Exopolysaccharide Isolated from Lactobacillus plantarum L-14 Has Anti-Inflammatory Effects via the Toll-Like Receptor 4 Pathway in LPS-Induced RAW 264.7 Cells
Abstract
:1. Introduction
2. Results
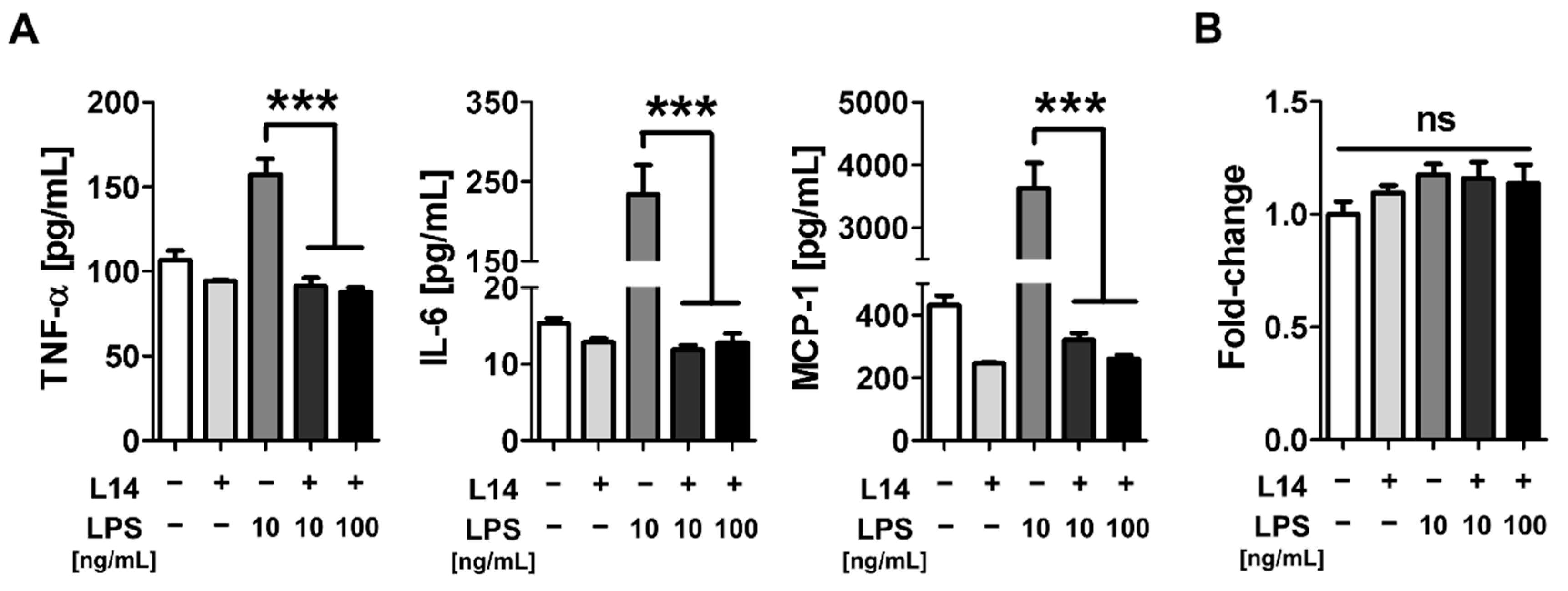
2.1. Co-Culture with L-14 Inhibited the Induction of Inflammation by LPS in RAW 264.7 Cells
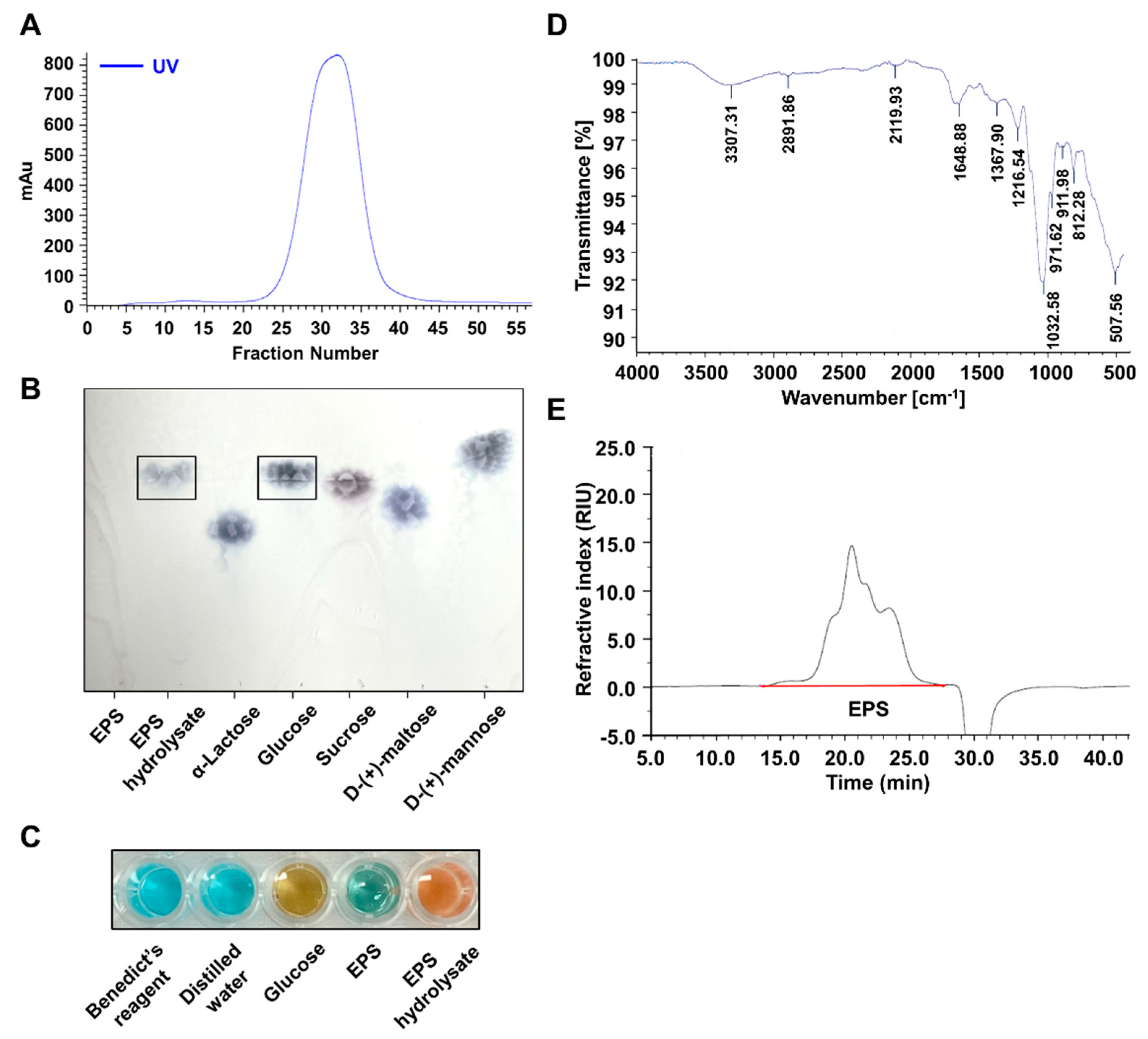
2.2. EPS Isolated from L-14 Was a Homogeneous Polysaccharide Primarily Composed of Glucose
2.3. EPS Isolated from L-14 Alleviated Morphological Changes Induced by LPS within Mouse Macrophages
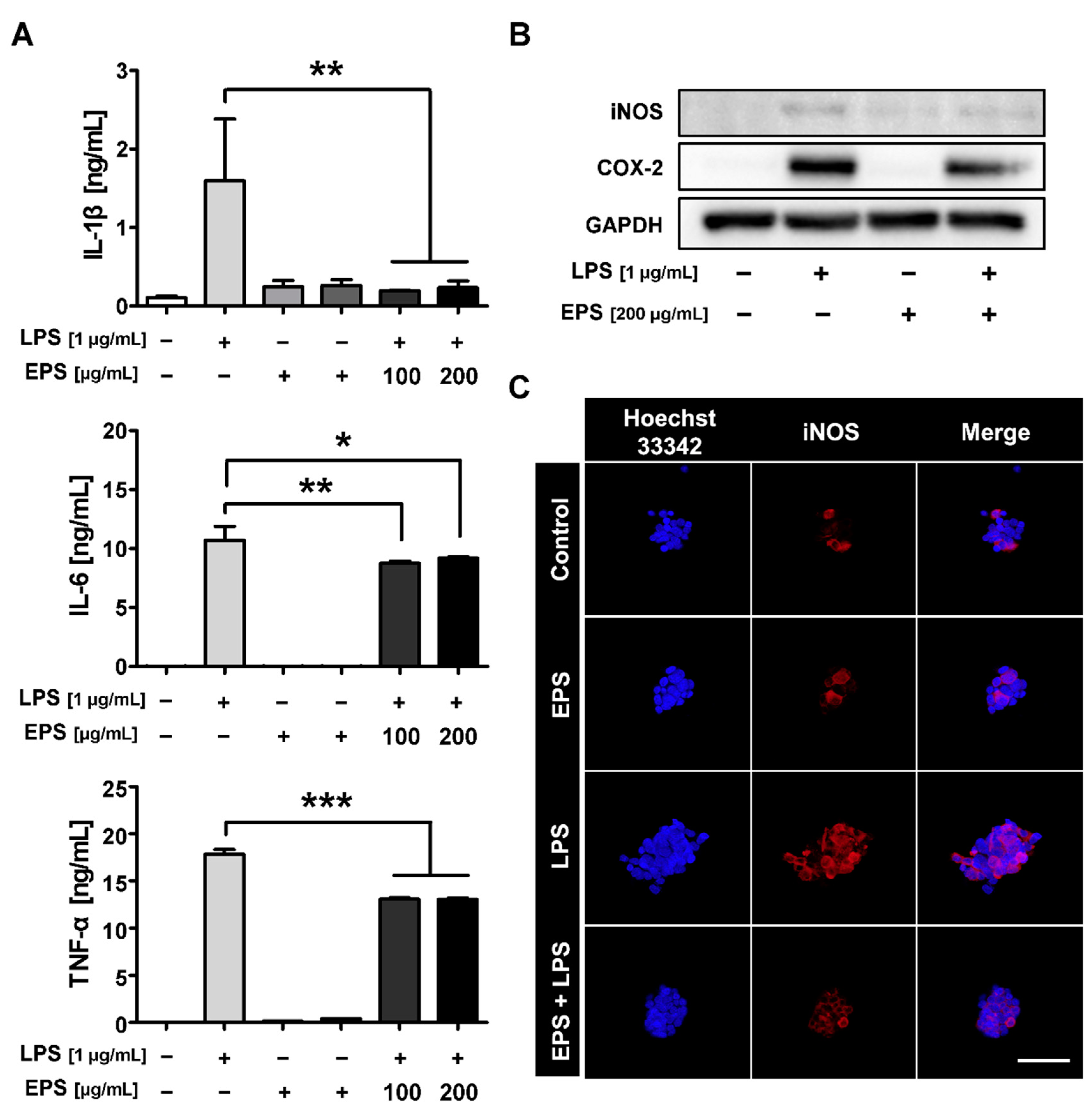
2.4. LPS-Induced Inflammatory Response Was Inhibited by EPS Pretreatment
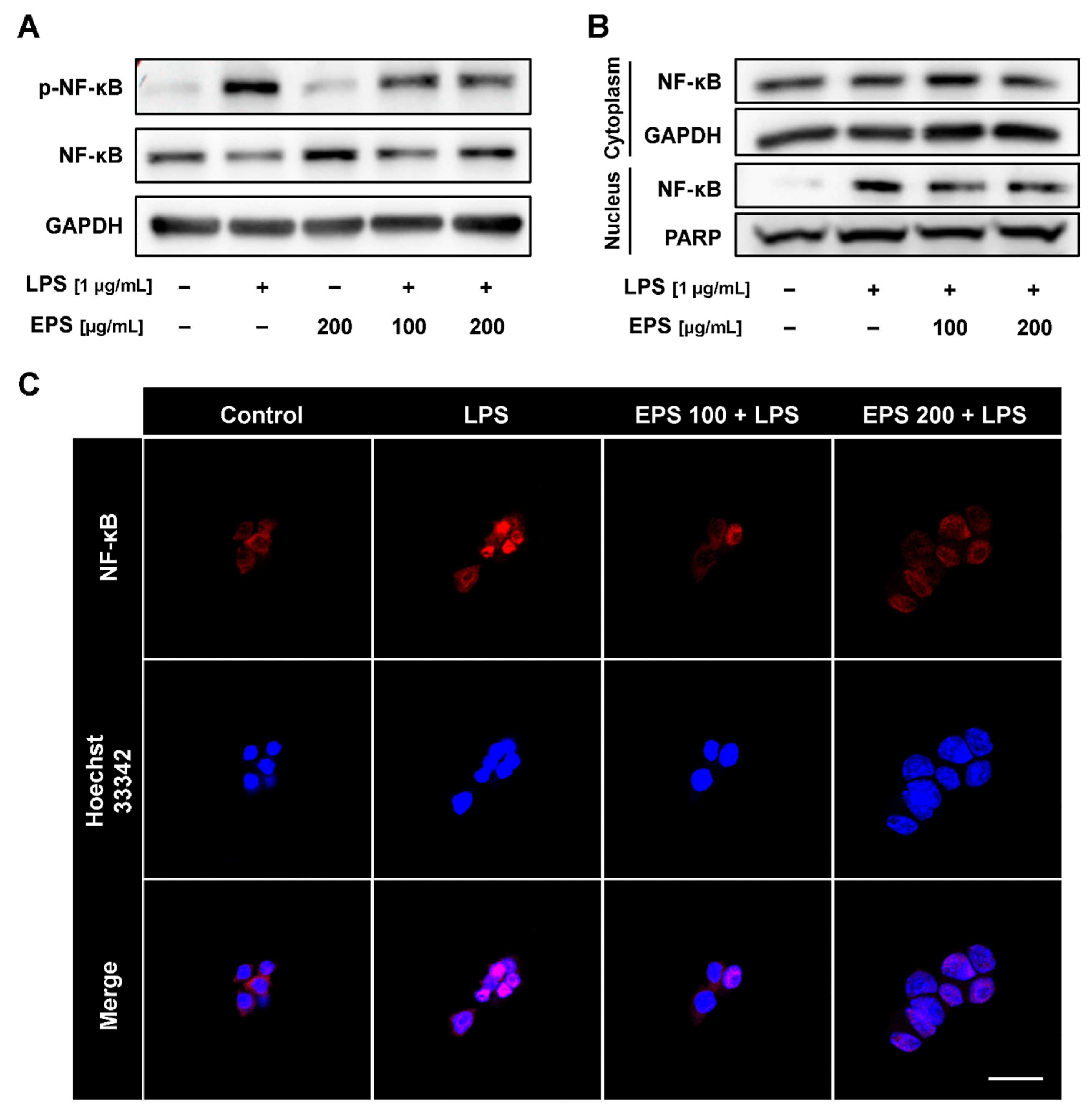
2.5. EPS Inhibited Nuclear Translocation of NF-κB Induced by LPS
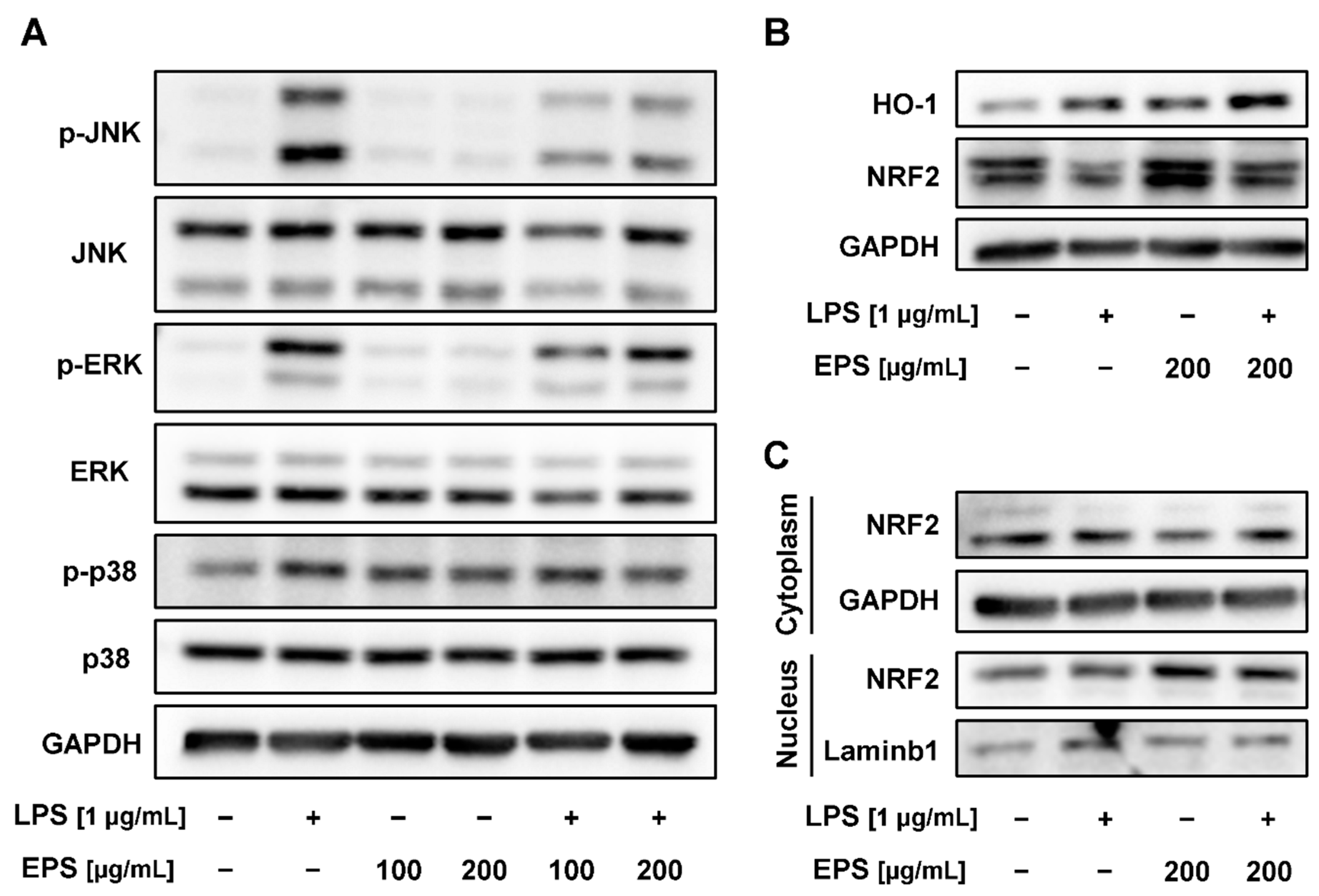
