Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Organ Weights, Fertility Parameters, Sperm Count, and Sperm Motility
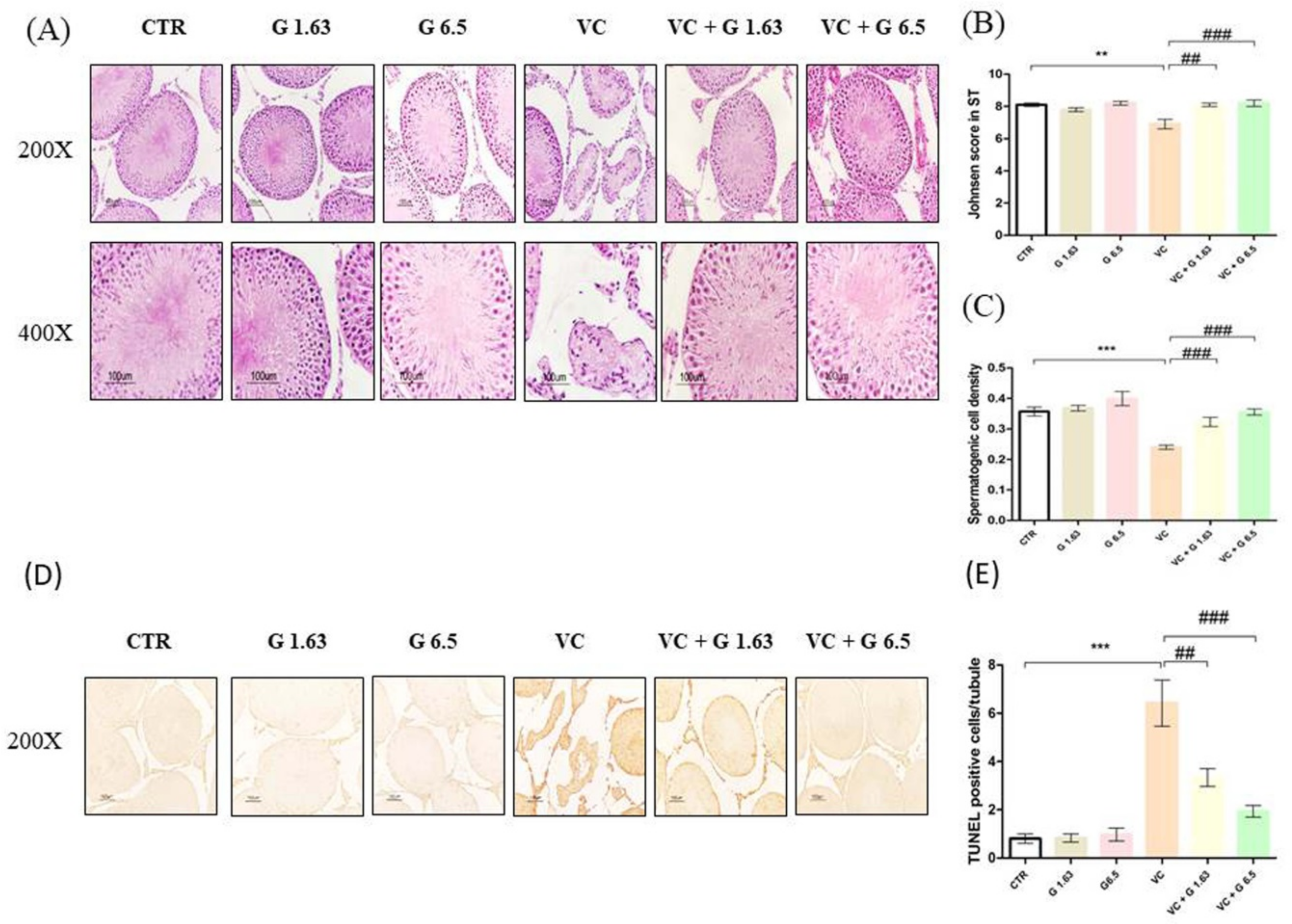
2.2. Gui-A-Gra Treatment Counteracts Damage to Seminiferous Tubules
2.3. Reproductive Hormone Levels and Inflammatory Markers
2.4. Determination of Testicular Lipid Peroxidation and Antioxidant Enzyme Activities
2.5. Western Blot and Immunohistochemistry of Proteins Expressed in Left Testis
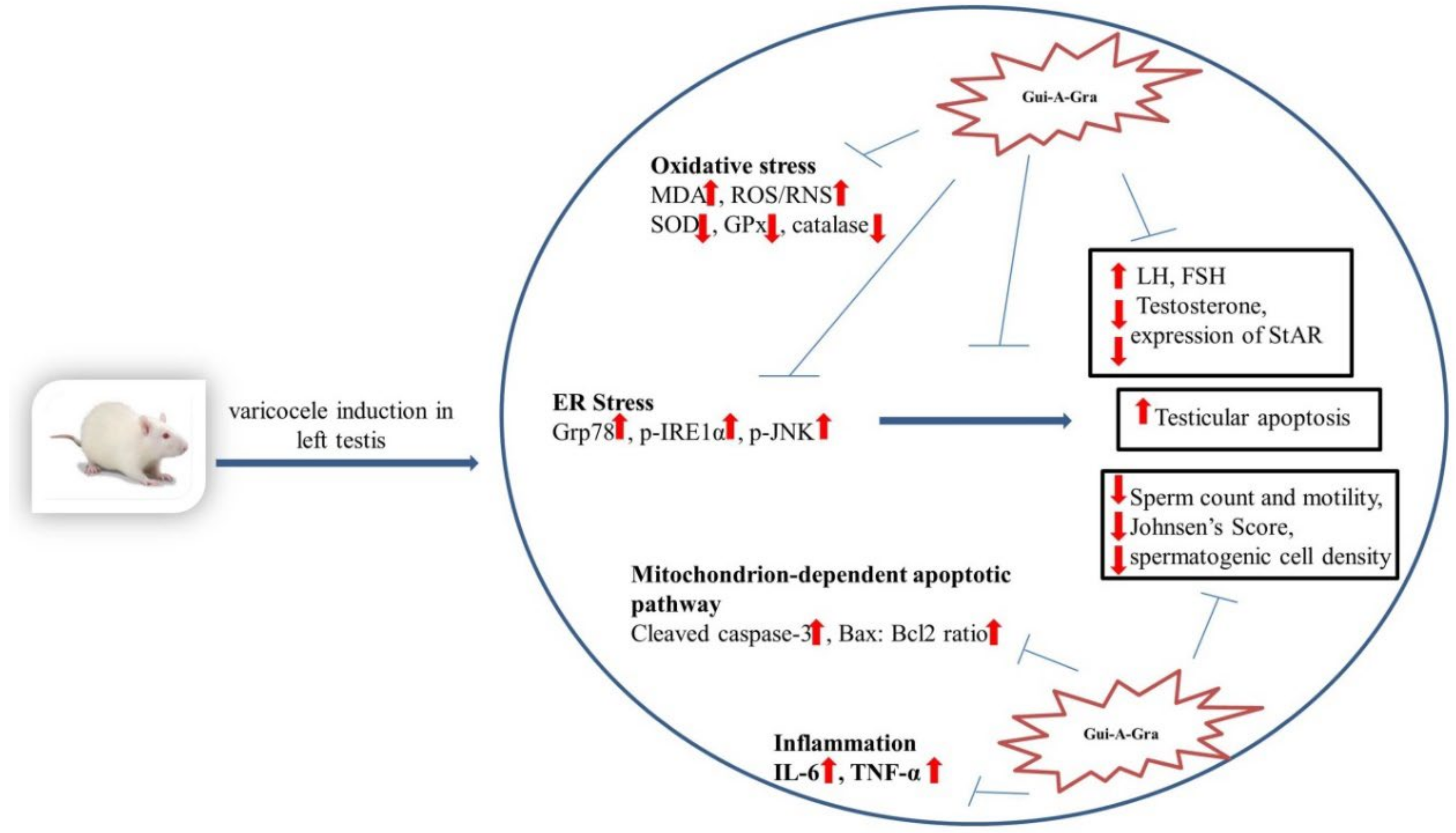
3. Discussion
4. Materials and Methods
4.1. Gui-A-Gra Material and Extract Preparation
4.2. Animal Care and Experimental Design
4.3. Assessment of Sperm Parameters
4.4. Hematoxylin and Eosin (H&E) and Terminal Deoxynucleotidyl Transferase-Mediated (dUTP) Nick-End Labeling (TUNEL) Staining
4.5. Serum Hormonal Assays
4.6. Measurements of Cytokines
4.7. Measurements of Lipid Peroxidation and Antioxidant Enzymes
4.8. Immunohistochemistry Staining
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTR | Control |
| Gui-A-Gra | Gryllus bimarculatus powder |
| G | Gui-A-Gra |
| G 1.63 | Gui-A-Gra 1.63 gm/kg |
| G 6.5 | Gui-A-Gra 6.5 gm/kg |
| VC | Varicocele |
| VC + G 1.63 | Varicocele + Gui-A-Gra 1.63 gm/kg |
| VC + G 6.5 | Varicocele + Gui-A-Gra 6.5 gm/kg |
| SD | Sprague-Dawley |
| ER | Endoplasmic reticulum |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | BCL 2 associated X protein |
| StAR | Steroidogenic acute regulatory protein |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| ROS/RNS | Reactive oxygen species/reactive nitrogen species |
| GPx | Glutathione peroxidase |
| GPx 4 | Glutathione peroxidase 4 |
| LH | Luteinizing hormone |
| FSH | Follicle stimulating hormone |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| Grp-78 | Glucose regulated protein-78 |
| p-JNK | Phosphorylated c-jun-N-terminal kinase |
| JNK | c-jun-N-terminal kinase |
| p-IRE1α | Phosphorylated inositol-requiring transmembrane Kinase 1α |
| IRE1α | Inositol-requiring transmembrane kinase 1α |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated (dUTP) nick-end labeling |
| H&E | Hematoxylin and eosin |
| STs | Seminiferous tubules |
| PBS | Phosphate buffer saline |
| DFC | Dichlorofluorescein |
| GMP | Good manufacturing practice |
| HACCP | Hazard analysis and critical control point |
| ANOVA | Analysis of variance |
| SEM | Standard error of the mean. |
