New Eugenol Derivatives with Enhanced Insecticidal Activity
Abstract
:1. Introduction
2. Results
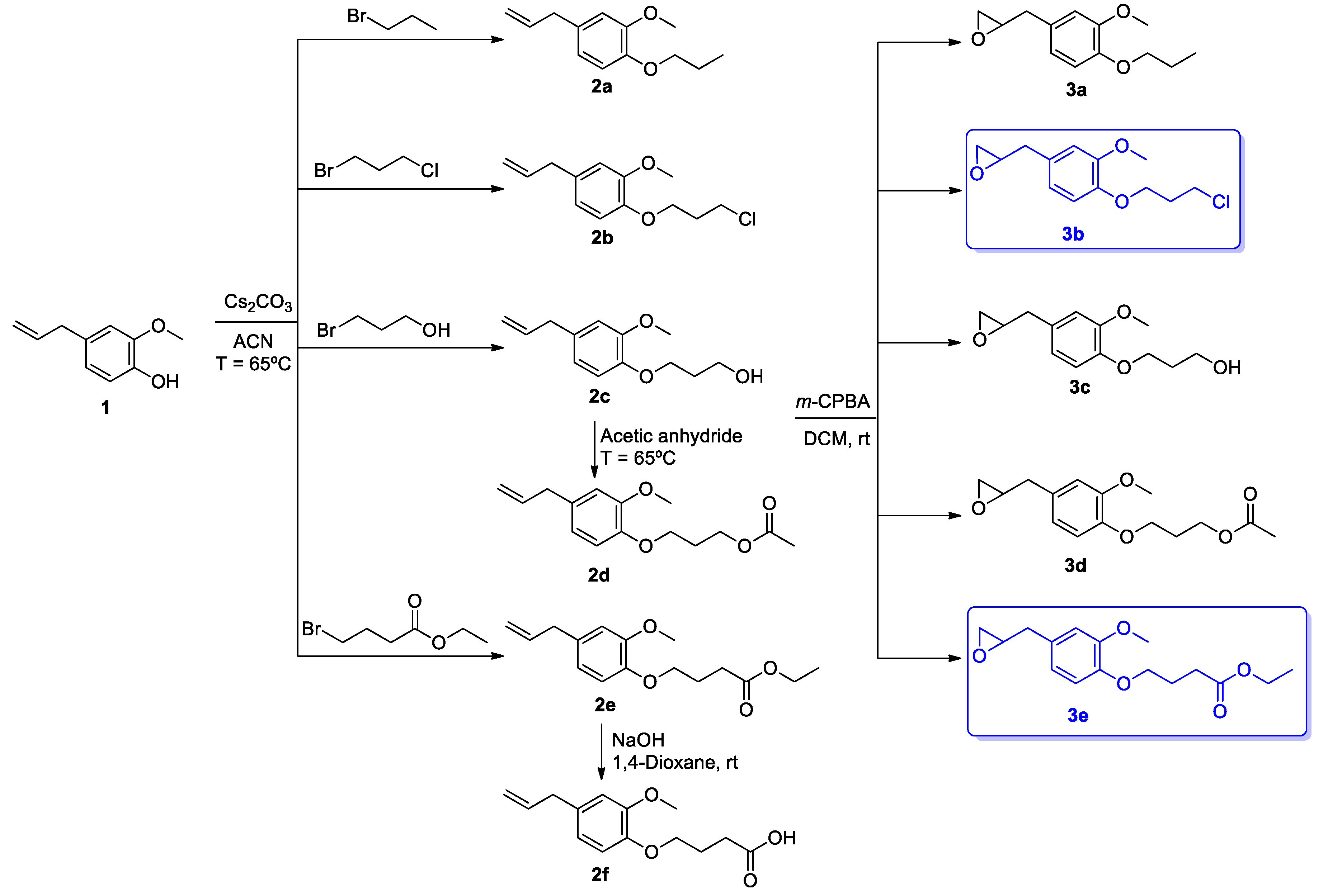
2.1. Synthesis of Eugenol Derivatives 2a–f and 3a–e
2.1.1. 4-Allyl-2-Methoxyphenol 1
2.1.2. 4-Allyl-2-Methoxy-1-Propoxybenzene 2a
2.1.3. 4-Allyl-1-(3-Chloropropoxy)-2-Methoxybenzene 2b
2.1.4. 3-(4-Allyl-2-Methoxyphenoxy)Propan-1-ol 2c
2.1.5. Ethyl 4-(4-Allyl-2-Methoxyphenoxy)Butanoate 2e
2.1.6. Methyl 4-(4-Allyl-2-Methoxyphenoxy)Butanoate 2d
2.1.7. 4-(4-Allyl-2-Methoxyphenoxy)Butanoic Acid 2f
2.1.8. 2-(3-Methoxy-4-propoxybenzyl)oxirane 3a
2.1.9. 2-(4-(3-Chloropropoxy)-3-Methoxybenzyl)Oxirane 3b
2.1.10. 3-(2-Methoxy-4-(Oxiran-2-Ylmethyl)Phenoxy)Propan-1-ol 3c
2.1.11. 3-(2-Methoxy-4-(Oxiran-2-Ylmethyl)Phenoxy)Propyl Acetate 3d
2.1.12. Ethyl 4-(2-Methoxy-4-(Oxiran-2-Ylmethyl)Phenoxy)Butanoate 3e
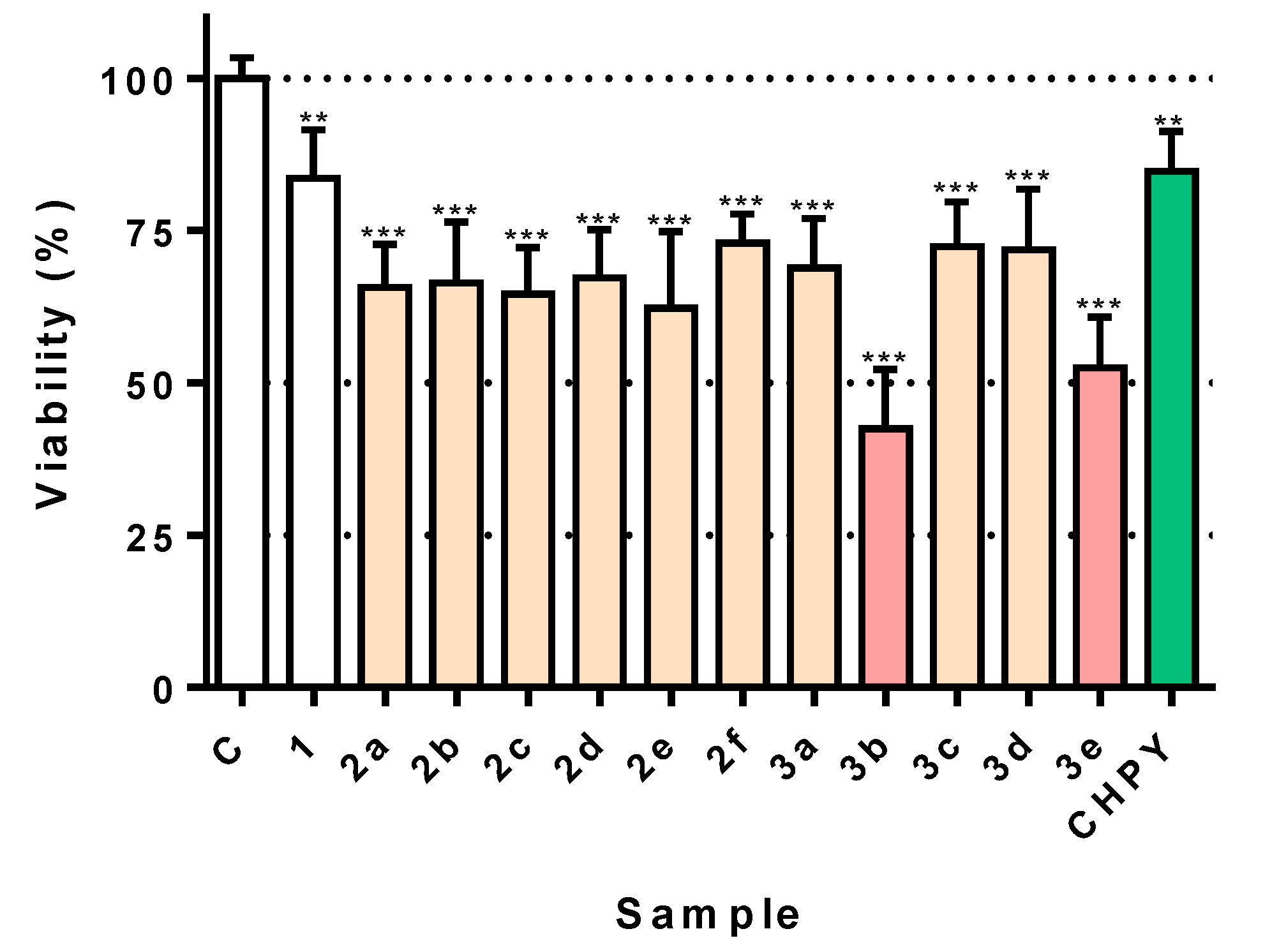
2.2. Screening of Toxicity Towards Insect Cells
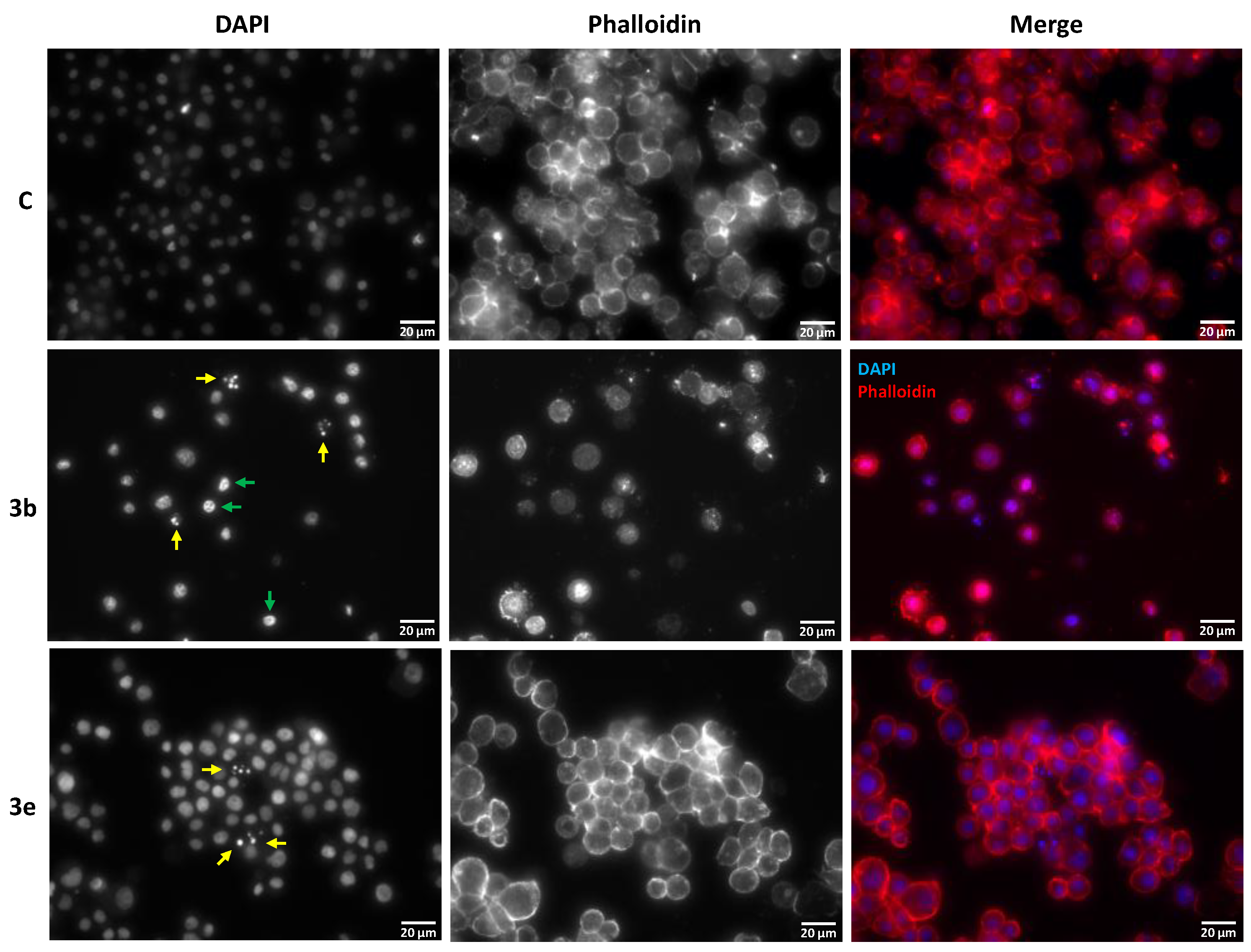
2.3. Impact of Eugenol Derivatives 3b and 3e in Insect Cell Morphology
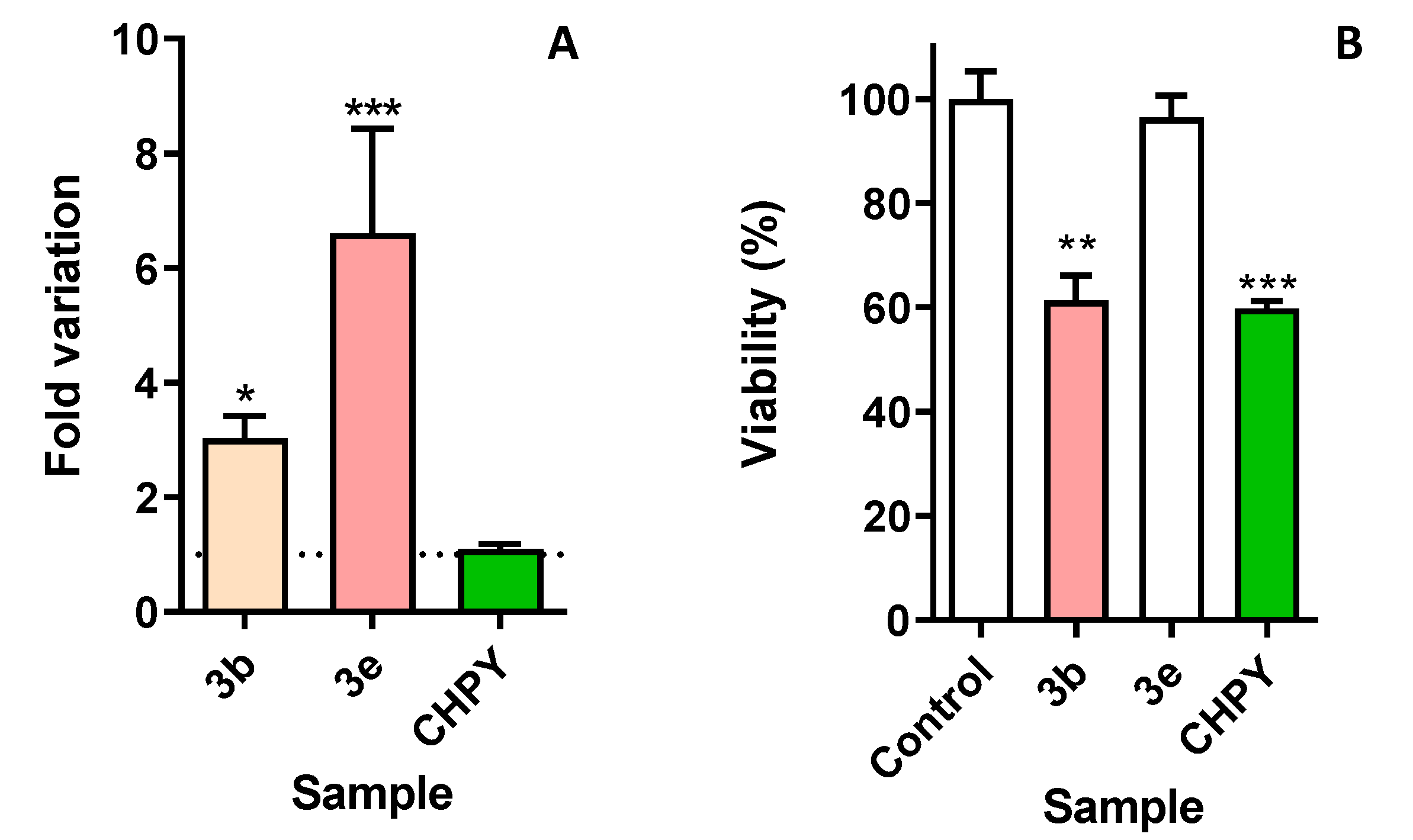
2.4. Oxiran-Bearing Eugenol Derivatives 3b and 3e Activate Caspase-Like Proteases in Sf9 Cells
2.5. Some Eugenol Derivatives are Selectively Toxic to Insect but Not Human Cells
3. Discussion
3.1. Synthesis of Eugenol Derivatives 2a–f and 3a–e
3.2. Differential Effect of Eugenol Derivatives Towards Insect Cells
3.3. Eugenol Derivatives Trigger a Process of Programmed Cell Death