2.6. EPS-Repressed Inflammatory Response via Regulation of MAPK and Nuclear Factor E2-Related Factor 2 (NRF2)/Heme Oxygenase-1 (HO-1) Pathways in RAW 264.7 Cells
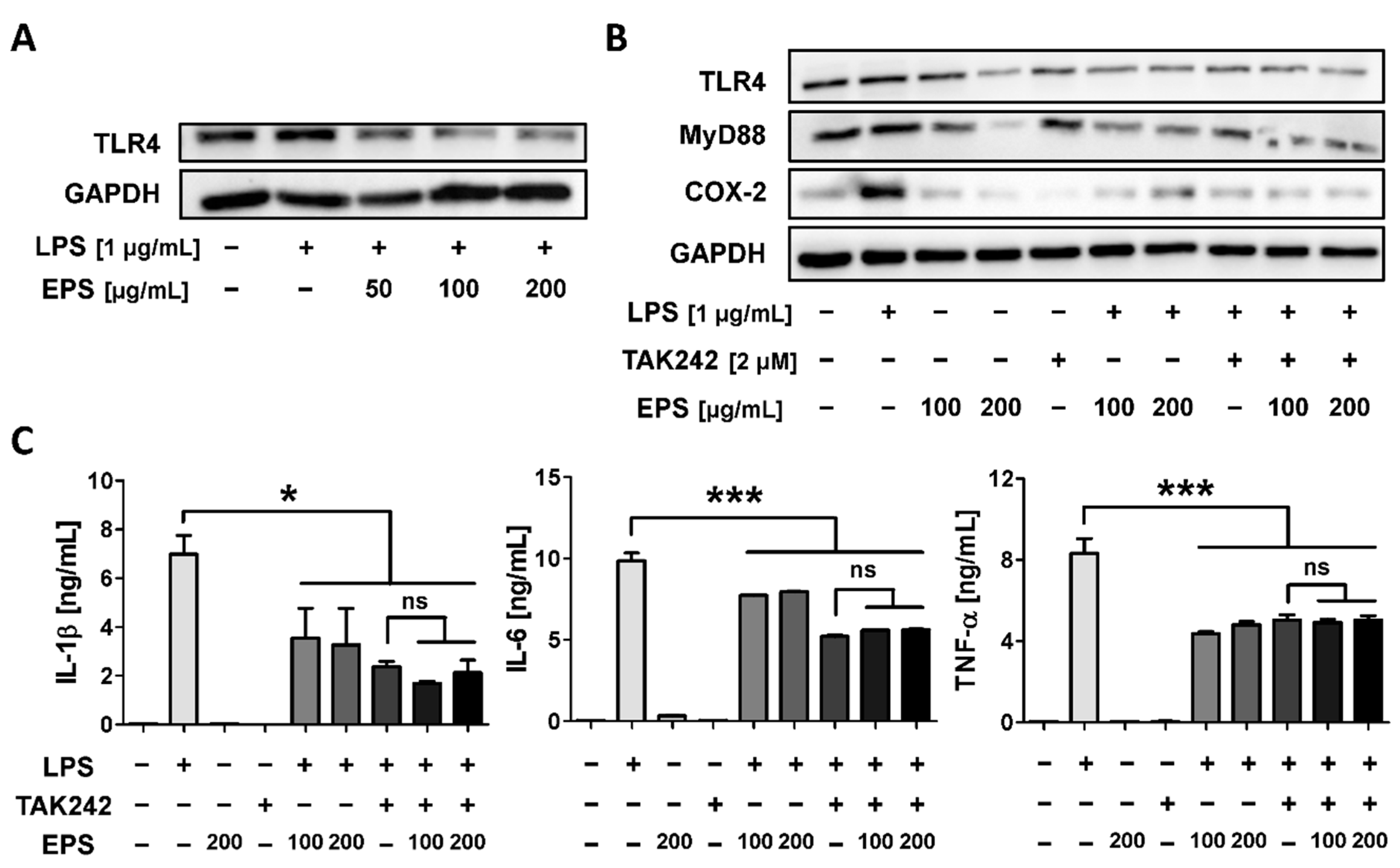
2.7. EPS Inhibits the Inflammatory Response by Suppressing the Interaction between LPS and TLR4
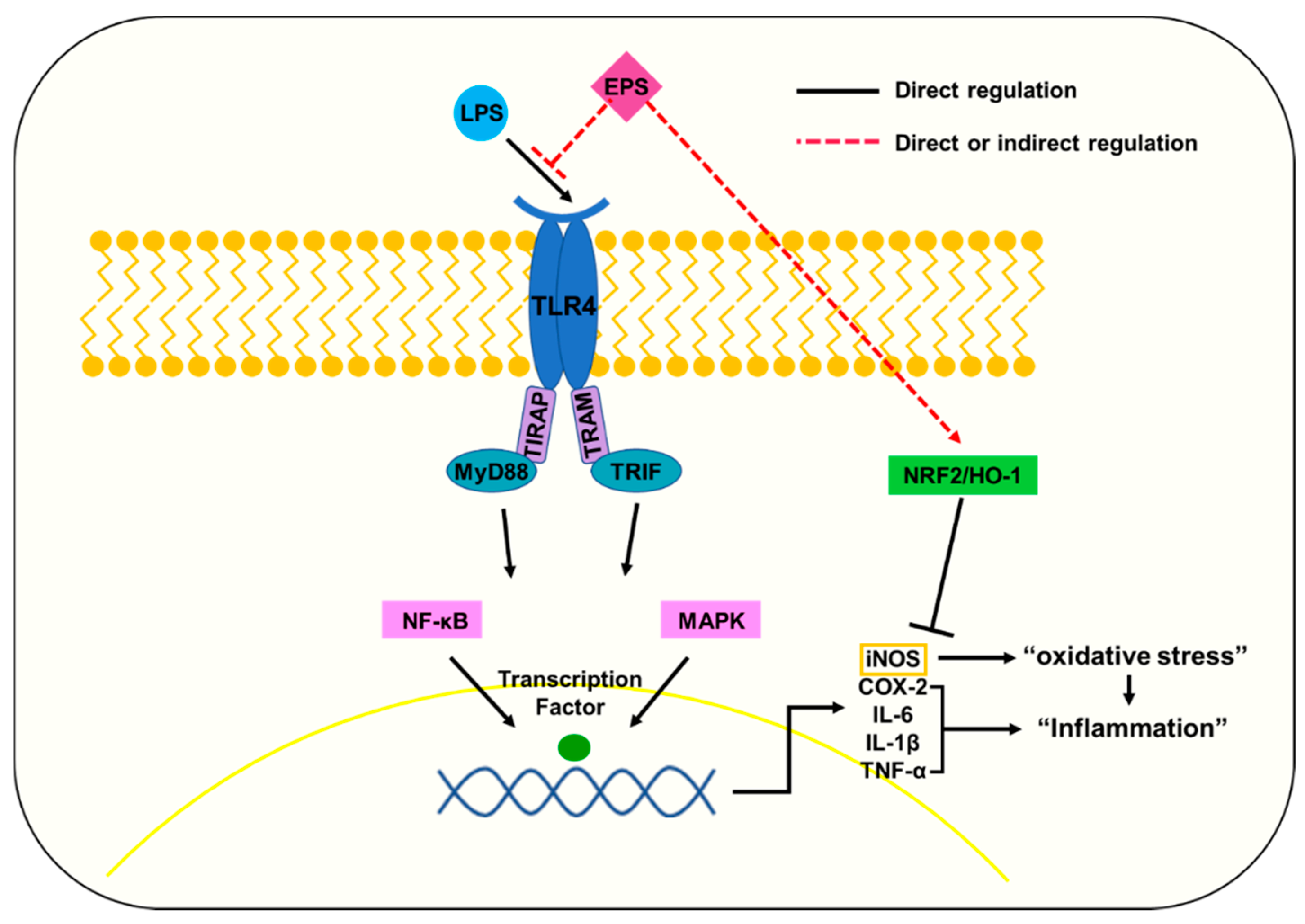
3. Discussion
4. Materials and Methods
4.1. Material
4.2. L-14 Culture and Exopolysaccharide Purification
4.3. FPLC
4.4. TLC and Benedict’s Test
4.5. FTIR and GPC
4.6. Cell Culture
4.7. Cell Viability Assay
4.8. ELISA
4.9. Crystal Violet Staining
4.10. Western Blot
4.11. IF Assay
4.12. Statistics
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| COX-2 | Cyclooxygenase-2 |
| DW | Distilled water |
| E. coli | Escherichia coli |
| ELISA | Enzyme-linked immunosorbent assay |