| p.o. | Per oral |
References
- Karna, K.K.; Choi, B.R.; You, J.H.; Shin, Y.S.; Cui, W.S.; Lee, S.W.; Kim, J.H.; Kim, C.Y.; Kim, H.K.; Park, J.K. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement. Altern. Med. 2019, 19, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karna, K.K.; Choi, B.R.; Kim, M.J.; Kim, H.K.; Park, J.K. The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat. Int. J. Mol. Sci. 2019, 20, 5785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, K.K.; Zhang, L.T.; Choi, B.R.; Karna, K.K.; You, J.H.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Zhao, C.; Chae, H.-J.; et al. Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm. Biol. 2018, 56, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Selvam, M.K.P.; Agarwal, A. Sperm and Seminal Plasma Proteomics: Molecular Changes Associated with Varicocele-Mediated Male Infertility. World J. Mens Health 2019, 38, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Paick, S.; Choi, W.S. Varicocele and Testicular Pain: A Review. World J. Mens Health 2019, 37, 4–11. [Google Scholar] [CrossRef]
- Selvam, M.K.P.; Samanta, L.; Agarwal, A. Functional Analysis of Differentially Expressed Acetylated Spermatozoal Proteins in Infertile Men with Unilateral and Bilateral Varicocele. Int. J. Mol. Sci. 2020, 21, 3155. [Google Scholar] [CrossRef]
- Selvam, M.K.P.; Agarwal, A.; Sharma, R.; Samanta, L.; Gupta, S.; Dias, T.R.; Martins, A.D. Protein Fingerprinting of Seminal Plasma Reveals Dysregulation of Exosome-Associated Proteins in Infertile Men with Unilateral Varicocele. World J. Mens Health 2019, 37. [Google Scholar] [CrossRef]
- Karna, K.K.; Shin, Y.S.; Choi, B.R.; Kim, H.K.; Park, J.K. The Role of Endoplasmic Reticulum Stress Response in Male Reproductive Physiology and Pathology: A Review. World J. Mens Health 2020, 38, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Lipshultz, L.I.; Chiba, K.; Ramasamy, R.; Lamb, D.J. The varicocele: Diagnostic dilemmas, therapeutic challenges and future perspectives. Asian J. Androl. 2016, 18, 276–281. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, W.; Chen, Q.; Li, L.; Cao, H.; Xu, C.L.; Chen, G.H.; Sun, Y.H. Effect of varicocelectomy on testis volume and semen parameters in adolescents: A meta-analysis. Asian J. Androl. 2015, 17, 1012–1016. [Google Scholar]
