Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating HIF-1α-NF-κB Crosstalk In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
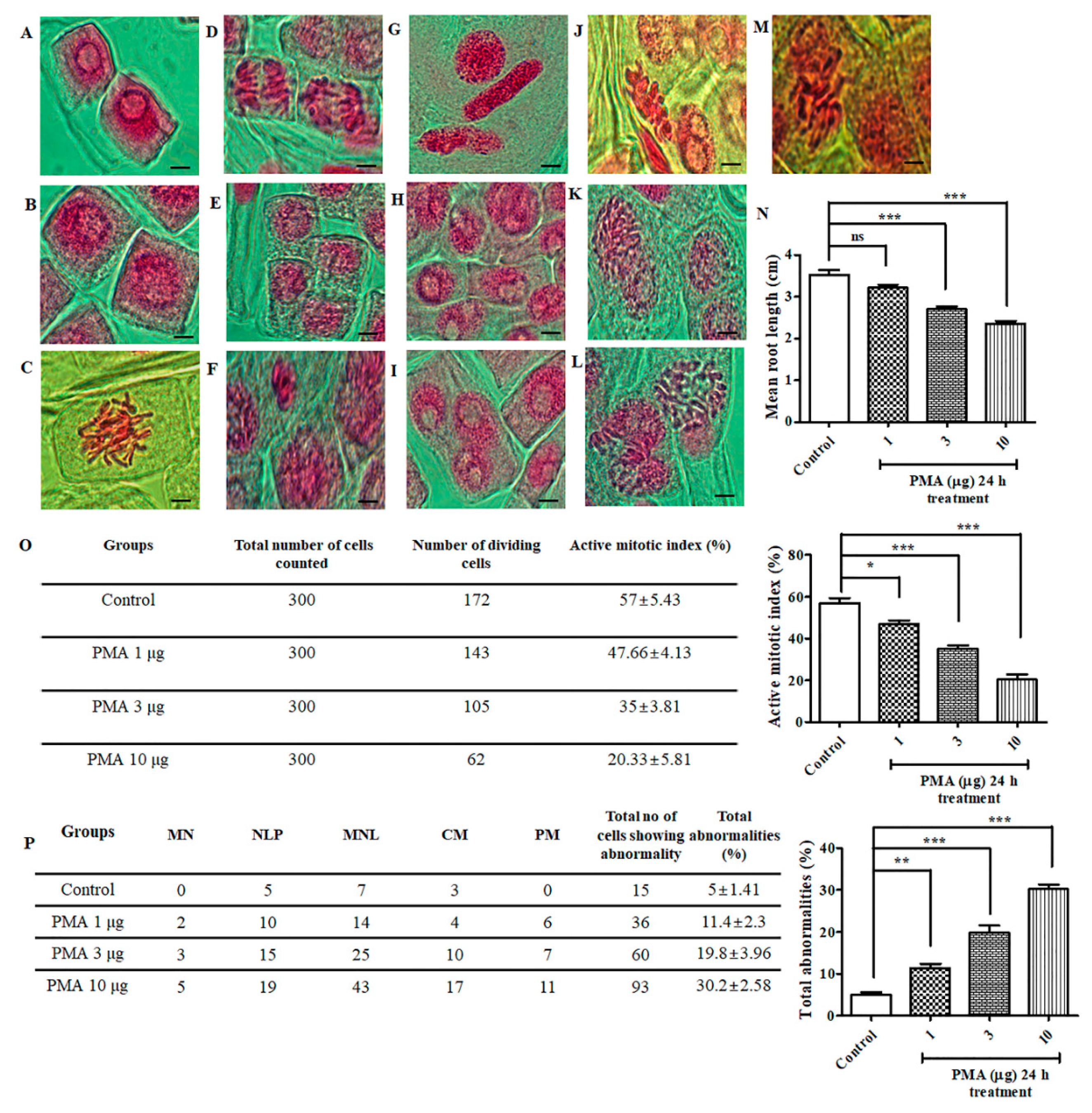
2.1. Effect of PMA on Allium cepa Root Tip
2.2. Effect of PMA on Zebrafish Embryonic Malformation
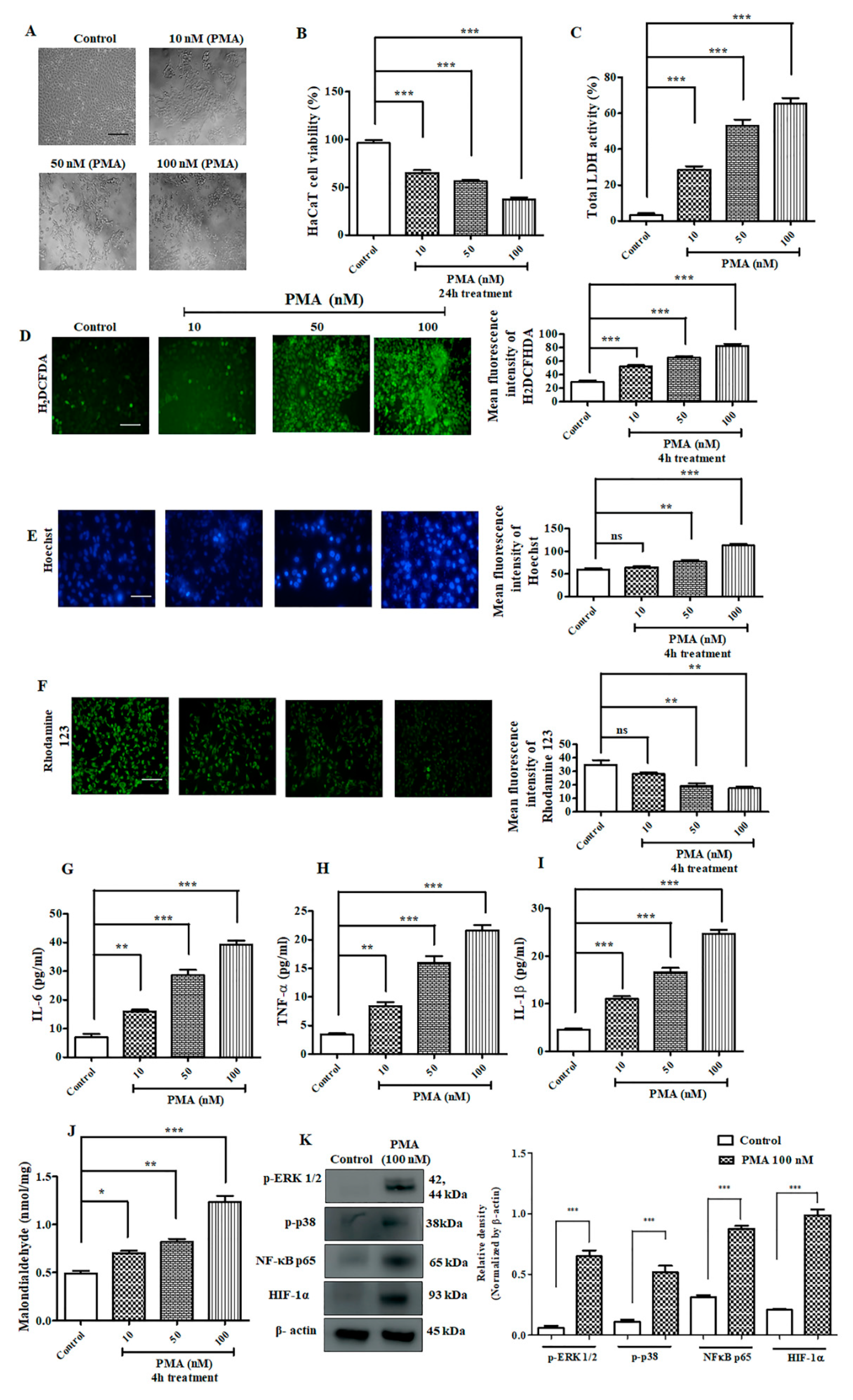
2.3. Effect of PMA on Immortalized Human Keratinocyte HaCaT Cells
2.4. PMA Treatment Aggravated ROS Generation and Inflammatory Cell Infiltration on Zebrafish Larvae
2.5. Antioxidant Potential and Cytotoxic Effects of Tangeretin (TAN) on Immortalized Human Keratinocyte Cells
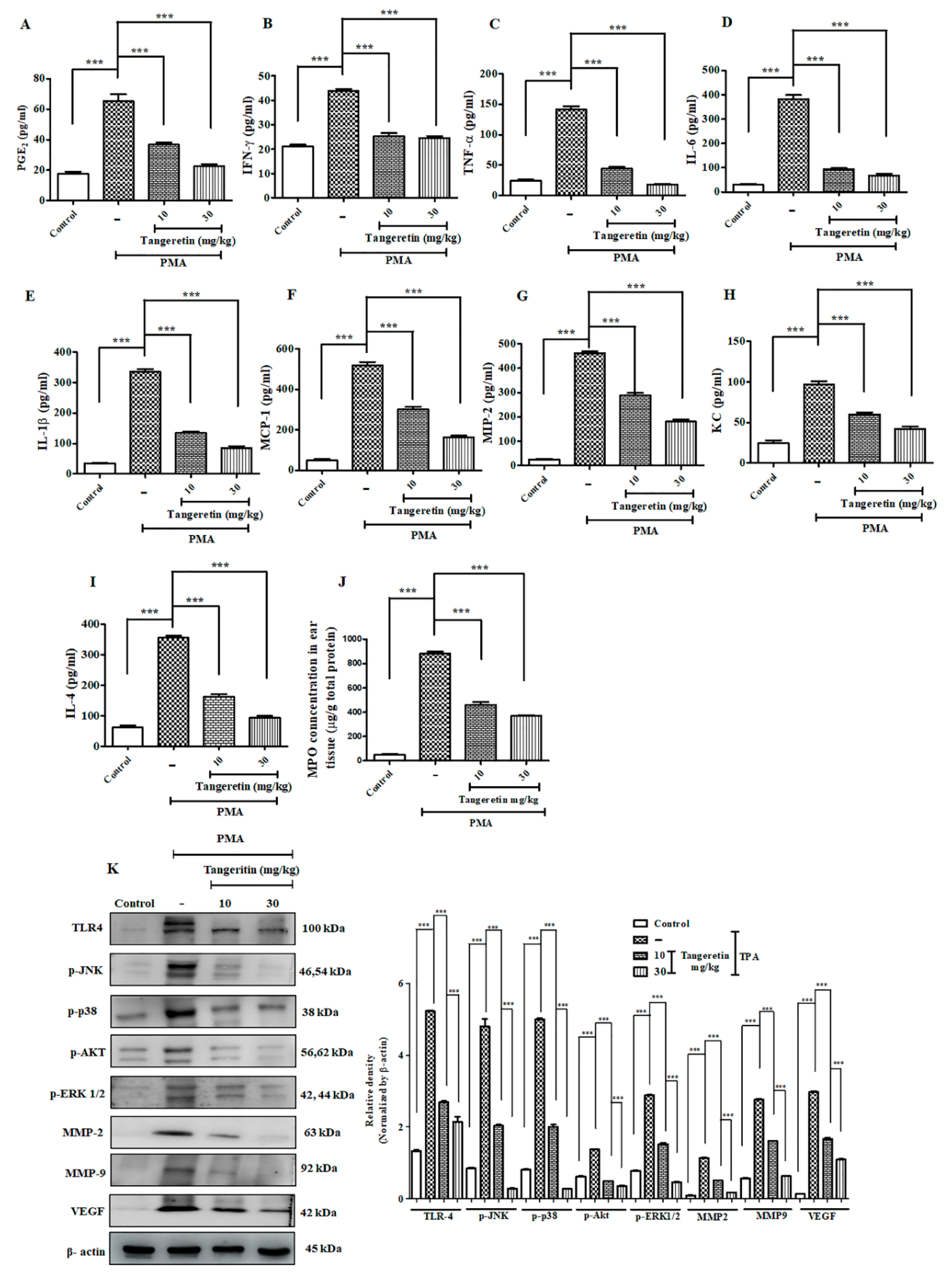
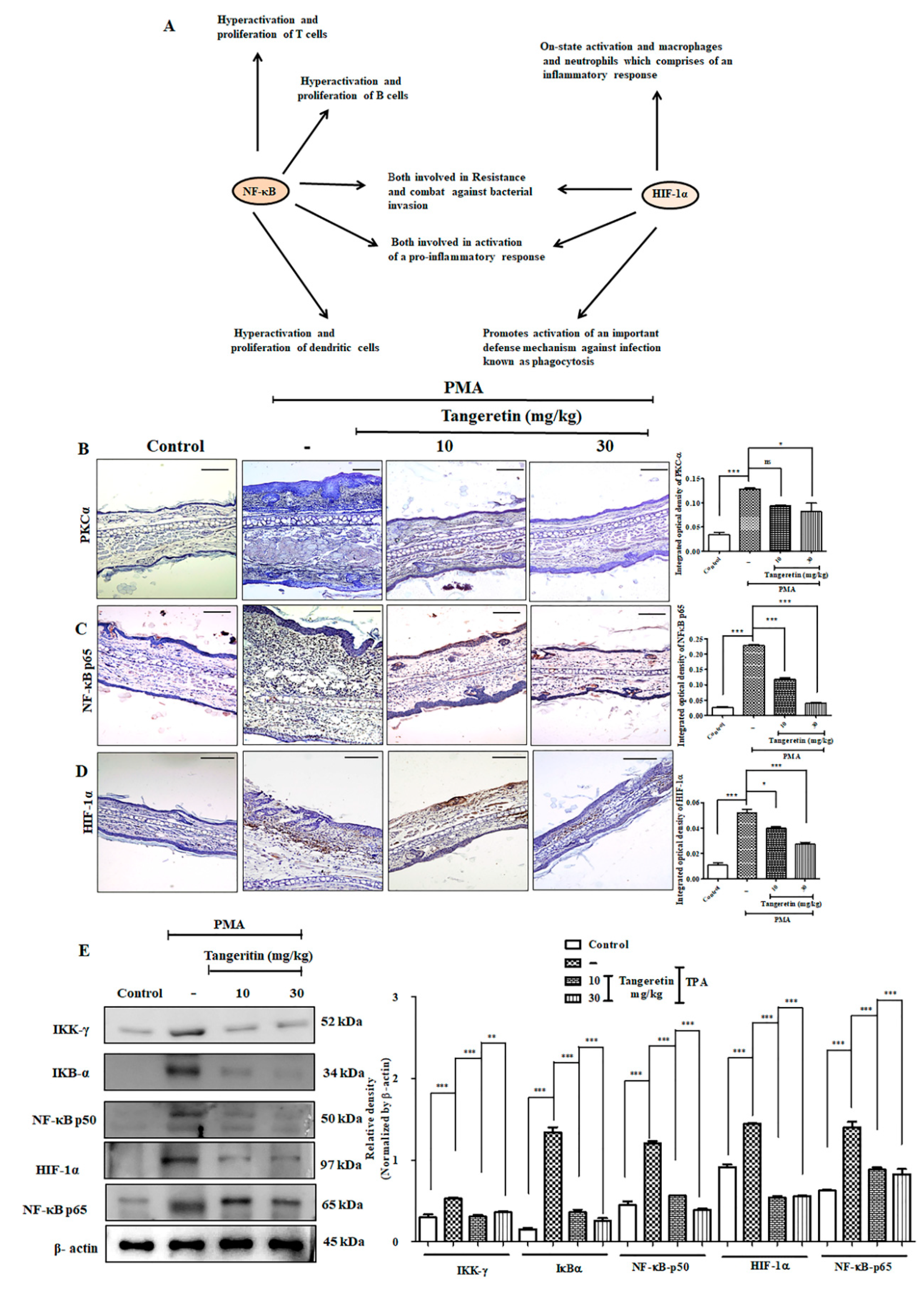
2.6. Tangeretin Counteracted PMA-Induced Epidermal Hyperplasia and Intra-Epidermal Neutrophilic Abscesses
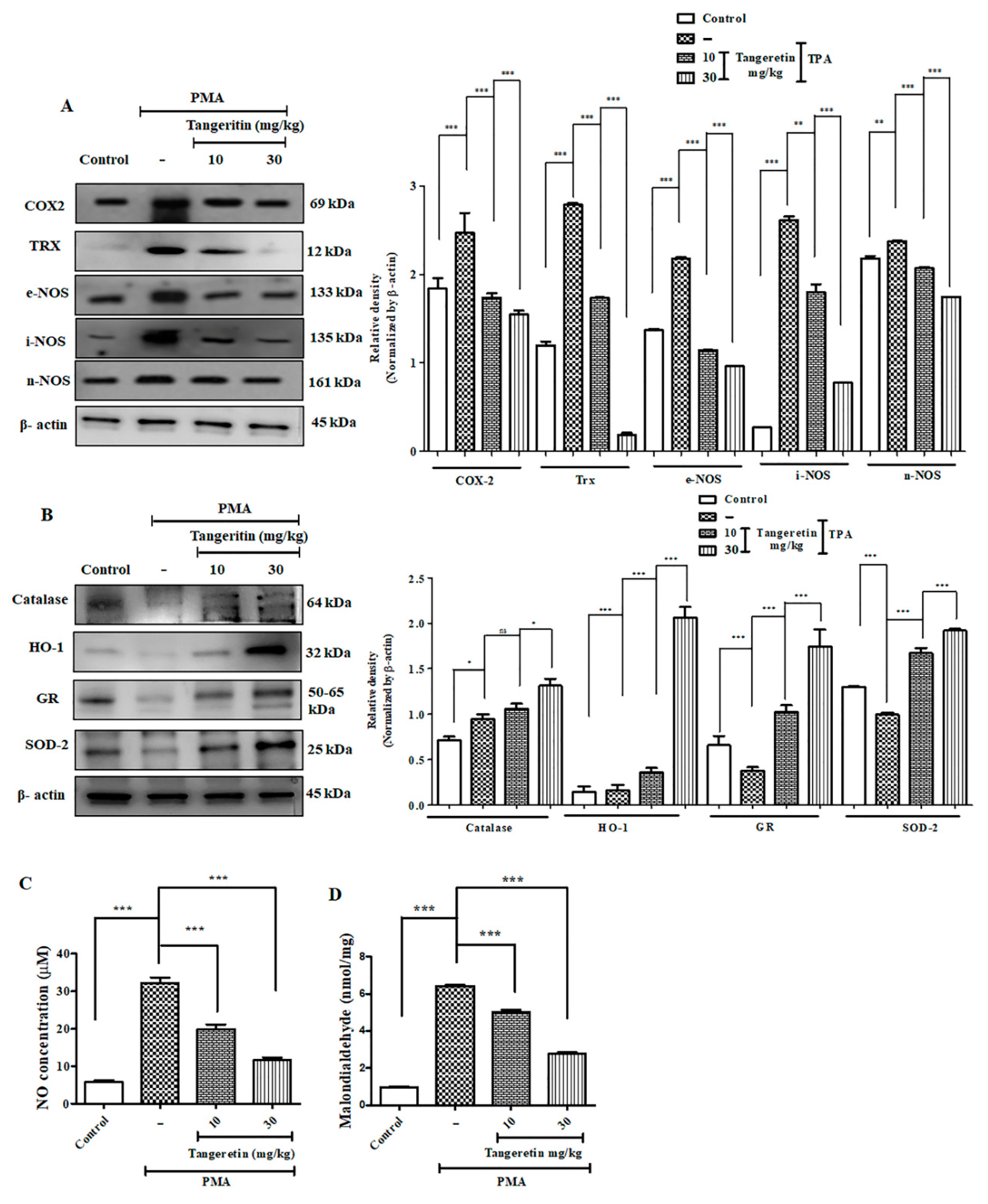
2.7. Tangeretin Suppressed PMA-Induced Oxidative Stress and Boosted Antioxidant Enzyme Response
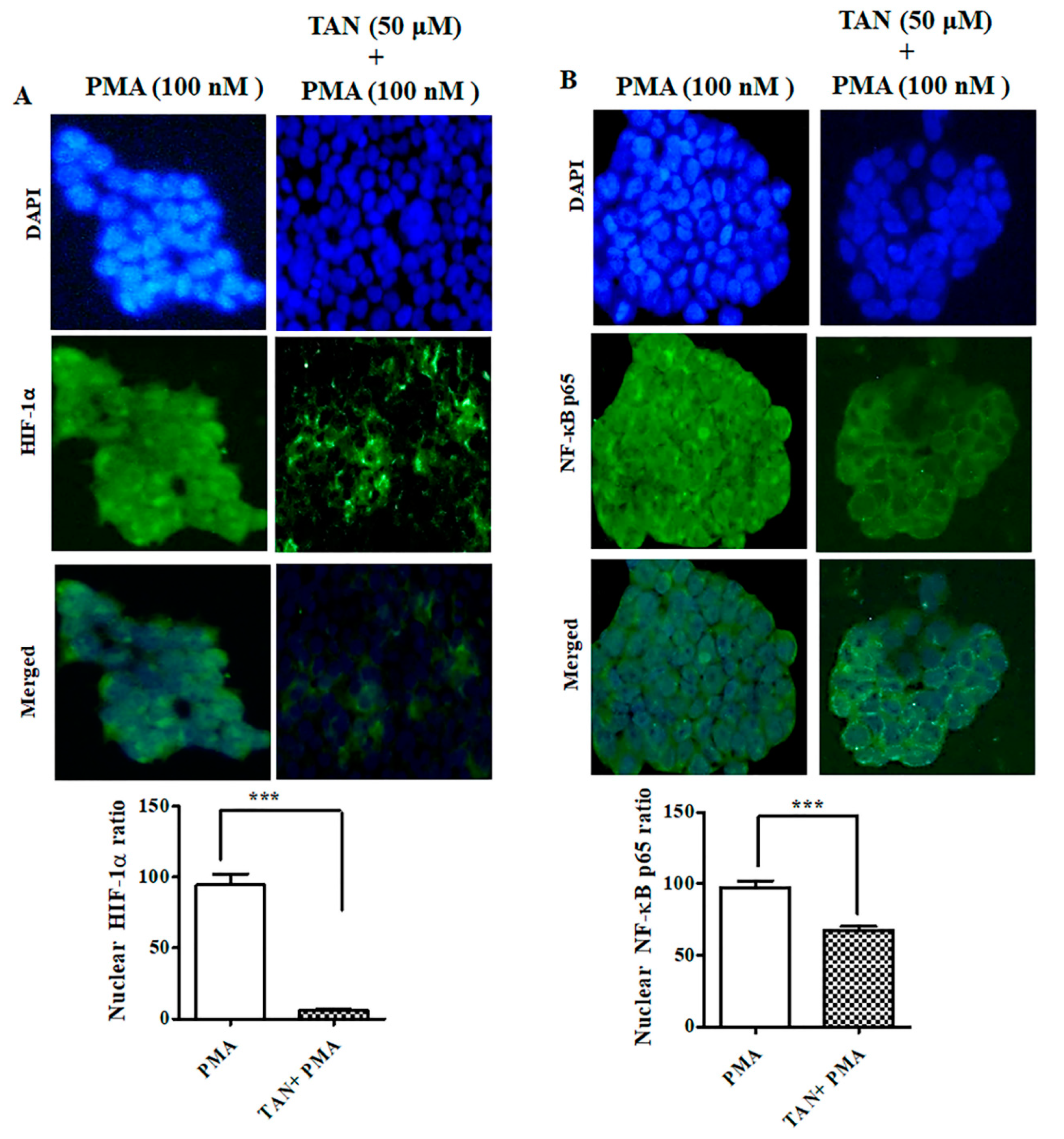
2.8. PMA Induced Nuclear Translocation and HIF-1α -NF-κB Crosstalk Kept at Bay by Tangeretin
3. Materials and Methods
3.1. Chemicals
3.2. Allium cepa Genotoxicity Analysis
3.3. Zebrafish Husbandry and PMA Toxicity Analysis
3.4. DPPH Assay
3.5. Cell Culture Studies and Toxicity Analysis
3.6. Hoechst-33342 Staining for Detection of Nuclear DNA Condensation
3.7. PMA-Induced Ear Inflammation
