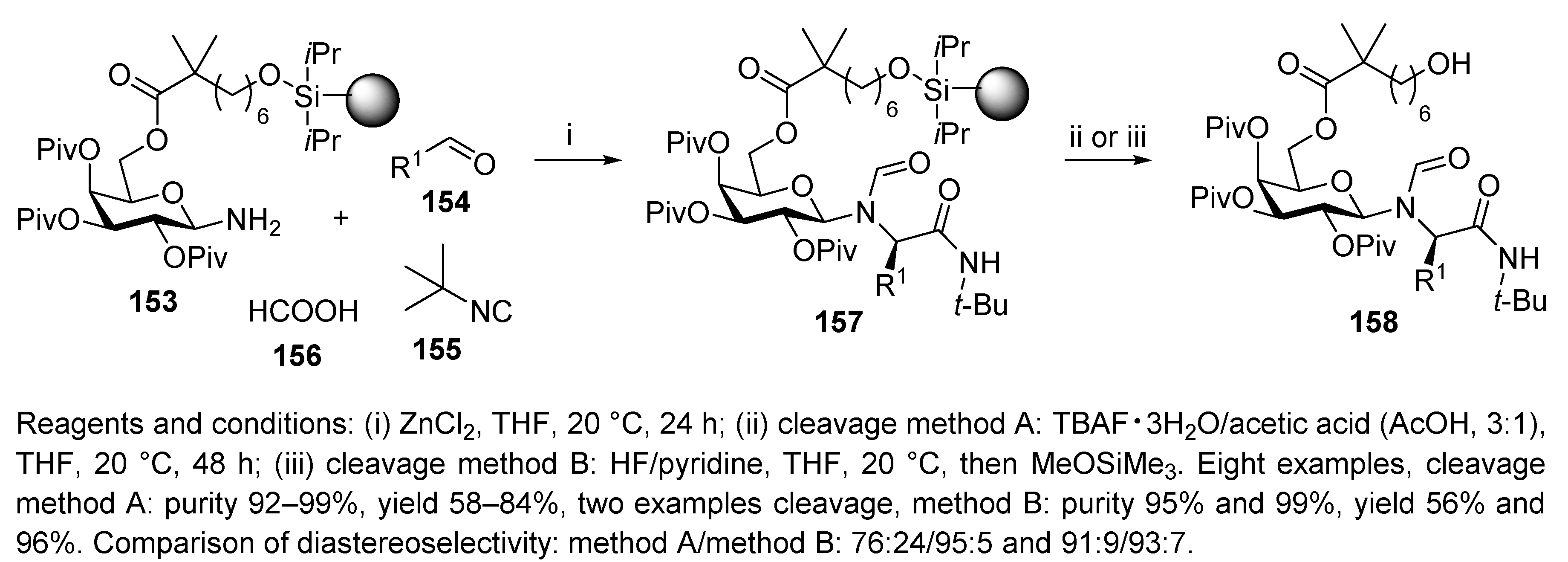

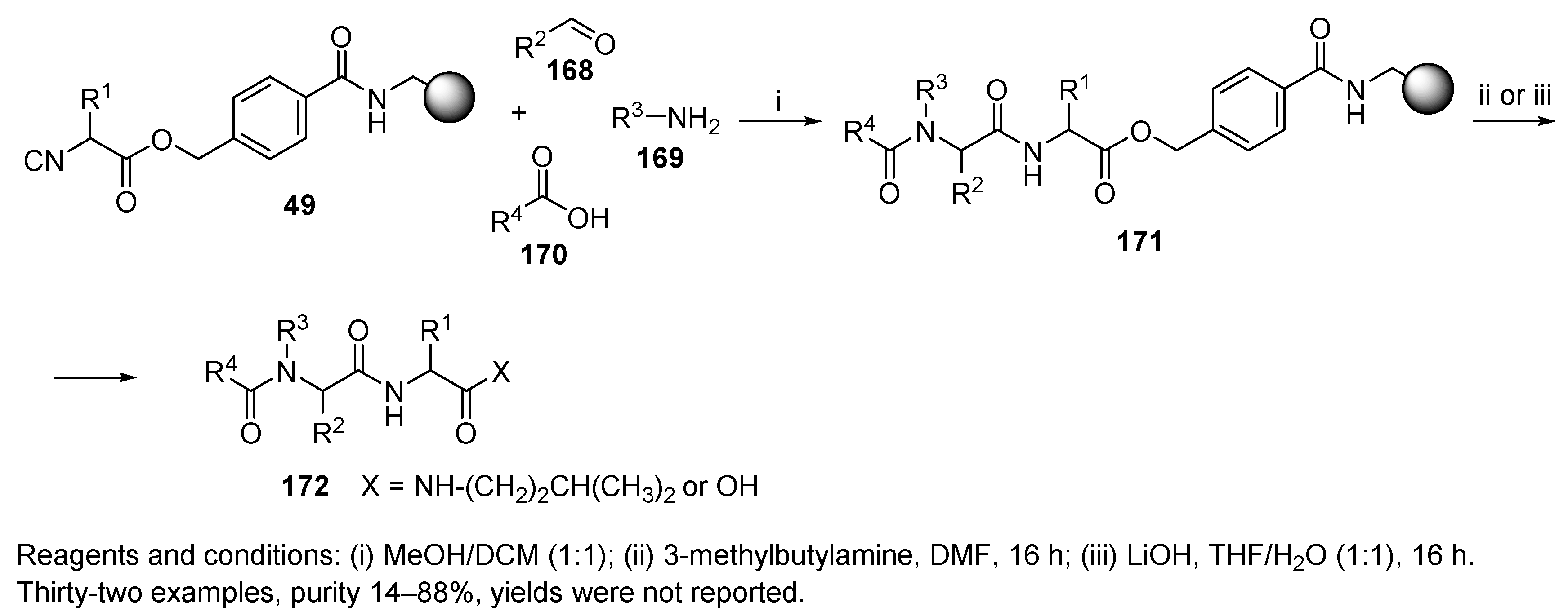









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cankařová, N.; Krchňák, V. Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application. Int. J. Mol. Sci. 2020, 21, 9160. https://doi.org/10.3390/ijms21239160
Cankařová N, Krchňák V. Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application. International Journal of Molecular Sciences. 2020; 21(23):9160. https://doi.org/10.3390/ijms21239160
Chicago/Turabian StyleCankařová, Naděžda, and Viktor Krchňák. 2020. "Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application" International Journal of Molecular Sciences 21, no. 23: 9160. https://doi.org/10.3390/ijms21239160
APA StyleCankařová, N., & Krchňák, V. (2020). Isocyanide Multicomponent Reactions on Solid Phase: State of the Art and Future Application. International Journal of Molecular Sciences, 21(23), 9160. https://doi.org/10.3390/ijms21239160