- Collodel, G.; Castellini, C.; Lee, J.C.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxidative Med. Cell. Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Lysiak, J.J. Oxidative stress: A common factor in testicular dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, M.; Shaygannia, E.; Rahmani, M.; Eskandari, A.; Golsefid, A.A.; Tavalaee, M.; Gharagozloo, P.; Drevet, J.R.; Nasr-Esfahani, M.H. Endoplasmic Reticulum Stress (ER Stress) and Unfolded Protein Response (UPR) Occur in a Rat Varicocele Testis Model. Oxidative Med. Cell. Longev. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Lee, T.H.; Cheng, W.H.; Jeng, S.Y. Involved intrinsic apoptotic pathway of testicular tissues in varicocele-induced rats. World J. Urol. 2009, 27, 527–532. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Song, M.-H.; Han, M.-H.; Kwak, K.-W.; Lee, S.; Kim, E.-S.; Park, K.-H.; Kim, W.-T.; Choi, J.-Y. Effect of different diets on growth and development of the two-spotted cricket, Gryllus bimaculatus (Orthoptera: Gryllidae). Int. J. Ind. Èntomol. 2016, 33, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Kim, Y.; Han, J.-S. Antioxidant Activities and Nutritional Components of Cricket (Gryllus bimaculatus) Powder and Protein Extract. Asian J. Beauty Cosmetol. 2020, 18, 163–172. [Google Scholar] [CrossRef]
- Cho, H.-R.; Lee, Y.-J.; Hong, J.-E.; Lee, S.-O. Enzymatic preparation and antioxidant activities of protein hydrolysates from Gryllus bimaculatus. Korean J. Food Sci. Technol. 2019, 51, 473–479. [Google Scholar]
- Kim, M.-W.; Song, Y.-S.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Park, Y.-K.; Kang, S.H.; Kim, S.-A.; Choi, C.; Jung, W.-J. Production of chitin and chitosan from the exoskeleton of adult two-spotted field crickets (Gryllus bimaculatus). Èntomol. Res. 2017, 47, 279–285. [Google Scholar] [CrossRef]
- Lee, S.R.; Yi, S.A.; Nam, K.H.; Park, J.G.; Hwang, J.S.; Lee, J.; Kim, K.H. (±)-Kituramides A and B, pairs of enantiomeric dopamine dimers from the two-spotted cricket Gryllus bimaculatus. Bioorg. Chem. 2020, 95, 103554. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Han, J.W.; Hwang, J.S.; Yun, E.Y.; Lee, B.M. Anti-inflammatory effect of glycosaminoglycan derived from Gryllus bimaculatus (a type of cricket, insect) on adjuvant-treated chronic arthritis rat model. J. Toxicol. Environ. Health A 2014, 77, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, G.H.; Lee, H.Y.; Hoang, T.H.; Chae, H.J. Glucose-lowering effect of Gryllus bimaculatus powder on streptozotocin-induced diabetes through the AKT/mTOR pathway. Food Sci. Nutr. 2020, 8, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.B.; Chang, M.H.; Park, J.