3.8. Hematoxylin and Eosin Staining
3.9. Western Blotting
3.10. Quantitative Determination of Inflammatory Markers through ELISA Assay
3.11. Myeloperoxidase Activity Assay
3.12. Nitric Oxide Assay
3.13. Malondialdehyde Assay for Estimation of Lipid Peroxidation
3.14. Immunohistochemistry
3.15. Intracellular ROS Generation Assay
3.16. Immunofluorescence Staining
3.17. RT-PCR Analysis
3.18. Statistical Significance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, F.J. Naturally Occurring Phorbol Esters; CRC Press Inc.: Boca Raton, FL, USA, 1986; ISBN 9781351083393. [Google Scholar]
- Beutler, J.A.; Alvarado-Lindner, A.B.; McCloud, T.G. Further studies on phorbol ester bioactivity in the Euphorbiaceae. Ann. Mol. Bot. Gard. 1996, 83, 530–533. [Google Scholar] [CrossRef]
- Taylor, S.E.; Gafur, M.A.; Choudhury, A.K.; Evans, F.J. Sapintoxin A, a new biologically active nitrogen containing phorbol ester. Experientia 1981, 37, 681–682. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Biddlestone, J.; Bandarra, D.; Rocha, S. The role of hypoxia in inflammatory disease (Review). Int. J. Mol. Med. 2015, 35, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-inducible factors as essential regulators of inflammation. Curr. Top. Microbiol. Immunol. 2010, 345, 105–120. [Google Scholar]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A mechanistic review of its pharmacological and therapeutic effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef]
