D-limonene Inhibits Pentylenetetrazole-Induced Seizure via Adenosine A2A Receptor Modulation on GABAergic Neuronal Activity
Abstract
1. Introduction
2. Results
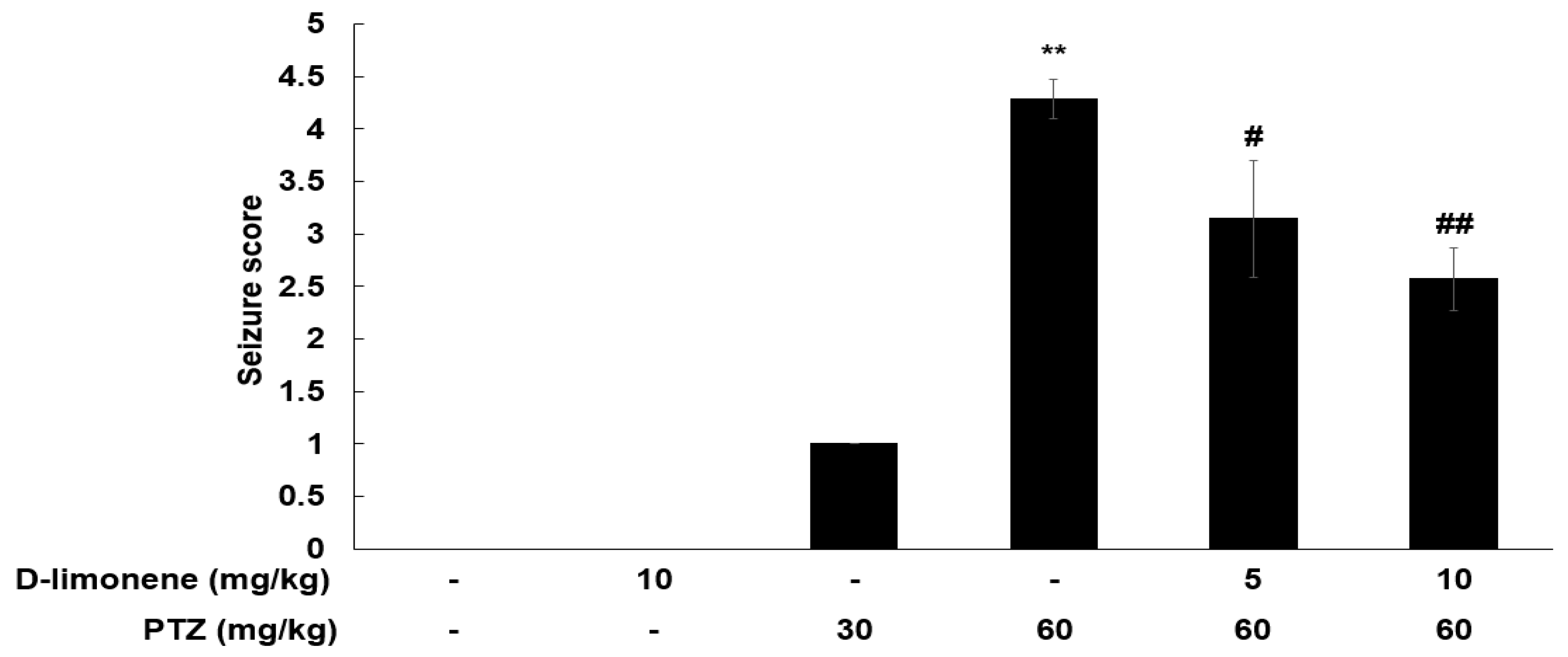
2.1. D-limonene Decreased PTZ-Induced Convulsion Dose Dependently
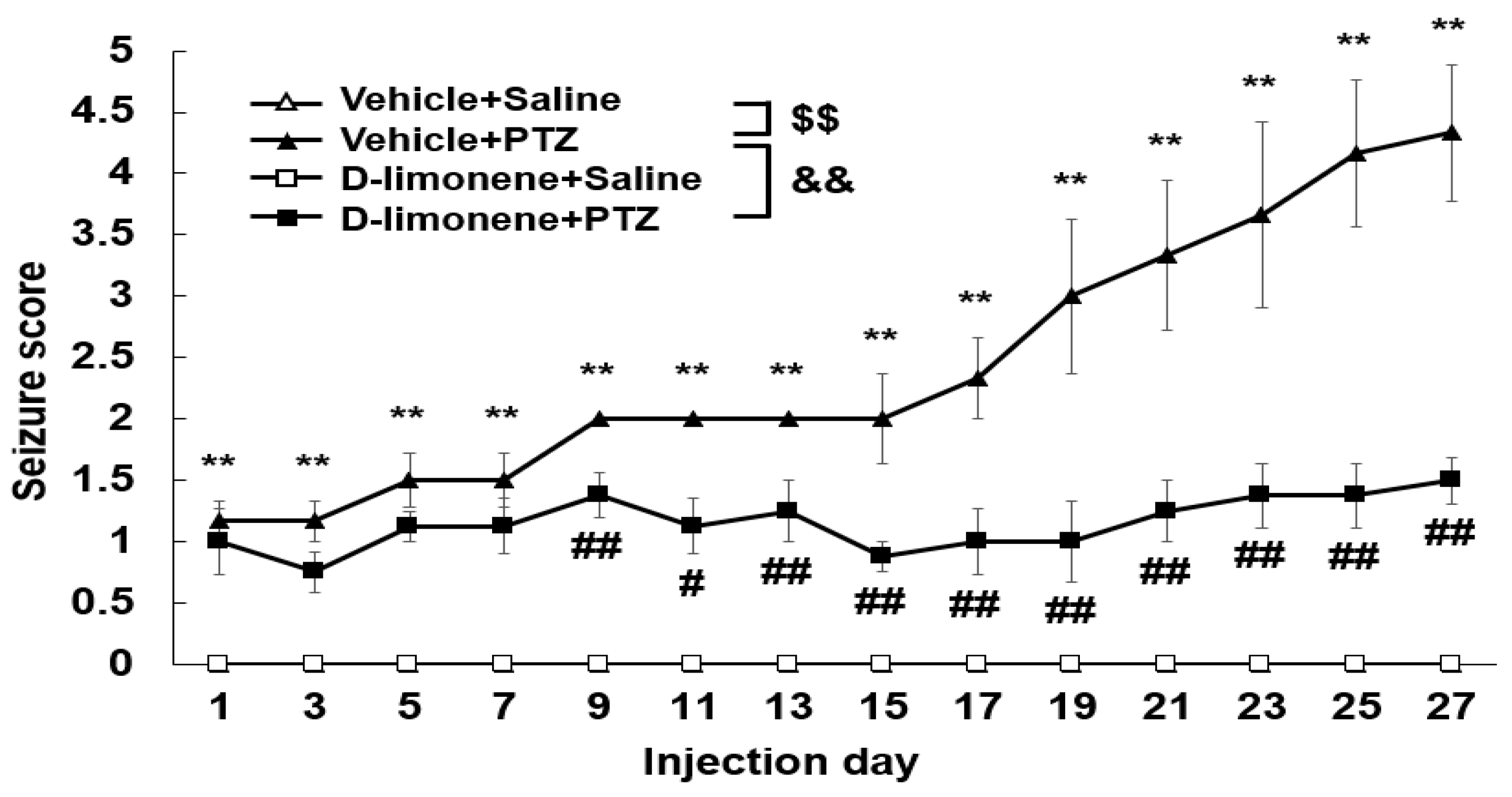
2.2. D-limonene Inhibited the Development of PTZ-Induced Kindling
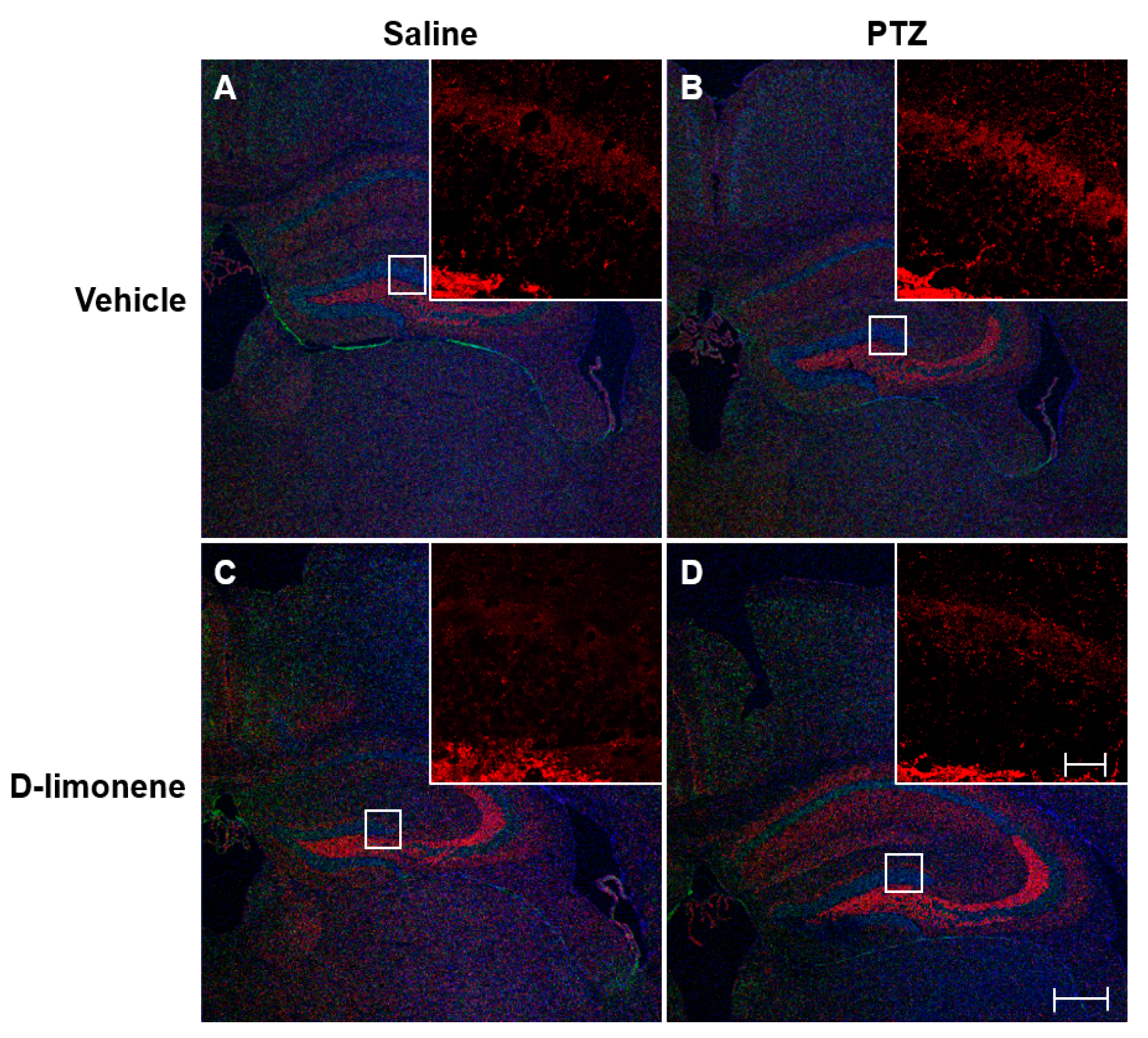
2.3. D-limonene Inhibited Axonal Sprouting in the Hippocampus
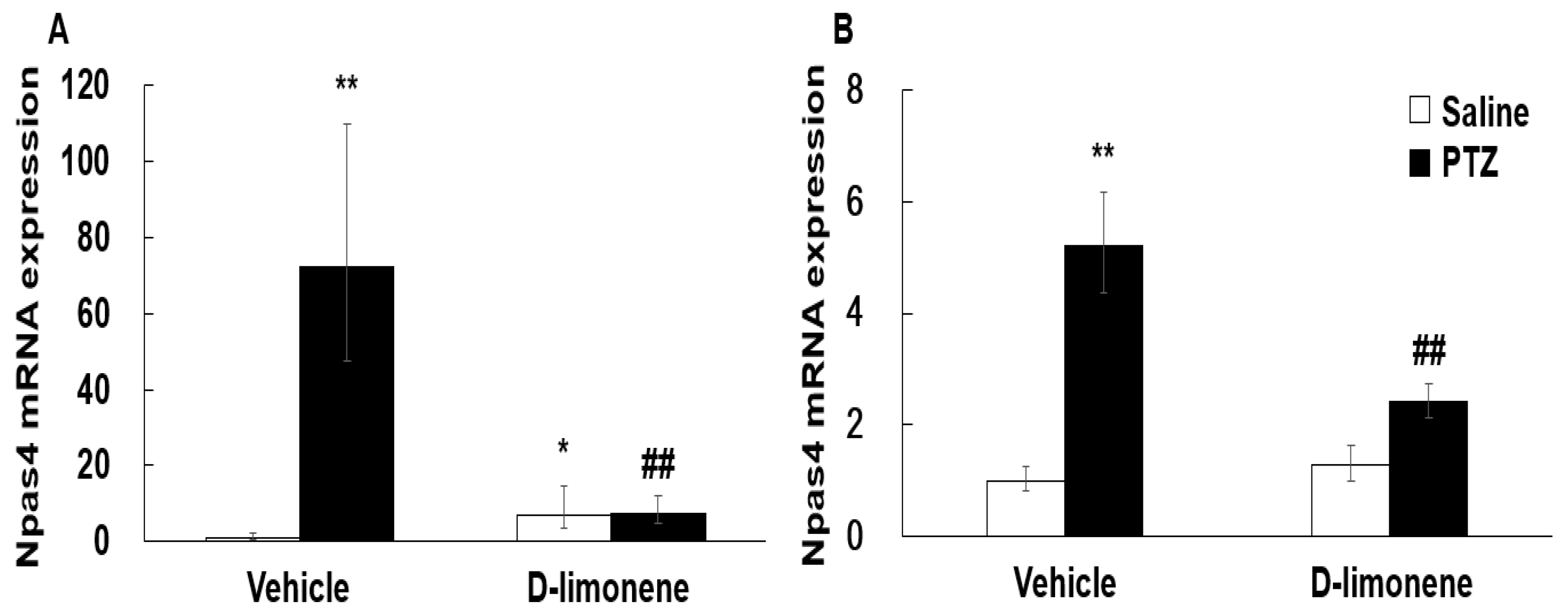
2.4. D-limonene Regulated GAD-67 and Npas4 Expression Levels
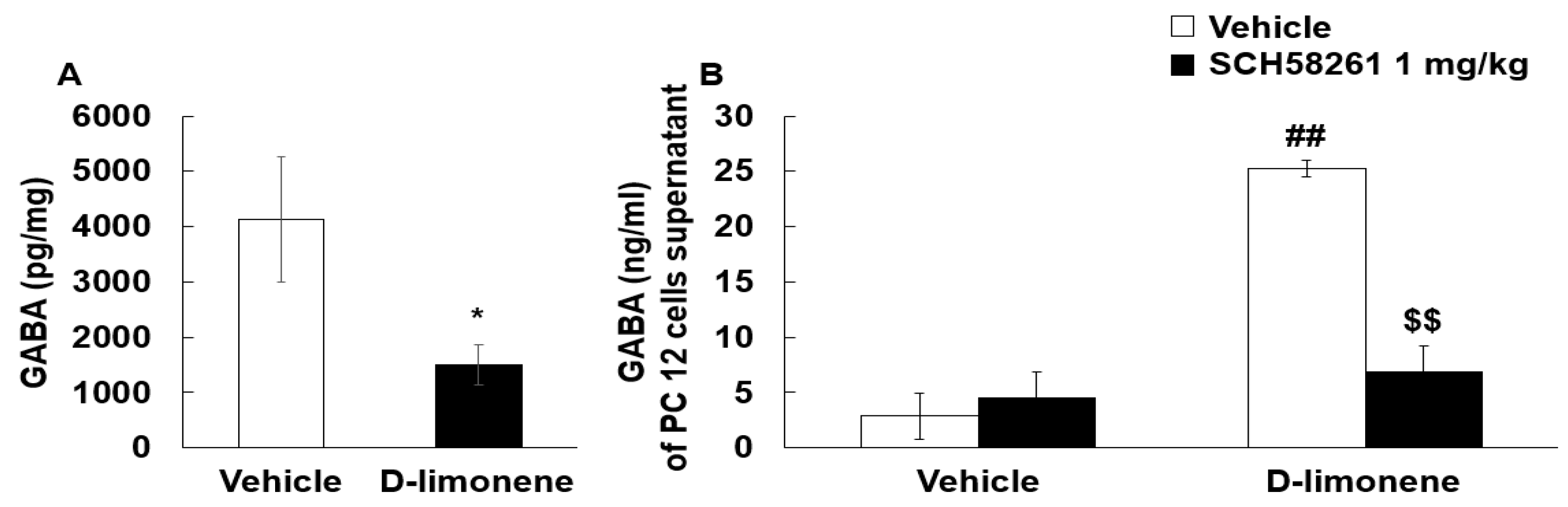
2.5. D-limonene Modulated GABA Levels in the Hippocampus and PC12 Cells
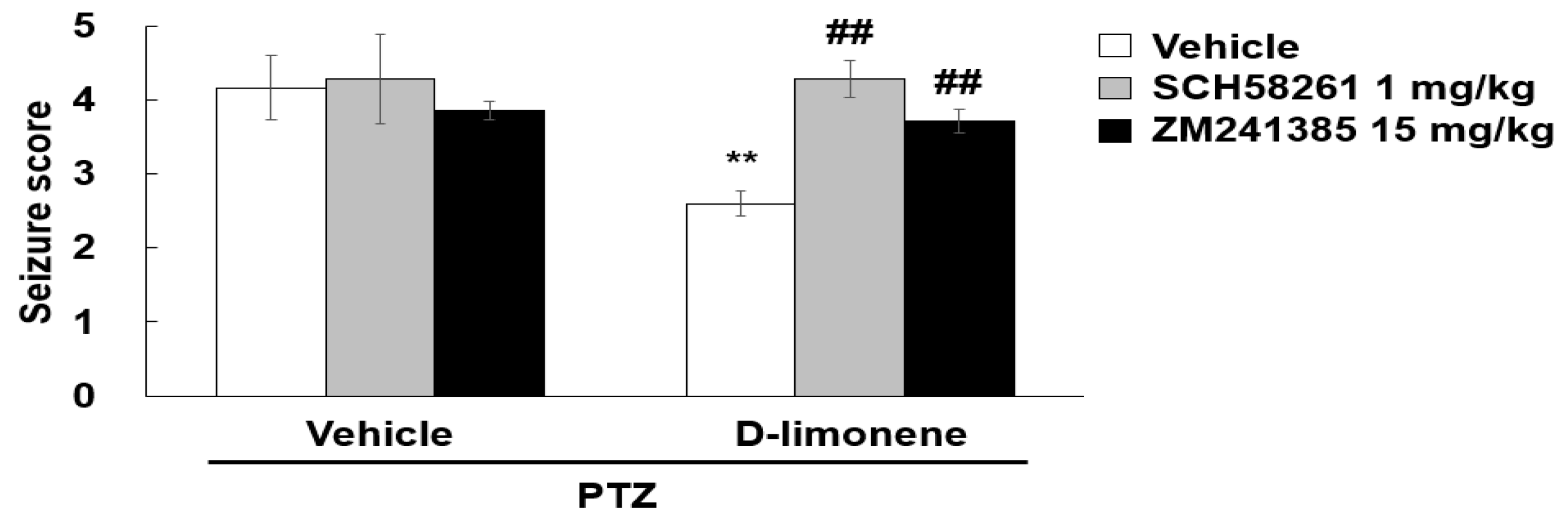
2.6. D-limonene Suppresses Seizure Score Induced by PTZ via Adenosine A2A Receptor
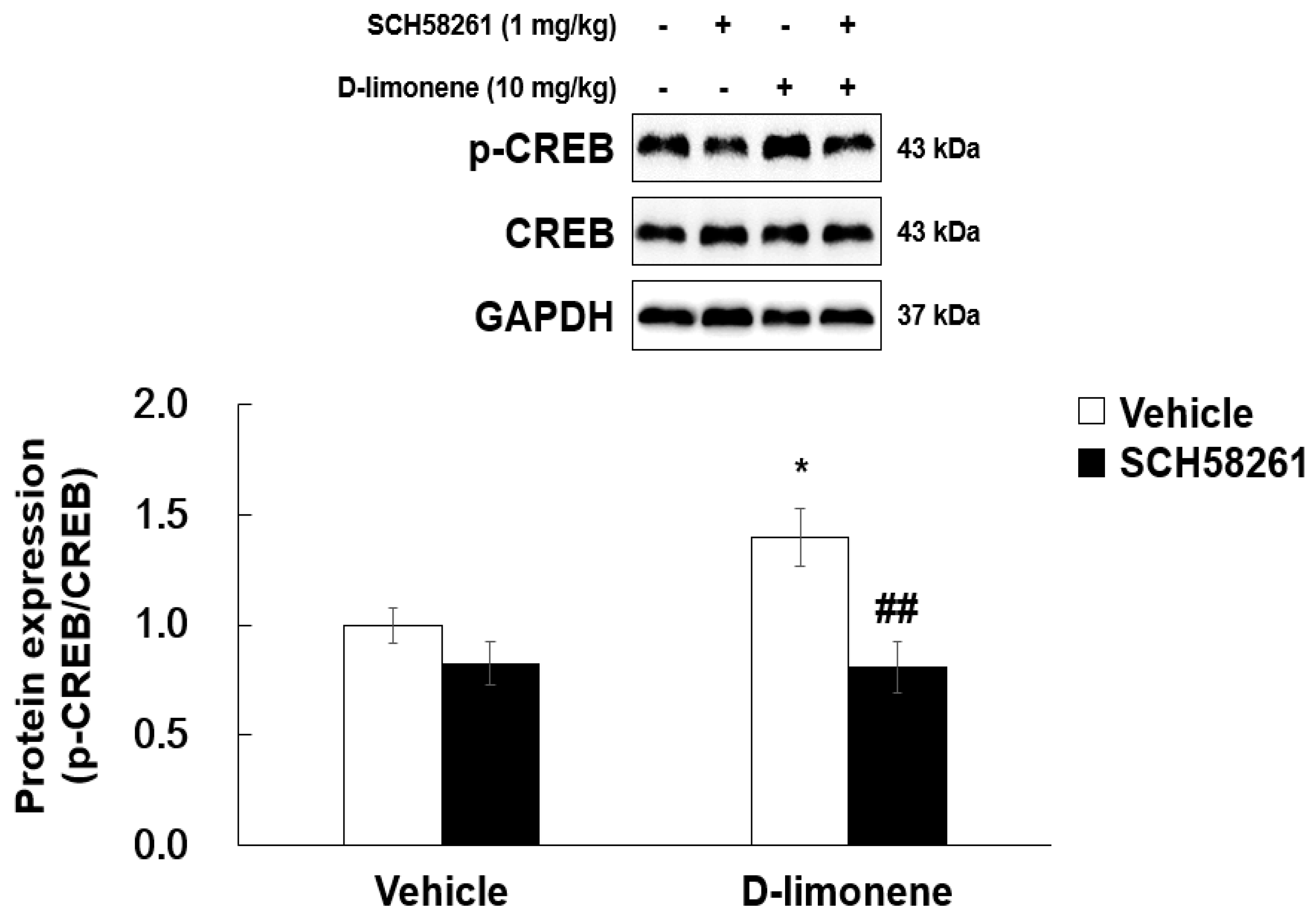
2.7. D-limonene Enhanced Phosphorylated cAMP Response Element-Binding Protein in the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Cell Culture