3.4. New Eugenol Derivatives are Not Toxic to Human Cells
4. Materials and Methods
4.1. Chemicals
4.2. Analytical Instruments
4.3. Synthesis of Eugenol Derivatives 2a–f and 3a–e
4.3.1. Extraction of Eugenol 1 from Syzygium Aromaticum
4.3.2. General Procedure for Synthesizing Compounds 2a–c and 2e
4.3.3. General Procedure for Synthesizing Compound 2d
4.3.4. General Procedure for Synthesizing Compound 2f
4.3.5. General Procedure for Synthesizing Compounds 3a–e
4.4. Preparation Methods
4.4.1. Cell Culture
4.4.2. Viability Assessment
4.4.3. Morphological Assessment
4.4.4. Caspase-Like Activity
4.4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| TLC | Thin-layer chromatography |
| NMR | Nuclear magnetic resonance |
| HRMS | High-resolution mass spectrometry |
| Sf9 | Spodoptera frugiperda |
References
- Pino-Otín, M.R.; Ballestero, D.; Navarro, E.; González-Coloma, A.; Val, J.; Mainar, A.M. Ecotoxicity of a novel biopesticide from Artemisia absinthium on non-target aquatic organisms. Chemosphere 2019, 216, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.A.T.A.; Silva, R.; Silva, A.G.; Milet-Pinheiro, P.; Paiva, P.M.G.; Navarro, D.M.A.F.; Silva, M.V.; Napoleao, T.H.; Correia, M.T.S. Chemical characterization and insecticidal effect against Sitophilus zeamais (maize weevil) of essential oil from Croton rudolphianus leaves. Crop Prot. 2020, 129, 105043. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Peres, M.C.; de Souza Costa, G.C.; dos Reis, L.E.L.; da Silva, L.D.; Peixoto, M.F.; Alves, C.C.F.; Forim, M.R.; Quintela, E.D.; Araújo, W.L.; Cazal, C.M. In natura and nanoencapsulated essential oils from Xylopia aromatica reduce oviposition of Bemisia tabaci in Phaseolus vulgaris. J. Pest. Sci. 2020, 93, 807–821. [Google Scholar] [CrossRef]
- Rong, S.; Xu, H.; Li, L.; Chen, R.; Gao, X.; Xu, Z. Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic. Biochem. Physiol. 2020, 162, 69–77. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Leong, W.-H.; The, S.-Y.; Hossain, M.M.; Nadarajaw, T.; Zabidi-Hussin, Z.; Chin, S.-Y.; Lai, K.-S.; Lim, S.-H.E. Application, monitoring and adverse effects in pesticide use: The importance of reinforcement of Good Agricultural Practices (GAPs). J. Environ. Manag. 2020, 260, 109987. [Google Scholar] [CrossRef]
- Mfarrej, M.F.B.; Rara, F.M. Competitive, sustainable natural pesticides. Acta Ecol. Sin. 2019, 39, 145–151. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Zhi, X.-Y.; Jiang, L.-Y.; Li, T.; Song, L.-L.; Wang, Y.; Cao, H.; Yang, C. Semisynthesis and insecticidal bioactivities of benzoxazole and benzoxazolone derivatives of honokiol, a naturally occurring neolignan derived from Magnolia officinalis. Bioorg. Med. Chem. Lett. 2020, 30, 27086. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Zhang, J.; Peng, J.-W.; Zhang, Z.-J.; Zhao, W.-B.; Wang, R.-X.; Ma, K.-Y.; Li, J.-C.; Liu, Y.-Q.; Zhao, Z.