- Dey, D.K.; Chang, S.N.; Vadlamudi, Y.; Park, J.G.; Kang, S.C. Synergistic therapy with tangeretin and 5-fluorouracil accelerates the ROS/JNK mediated apoptotic pathway in human colorectal cancer cell. Food Chem. Toxicol. 2020, 143, 111529. [Google Scholar] [CrossRef]
- Ridd, K.; Dhir, S.; Smith, A.G.; Gant, T.W. Defective TPA signalling compromises HaCat cells as a human in vitro skin carcinogenesis model. Toxicol. In Vitro 2010, 24, 910–915. [Google Scholar] [CrossRef]
- Reumaux, D.; de Boer, M.; Meijer, A.B.; Duthilleul, P.; Roos, D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J. Leukoc. Biol. 2003, 73, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chiou, Y.S.; Jiang, Y.; Pan, M.H.; Lin, Z.; Huang, Q. Safety evaluation of tangeretin and the effect of using emulsion-based delivery system: Oral acute and 28-day sub-acute toxicity study using mice. Food Res. Int. 2015, 74, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lim, T.G.; Lee, K.M.; Jeon, A.J.; Kim, S.Y.; Lee, K.W. Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation. J. Agric. Food Chem. 2011, 59, 222–228. [Google Scholar] [CrossRef]