4.4. Pentylenetetrazole-Induced Convulsion and Kindling Model
4.5. Effects of D-limonene on PTZ-Induced Convulsion and Kindling
4.6. Immunoblotting
4.7. RNA Extraction and Real-Time RT-PCR
4.8. Immunohistochemistry
4.9. High-Performance Liquid Chromatography Analysis
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CREB | cAMP response element-binding protein |
| GABA | Gamma-aminobutyric acid |
| GAD-67 | Glutamate decarboxylase-67 |
| HPLC | High-performance liquid chromatography |
| Npas4 PC12 cell PTZ | Neuronal PAS Domain Protein 4 Pheochromocytoma cells derived from the adrenal gland of Rattus norvegicus Pentylenetetrazole |
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in Adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Flamm, W.G.; Lehman-McKeeman, L.D. The human relevance of the renal tumor-inducing potential of d-limonene in male rats: Implications for risk assessment. Regul. Toxicol. Pharmacol. 1991, 13, 70–86. [Google Scholar] [CrossRef]
- De Almeida, A.A.C.; Costa, J.P.; de Carvalho, R.B.F.; de Sousa, D.P.; de Freitas, R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012, 1448, 56–62. [Google Scholar] [CrossRef]
- Amaral, J.F.D.; Silva, M.I.G.; Neto, M.R.A.D.A.; Neto, P.F.T.; Moura, B.A.; De Melo, C.T.V.; De Araújo, F.L.O.; De Sousa, D.P.; De Vasconcelos, P.F.; De Vasconcelos, S.M.M.; et al. Antinociceptive effect of the monoterpene R-()-limonene in mice. Biol. Pharm. Bull. 2007, 30, 1217–1220. [Google Scholar] [CrossRef]
- Lima, N.G.P.B.; Sousa, D.P.D.; Pimenta, F.C.F.; Alves, M.F.; Souza, F.S.D.; Macedo, R.O.; Cardoso, R.B.; Morais, L.C.S.L.d.; Diniz, M.d.F.F.M.; Almeida, R.N.d. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol. Biochem. Behav. 2013, 103, 450–454. [Google Scholar] [CrossRef]
- Zhou, W.; Yoshioka, M.; Yokogoshi, H. Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol. 2009, 55, 367–373. [Google Scholar] [CrossRef]
- Tujioka, K.; Okuyama, S.; Yokogoshi, H.; Fukaya, Y.; Hayase, K.; Horie, K.; Kim, M. Dietary gamma-aminobutyric acid affects the brain protein synthesis rate in young rats. Amino Acids 2007, 32, 255–260. [Google Scholar] [CrossRef]