-M.; et al. Discovery of luotonin A analogues as potent fungicides and insecticides: Design, synthesis and biological evaluation inspired by natural alkaloid. Eur. J. Med. Chem. 2020, 194, 112253. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Njume, L.E.; Malange, Y.I.; Günther, S.; Sippl, W.; Yong, J.N. The Chemistry and biological activities of natural products from Northern African plant families: From Taccaceae to Zygophyllaceae. Nat. Prod. Bioprospect. 2016, 6, 63–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Abbas, R.Z.; Israr, M.; Abbas, A.; Mehmood, K.; Khan, M.K.; Sindhu, Z.D.; Hussaind, R.; Saleemie, M.K.; Shaha, S. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol. 2020, 283, 109178. [Google Scholar] [CrossRef]
- Saroj, A.; Oriyomi, O.V.; Nayak, A.K.; Haider, S.Z. Phytochemicals of plant-derived essential oils: A novel green approach against pests. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 65–79. [Google Scholar] [CrossRef]
- Matos, L.F.; Barbosa, D.R.S.; Lima, E.C.; Dutra, K.A.; Navarro, D.M.A.F.; Alves, J.L.R.; Silva, G.N. Chemical composition and insecticidal effect of essential oils from Illicium verum and Eugenia caryophyllus on Callosobruchus maculatus in cowpea. Ind. Crops Prod. 2020, 145, 112088. [Google Scholar] [CrossRef]
- Vargas-Méndez, L.Y.; Sanabria-Flórez, P.L.; Saavedra-Reyes, L.M.; Merchan-Arenas, D.R.; Kouznetsov, V.V. Bioactivity of semisynthetic eugenol derivatives against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae infesting maize in Colombia. Saudi J. Biol. Sci. 2019, 26, 1613–1620. [Google Scholar] [CrossRef]