- Lebwohl, M. Psoriasis. Ann. Intern. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.L.; Steiner, S.; Havens, M.; Tramposch, K.M. Mouse skin inflammation induced by multiple topical applications of 12-O-tetradecanoylphorbol-13-acetate. Ski. Pharmacol. 1991, 4, 262–271. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, E.J.; Park, J.S.; Jang, S.E.; Kim, D.H.; Kim, H.S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J. Neuroimmune Pharmacol. 2016, 11, 294–305. [Google Scholar] [CrossRef]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-κB. Exp. Mol. Pathol. 2013, 94, 419–429. [Google Scholar] [CrossRef]
- Liang, F.; Fang, Y.; Cao, W.; Zhang, Z.; Pan, S.; Xu, X. Attenuation of tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in HepG2 cells by tangeretin: Relevance of the Nrf2-ARE and MAPK signaling pathways. J. Agric. Food Chem. 2018, 66, 6317–6325. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. A review of recent studies on the metabolism of exogenous and endogenous malondialdehyde. Xenobiotica 1990, 20, 901–907. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef]
- Bandarra, D.; Rocha, S. A tale of two transcription factors: NF-kB and HIF crosstalk. OA Mol. Cell Biol. 2013, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Walmsley, S.R.; Print, C.; Farahi, N.; Peyssonnaux, C.; Johnson, R.S.; Cramer, T.; Sobolewski, A.; Condliffe, A.M.; Cowburn, A.S.; Johnson, N.; et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 2005, 201, 105–115. [Google Scholar] [CrossRef]