| EPS | Exopolysaccharide |
| FPLC | Fast protein liquid chromatography |
| FTIR | Fourier-transform infrared spectroscopy |
| GPC | Gel permeation chromatography |
| HO-1 | Heme oxygenase-1 |
| HoPS | Homopolysaccharide |
| IF | Immunofluorescence |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| L. plantarum | Lactobacillus plantarum |
| LAB | Lactic acid bacteria |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDD | Major depression disorder |
| MyD88 | Myeloid differentiation factor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NRF2 | Nuclear factor E2-related factor 2 |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| SD | Standard deviation |
| TLC | Thin layer chromatography |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor-α |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
References
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Gaestel, M.; Kotlyarov, A.; Kracht, M. Targeting innate immunity protein kinase signalling in inflammation. Nat. Rev. Drug Discov. 2009, 8, 480–499. [Google Scholar] [CrossRef]
- Huang, H.; Fang, M.; Jostins, L.; Mirkov, M.U.; Boucher, G.; Anderson, C.A.; Andersen, V.; Cleynen, I.; Cortes, A.; Crins, F. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 2017, 547, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Anda-Jáuregui, G.; Guo, K.; McGregor, B.A.; Hur, J. Exploration of the anti-inflammatory drug space through network pharmacology: Applications for drug repurposing. Front. Physiol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, K.; Maes, M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Li, P.-Y.; Liang, Y.-C.; Sheu, M.