- Akhtar, Y.; Yeoung, Y.-R.; Isman, M.B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev. 2008, 7, 77–88. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Mazzeo, P.P.; Carraro, C.; Monica, A.; Capucci, D.; Pelagatti, P.; Bianchi, F.; Agazzi, S.; Careri, M.; Raio, A.; Carta, M.; et al. Designing a palette of cocrystals based on essential oil constituents for agricultural applications. ACS Sustain. Chem. Eng. 2019, 7, 17929–17940. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef]
- Dianez, F.; Santos, M.; Parra, C.; Navarro, M.J.; Blanco, R.; Gea, F.J. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liang, X.; Ou, Z.; Ye, M.; Shi, Y.; Chen, Y.; Zhao, J.; Zheng, D.; Xiang, H. Screening of chemical composition, anti-arthritis, antitumor and antioxidant capacities of essential oils from four Zingiberaceae herbs. Ind. Crops Prod. 2020, 149, 112342. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Mossa, A.T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef] [Green Version]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Kaufman, T.S. The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Gazolla, P.A.R.; Silva, A.M.; Borsodi, M.P.G.; Bergmann, B.R.; Ferreira, R.S.; Vaz, B.G.; Vasconcelos, G.A.; Lima, W.P. Synthesis and leishmanicidal activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur. J. Med. Chem. 2018, 146, 274–286. [Google Scholar] [CrossRef]