- Ciappina, A.L.; Ferreira, F.A.; Pereira, I.R.; Sousa, T.R.; Matos, F.S.; Reis, P.R.; Gonçalves, P.J.; Bailão, E.F.; Almeida, L.M. Toxicity of Jatropha curcas L. latex in Allium cepa test. Biosci. J. 2017, 33. [Google Scholar] [CrossRef]
- Dey, D.K.; Kang, S.C. Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division, and triggers inflammatory response in zebrafish larvae. Sci. Total Environ. 2020, 737, 139704. [Google Scholar] [CrossRef]
- Hrubik, J.; Glisic, B.; Samardzija, D.; Stanic, B.; Pogrmic-Majkic, K.; Fa, S.; Andric, N. Effect of PMA-induced protein kinase C activation on development and apoptosis in early zebrafish embryos. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 190, 21–24. [Google Scholar] [CrossRef]
- Dey, D.K.; Khan, I.; Kang, S.C. Anti-bacterial susceptibility profiling of Weissella confusa DD_A7 against the multidrug-resistant ESBL-positive E. coli. Microb. Pathog. 2019, 128, 119–130. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Bhardwaj, M.; Pal Khaket, T.; Kang, S.C. Carvacrol nanoemulsion evokes cell cycle arrest, apoptosis induction and autophagy inhibition in doxorubicin resistant-A549 cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.N.; Khan, I.; Dey, D.K.; Cho, K.H.; Hwang, B.S.; Bae, K.B.; Kang, S.C.; Park, J.G. Decursinol