-W.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, H.; Ogawa, Y.; Yoshida, H. Relationship between testicular volume and varicocele in patients with infertility. Urology 2008, 71, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, A.W.; Wang, R. Varicocele and testicular function. Asian J. Androl. 2015, 17, 659–667. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Antonuccio, P.; Micali, A.; Puzzolo, D.; Romeo, C.; Vermiglio, G.; Squadrito, V.; Freni, J.; Pallio, G.; Trichilo, V.; Righi, M.; et al. Nutraceutical Effects of Lycopene in Experimental Varicocele: An “In Vivo” Model to Study Male Infertility. Nutrients 2020, 12, 1536. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jo, N.H.; Park, J.K.; Gye, M.C. Changes in Inflammatory Cytokines Accompany Deregulation of Claudin-11, Resulting in Inter-Sertoli Tight Junctions in Varicocele Rat Testes. J. Urol. 2016, 196, 1303–1312. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [Green Version]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Nasebakht, A.; Valizadeh, R.; Fallah, M.M.; Afshari, A.T.; Rahimi, M.M.; Daneshyar, C. A preliminary evaluation of serum level of testosterone, LH, and FSH in patients with varicocele after varicocelectomy as a kidney-related disease. Ther. Clin. Risk Manag. 2018, 14, 1585–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogan, F.; Armagan, A.; Oksay, T.; Akman, T.; Aylak, F.; Bas, E. Impact of micronised purified flavonoid fraction on increased malondialdehyde and decreased metalloproteinase-2 and metalloproteinase-9 levels in varicocele: Outcome of an experimentally induced varicocele. Andrologia 2014, 46, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.L.; Esteves, S.C.; Agarwal, A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J. Androl. 2016, 18, 186–193. [Google Scholar]
- Köksal, I.T.; Tefekli, A.; Usta, M.; Erol, H.; Abbasoglu, S.; Kadioglu, A. The role of reactive oxygen species in testicular dysfunction associated with varicocele. BJU Int. 2000, 86, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Shokoohi, M.; Khaki, A.; Shoorei, H.; Khaki, A.A.; Moghimian, M.; Abtahi-Eivary, S.H. Hesperidin attenuated apoptotic-related genes in testicle of a male rat model of varicocoele. Andrology 2020, 8, 249–258. [Google Scholar] [CrossRef]