- Singh, N.; Rao, A.S.; Nandal, A.; Kumar, S.; Yadav, S.S.; Ganaie, S.A.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl—A versatile spice used in food and nutrition. Food Chem. 2020, 338, 127773. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y. Eugenol nanoemulsion stabilized with zein and sodium caseinate by self-assembly. J. Agric. Food Chem. 2017, 65, 2990–2998. [Google Scholar] [CrossRef]
- Silva, F.F.M.; Monte, F.J.Q.; Lemos, T.L.G.; Nascimento, P.G.G.; Costa, A.K.M.; Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Analysis of the synergistic antifungal mechanism of eugenol and citral. LWT Food Sci. Technol. 2020, 123, 109128. [Google Scholar] [CrossRef]
- Novato, T.; Gomes, G.A.; Zeringóta, V.; Franco, C.T.; Oliveira, D.R.; Melo, D.; Carvalho, M.G.; Daemon, E.; Monteiro, C.M.O. In vitro assessment of the acaricidal activity of carvacrol, thymol, eugenol and their acetylated derivatives on Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 2018, 260, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Tung, S.-H.; Jeng, R.-J.; Abu-Omar, M.M.; Lin, C.-H. A facile strategy to achieve fully bio-based epoxy thermosets from eugenol. Green Chem. 2019, 21, 4475–4488. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, J.; Sethuraman, V.; Cui, G.; Yi, X.; Zhong, G. Transcriptome analysis of Spodoptera frugiperda Sf9 cells reveals putative apoptosis-related genes and a preliminary apoptosis mechanism induced by azadirachtin. Sci. Rep. 2017, 7, 13231. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Chouiter, M.I.; Boulebd, H.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Belfaitah, A.; Silva, A.M.S. New chalcone-type compounds and 2-pyrazoline derivatives: Synthesis and caspase-dependent anticancer activity. Future Med. Chem. 2020, 12, 493–509. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, M.J.G.; Pereira, R.B.; Pereira, D.M.; Fortes, A.G.; Castanheira, E.M.S.; Gonçalves, M.S.T. New Eugenol Derivatives with Enhanced Insecticidal Activity. Int. J. Mol. Sci. 2020, 21, 9257. https://doi.org/10.3390/ijms21239257
Fernandes MJG, Pereira RB, Pereira DM, Fortes AG, Castanheira EMS, Gonçalves MST. New Eugenol Derivatives with Enhanced Insecticidal Activity. International Journal of Molecular Sciences. 2020; 21(23):9257. https://doi.org/10.3390/ijms21239257
Chicago/Turabian StyleFernandes, Maria José G., Renato B. Pereira, David M. Pereira, A. Gil Fortes, Elisabete M. S. Castanheira, and M. Sameiro T. Gonçalves. 2020. "New Eugenol Derivatives with Enhanced Insecticidal Activity" International Journal of Molecular Sciences 21, no. 23: 9257. https://doi.org/10.3390/ijms21239257
APA StyleFernandes, M. J. G., Pereira, R. B., Pereira, D. M., Fortes, A. G., Castanheira, E. M. S., & Gonçalves, M. S. T. (2020). New Eugenol Derivatives with Enhanced Insecticidal Activity. International Journal of Molecular Sciences, 21(23), 9257. https://doi.org/10.3390/ijms21239257