angelate ameliorates 12-O-tetradecanoyl phorbol-13-acetate (TPA) -induced NF-κB activation on mice ears by inhibiting exaggerated inflammatory cell infiltration, oxidative stress and pro-inflammatory cytokine production. Food Chem. Toxicol. 2019, 132, 110699. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, H.J.; Hong, S.T. Iron-dextran as a thermosensitizer in radiofrequency hyperthermia for cancer treatment. Appl. Biol. Chem. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Choi, D.H.; Hwang, H.S. Anti-inflammation activity of brazilin in TNF-α induced human psoriasis dermatitis skin model. Appl. Biol. Chem. 2019, 62, 46. [Google Scholar] [CrossRef] [Green Version]
- Dey, D.K.; Chang, S.N.; Kang, S.C. The inflammation response and risk associated with aflatoxin B1 contamination was minimized by insect peptide CopA3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2020, 268, 115713. [Google Scholar] [CrossRef]
- Kang, J.; Song, J.; Shen, S.; Li, B.; Yang, X.; Chen, M. Diisononyl phthalate aggravates allergic dermatitis by activation of NF-kB. Oncotarget 2016, 7, 85472–85482. [Google Scholar] [CrossRef]
- Singh, M.P.; Chauhan, A.K.; Kang, S.C. Morin hydrate ameliorates cisplatin-induced ER stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int. Immunopharmacol. 2018, 56, 156–167. [Google Scholar] [CrossRef]