- Nallella, K.P.; Allamaneni, S.S.; Pasqualotto, F.F.; Sharma, R.K.; Thomas, A.J., Jr.; Agarwal, A.J.U. Relationship of interleukin-6 with semen characteristics and oxidative stress in patients with varicocele. Urology 2004, 64, 1010–1013. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Hedger, M.P. Inflammatory networks in the control of spermatogenesis: Chronic inflammation in an immunologically privileged tissue? Adv. Exp. Med. Biol. 2008, 636, 92–114. [Google Scholar]
- Wang, H.; Lv, Y.; Hu, K.; Feng, T.; Jin, Y.; Wang, Y.; Huang, Y.; Chen, B. Seminal plasma leptin and spermatozoon apoptosis in patients with varicocele and leucocytospermia. Andrology 2014, 47, 655–661. [Google Scholar] [CrossRef]
- Karna, K.K.; Choi, B.R.; You, J.H.; Shin, Y.S.; Soni, K.K.; Cui, W.S.; Lee, S.W.; Kim, C.Y.; Kim, H.K.; Park, J.K.; et al. Cross-talk between ER stress and mitochondrial pathway mediated adriamycin-induced testicular toxicity and DA-9401 modulate adriamycin-induced apoptosis in Sprague–Dawley rats. Cancer Cell Int. 2019, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.K.; Shin, Y.S.; Choi, B.R.; Karna, K.K.; Kim, H.K.; Lee, S.W.; Kim, C.Y.; Park, J.K. Protective effect of DA-9401 in finasteride-induced apoptosis in rat testis: Inositol requiring kinase 1 and c-Jun N-terminal kinase pathway. Drug Des. Dev. Ther. 2017, 11, 2969–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, K.K.; Zhang, L.T.; You, J.H.; Lee, S.W.; Kim, C.Y.; Cui, W.S.; Chae, H.J.; Kim, H.K.; Park, J.K. The effects of MOTILIPERM on cisplatin induced testicular toxicity in Sprague–Dawley rats. Cancer Cell Int. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.K.; Kim, H.K.; Choi, B.R.; Karna, K.K.; You, J.H.; Cha, J.S.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Park, J.K. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: Reactive oxygen species and endoplasmic reticulum stress. Drug Des. Dev. Ther. 2016, 10, 3959–3968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karna, K.K.; Soni, K.K.; You, J.H.; Choi, N.Y.; Kim, H.K.; Kim, C.Y.; Lee, S.W.; Shin, Y.S.; Park, J.K. MOTILIPERM Ameliorates Immobilization Stress-Induced Testicular Dysfunction Via Inhibition of Oxidative Stress and Modulation of the Nrf2/HO-1 Pathway in SD Rats. Int. J. Mol. Sci. 2020, 21, 4750. [Google Scholar] [CrossRef] [PubMed]