-J.; Huang, S.-S.; Chao, C.-Y.; Kuo, Y.-H.; Huang, G.-J. Alpinumisoflavone attenuates lipopolysaccharide-induced acute lung injury by regulating the effects of anti-oxidation and anti-inflammation both in vitro and in vivo. RSC Adv. 2018, 8, 31515–31528. [Google Scholar] [CrossRef] [Green Version]
- Yücel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.-H.; Cyganek, L.; Utikal, J. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 2935. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Huang, S.; He, H.; Lei, T.; Saaoud, F.; Yu, X.-Q.; Melnick, A.; Kumar, A.; Papasian, C.J. Central role of myeloid MCPIP1 in protecting against LPS-induced inflammation and lung injury. Signal Transduct. Target. Ther. 2017, 2, 17066. [Google Scholar] [CrossRef]
- Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. WHO/FAO. 2001, Volume 5, pp. 1–10. Available online: http://pc.ilele.hk/public/pdf/20190225/bd3689dfc2fd663bb36def1b672ce0a4.pdf (accessed on 26 October 2020).
- Kamiński, M.; Łoniewski, I.; Marlicz, W. Global internet data on the interest in antibiotics and probiotics generated by Google Trends. Antibiotics 2019, 8, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natural Products Insider. Available online: https://www.naturalproductsinsider.com/digestive-health/new-market-profile-probiotics-consumption (accessed on 12 July 2019).
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L. In vitro study of postbiotics from Lactobacillus plantarum RG14 on rumen fermentation and microbial population. Rev. Bras. Zootecn. 2018, 47. [Google Scholar] [CrossRef] [Green Version]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Alitheen, N.B.M.; Yeap, S.K.; Mutalib, N.E.A.; Rahim, R.A.