- Bahr, T.A.; Rodriguez, D.; Beaumont, C.; Allred, K. The effects of various essential oils on epilepsy and acute seizure: A systematic review. Evid.-Based Complement Alternat. Med. 2019, 2019, 6216745. [Google Scholar] [CrossRef]
- Dhir, A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr. Protoc. Neurosci. 2012, 58, 9–37. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, J.H.; Yaoyao, J.; Jun, H.J.; Lee, S.J. Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A2A receptors. Biochem. Biophys. Res. Commun. 2011, 404, 345–348. [Google Scholar] [CrossRef]
- Canas, P.M.; Porciúncula, L.O.; Simões, A.P.; Augusto, E.; Silva, H.B.; Machado, N.J.; Gonçalves, N.; Alfaro, T.M.; Gonçalves, F.Q.; Araújo, I.M.; et al. Neuronal adenosine A2A receptors are critical mediators of neurodegeneration triggered by convulsions. eNeuro 2018, 5, ENEURO.0385-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.M.; Mishra, C.B.; Jha, P.K.; Barodia, S.K. Synthesis of novel 7-imino-2-thioxo-3,7-dihydro-2H-thiazolo [4,5-d] pyrimidine derivatives as adenosine A2A receptor antagonists. Bioorgan. Med. Chem. Lett. 2010, 20, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, V.-P.; Griffith, M.T.; Hanson, M.A.; Cherezov, V.; Chien, E.Y.T.; Lane, J.R.; Ijzerman, A.P.; Stevens, R.C. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 2008, 322, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Sutula, T.; He, X.X.; Cavazos, J.; Scott, G. Synaptic Reorganization in the hippocampus induced by abnormal functional activity. Science 1988, 239, 1147–1150. [Google Scholar] [CrossRef]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef]
- Mello, L.E.; Cavalheiro, E.A.; Tan, A.M.; Kupfer, W.R.; Pretorius, J.K.; Babb, T.L.; Finch, D.M. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: Cell loss and mossy fiber sprouting. Epilepsia 1993, 34, 985–995. [Google Scholar] [CrossRef]