| Parameters | CTR | G1.63 | G6.5 | VC | VC + G1.63 | VC + G6.5 |
|---|---|---|---|---|---|---|
| Body weight (sacrifice; g) | 457.41 ± 8.5 | 450.75 ± 9.90 | 476.16 ± 9.64 | 438.91 ± 6.11 | 448.91 ± 7.97 | 450.41 ± 9.79 |
| Testis weight (g) | 2.15 ± 0.04 | 2.10 ± 0.06 | 2.21 ± 0.03 | 2.01 ± 0.05 | 2.15 ± 0.03 | 2.10 ± 0.05 |
| Epididymis weight (g) | 0.80 ± 0.02 | 0.93 ± 0.01 | 0.89 ± 0.01 | 0.83 ± 0.01 | 0.83 ± 0.01 | 0.82 ± 0.01 |
| Prostate weight (g) | 0.96 ± 0.02 | 0.99 ± 0.02 | 1.03 ± 0.04 | 1.01 ± 0.03 | 0.96 ± 0.03 | 1.09 ± 0.06 |
| Seminal Vesicle weight (g) | 1.94 ± 0.07 | 2.27 ± 0.08 | 2.31 ± 0.06 | 2.10 ± 0.09 | 2.14 ± 0.07 | 2.08 ± 0.06 |
| Penis weight (g) | 0.38 ± 0.01 | 0.40 ± 0.01 | 0.38 ± 0.01 | 0.35 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.01 |
| Kidney weight (g) | 1.33 ± 0.02 | 1.40 ± 0.03 | 1.34 ± 0.01 | 1.33 ± 0.02 | 1.38 ± 0.03 | 1.39 ± 0.04 |
| Parameters | CTR | G1.63 | G6.5 | VC | VC + G1.63 | VC + G6.5 |
|---|---|---|---|---|---|---|
| Sperm count (106 /mL) | ||||||
| Vas deferens | 56.65 ± 2.39 | 50.37 ± 2.82 | 62.58 ± 1.29 | 45.91 ± 1.80 ** | 54.08 ± 1.84 | 72.41 ± 1.05 ### |
| Epididymis | 60.16 ± 2.72 | 54.79 ± 2.18 | 64.95 ± 1.25 | 54.58 ± 2.14 | 55.54 ± 1.97 | 77.50 ± 1.43 ### |
| Sperm motility (%) | ||||||
| Vas deferens | 70.93 ± 3.75 | 62.07 ± 1.95 | 58.62 ± 1.29 | 68.82 ± 1.33 | 78.21 ± 2.40 # | 81.37 ± 1.63 ## |
| Epididymis | 68.81 ± 3.11 | 61.64 ± 2.31 | 58.50 ± 1.71 | 66.83 ± 1.83 | 81.80 ± 1.62 ### | 83.70 ± 1.46 ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karna, K.K.; Choi, N.Y.; Kim, C.Y.; Kim, H.K.; Shin, Y.S.; Park, J.K. Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway. Int. J. Mol. Sci. 2020, 21, 9231. https://doi.org/10.3390/ijms21239231
Karna KK, Choi NY, Kim CY, Kim HK, Shin YS, Park JK. Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway. International Journal of Molecular Sciences. 2020; 21(23):9231. https://doi.org/10.3390/ijms21239231
Chicago/Turabian StyleKarna, Keshab Kumar, Na Young Choi, Chul Young Kim, Hye Kyung Kim, Yu Seob Shin, and Jong Kwan Park. 2020. "Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway" International Journal of Molecular Sciences 21, no. 23: 9231. https://doi.org/10.3390/ijms21239231