- Singh, M.P.; Khaket, T.P.; Bajpai, V.K.; Alfarraj, S.; Kim, S.G.; Chen, L.; Huh, Y.S.; Han, Y.K.; Kang, S.C. Morin hydrate sensitizes hepatoma cells and xenograft tumor towards cisplatin by downregulating PARP-1-HMGB1 mediated autophagy. Int. J. Mol. Sci. 2020, 21, 8253. [Google Scholar] [CrossRef]
- Cabuga, C.C., Jr.; Joy Abelada, J.Z.; Rose Apostado, R.Q.; Joy Hernando, B.H.; Erick Lador, J.C.; Lloyd Obenza, O.P.; James Presilda, C.R.; Havana, H.C. Allium cepa test: An evaluation of genotoxicity. Proc. Int. Acad. Ecol. Environ. Sci. 2017, 7, 12–19. [Google Scholar]
- Pereira, A.C.; Gomes, T.; Ferreira Machado, M.R.; Rocha, T.L. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: From morphological to molecular approach. Environ. Pollut. 2019, 252, 1841–1853. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.E.; Ryu, K.R.; Park, S.H.; Chung, S.; Teruya, Y.; Han, M.J.; Woo, J.T.; Kim, D.H. Nobiletin and tangeretin ameliorate scratching behavior in mice by inhibiting the action of histamine and the activation of NF-κB, AP-1 and p38. Int. Immunopharmacol. 2013, 7, 502–507. [Google Scholar] [CrossRef]
- Eun, S.H.; Woo, J.T.; Kim, D.H. Tangeretin Inhibits IL-12 Expression and NF-κB Activation in Dendritic Cells and Attenuates Colitis in Mice. Planta Med. 2017, 234, 527–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.N.; Dey, D.K.; Oh, S.T.; Kong, W.H.; Cho, K.H.; Al-Olayan, E.M.; Hwang, B.S.; Kang, S.C.; Park, J.G. Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating HIF-1α-NF-κB Crosstalk In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 9261. https://doi.org/10.3390/ijms21239261
Chang SN, Dey DK, Oh ST, Kong WH, Cho KH, Al-Olayan EM, Hwang BS, Kang SC, Park JG. Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating HIF-1α-NF-κB Crosstalk In Vitro and In Vivo. International Journal of Molecular Sciences. 2020; 21(23):9261. https://doi.org/10.3390/ijms21239261
Chicago/Turabian StyleChang, Sukkum Ngullie, Debasish Kumar Dey, Seong Taek Oh, Won Ho Kong, Kiu Hyung Cho, Ebtesam M. Al-Olayan, Buyng Su Hwang, Sun Chul Kang, and Jae Gyu Park. 2020. "Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating HIF-1α-NF-κB Crosstalk In Vitro and In Vivo" International Journal of Molecular Sciences 21, no. 23: 9261. https://doi.org/10.3390/ijms21239261
APA StyleChang, S. N., Dey, D. K., Oh, S. T., Kong, W. H., Cho, K. H., Al-Olayan, E. M., Hwang, B. S., Kang, S. C., & Park, J. G. (2020). Phorbol 12-Myristate 13-Acetate Induced Toxicity Study and the Role of Tangeretin in Abrogating HIF-1α-NF-κB Crosstalk In Vitro and In Vivo. International Journal of Molecular Sciences, 21(23), 9261. https://doi.org/10.3390/ijms21239261