- Almeida, A.A.C.; Ferreira, J.R.O.; de Carvalho, R.B.F.; Rizzo, M.D.S.; Lopes, L.D.S.; Dittz, D.; Castro, E.S.J.M.; Ferreira, P.M.P. Non-clinical toxicity of (+)-limonene epoxide and its physio-pharmacological properties on neurological disorders. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2301–2314. [Google Scholar] [CrossRef]
- Corsi, C.; Melani, A.; Bianchi, L.; Pepeu, G.; Pedata, F. Effect of adenosine A2A receptor stimulation on GABA release from the striatum of young and aged rats in vivo. Neuroreport 1999, 10, 3933–3937. [Google Scholar] [CrossRef]
- Cunha, R.A.; Ribeiro, J.A. Purinergic modulation of [3H]GABA release from rat hippocampal nerve terminals. Neuropharmacology 2000, 39, 1156–1167. [Google Scholar] [CrossRef]
- Bogenpohl, J.W.; Ritter, S.L.; Hall, R.A.; Smith, Y. Adenosine A2A Receptor in the monkey basal ganglia: Ultrastructural localization and colocalization with the metabotropic glutamate receptor 5 in the striatum. J. Comp. Neurol. 2012, 520, 570–589. [Google Scholar] [CrossRef]
- Sun, X.; Lin, Y. Npas4: Linking neuronal activity to memory. Trends Neurosci. 2016, 39, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Maya-Vetencourt, J.F.; Tiraboschi, E.; Greco, D.; Restani, L.; Cerri, C.; Auvinen, P.; Maffei, L.; Castrén, E. Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J. Physiol. 2012, 590, 4777–4787. [Google Scholar] [CrossRef] [PubMed]
- Bshesh, K.; Zhao, B.; Spight, D.; Biaggioni, I.; Feokistov, I.; Denenberg, A.; Wong, H.R.; Shanley, T.P. The A2A receptor mediates an endogenous regulatory pathway of cytokine expression in THP-1 cells. J. Leukoc. Biol. 2002, 72, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Nagai, T.; Furukawa-Hibi, Y.; Kuroda, K.; Kaibuchi, K.; Greenberg, M.E.; Yamada, K. Neuronal per Arnt Sim (PAS) domain protein 4 (NPAS4) regulates neurite outgrowth and phosphorylation of synapsin I. J. Biol. Chem. 2013, 288, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, 10, e56573. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.; Song, Y.; Gu, S.M.; Min, H.K.; Hong, J.T.; Cha, H.J.; Yun, J. D-limonene Inhibits Pentylenetetrazole-Induced Seizure via Adenosine A2A Receptor Modulation on GABAergic Neuronal Activity. Int. J. Mol. Sci. 2020, 21, 9277. https://doi.org/10.3390/ijms21239277
Seo S, Song Y, Gu SM, Min HK, Hong JT, Cha HJ, Yun J. D-limonene Inhibits Pentylenetetrazole-Induced Seizure via Adenosine A2A Receptor Modulation on GABAergic Neuronal Activity. International Journal of Molecular Sciences. 2020; 21(23):9277. https://doi.org/10.3390/ijms21239277
Chicago/Turabian StyleSeo, Sowoon, Yunjeong Song, Sun Mi Gu, Hyun Kyu Min, Jin Tae Hong, Hye Jin Cha, and Jaesuk Yun. 2020. "D-limonene Inhibits Pentylenetetrazole-Induced Seizure via Adenosine A2A Receptor Modulation on GABAergic Neuronal Activity" International Journal of Molecular Sciences 21, no. 23: 9277. https://doi.org/10.3390/ijms21239277
APA StyleSeo, S., Song, Y., Gu, S. M., Min, H. K., Hong, J. T., Cha, H. J., & Yun, J. (2020). D-limonene Inhibits Pentylenetetrazole-Induced Seizure via Adenosine A2A Receptor Modulation on GABAergic Neuronal Activity. International Journal of Molecular Sciences, 21(23), 9277. https://doi.org/10.3390/ijms21239277