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for preventing and treating common infectious diseases in children: A systematic review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Jin, M.-M.; Meng, J.; Gao, S.-M.; Lu, R.-R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Albarracin, L.; Kobayashi, H.; Iida, H.; Komatsu, R.; Kober, A.H.; Ikeda-Ohtsubo, W.; Suda, Y.; Aso, H.; Makino, S. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol. Immunol. 2018, 93, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Pană, A.-M.; Rusnac, L.-M.; Bandur, G.; Silion, M.; Deleanu, C.; Bălan, M. Novel D-glucose and D-mannose based oligomers: Synthesis and characterization. E-Polymers 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Nataraj, S.; Schomäcker, R.; Kraume, M.; Mishra, I.; Drews, A. Analyses of polysaccharide fouling mechanisms during crossflow membrane filtration. J. Membr. Sci. 2008, 308, 152–161. [Google Scholar] [CrossRef]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman spectroscopies as tools for biofilm characterization created by cariogenic streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 2014, 20, 2515–2532. [Google Scholar] [CrossRef]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Tech. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M. Lactobacillus delbrueckii TUA 4408 L and its extracellular polysaccharides attenuate enterotoxigenic E scherichia coli-induced inflammatory response in porcine intestinal epitheliocytes via T oll-like receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Friedman, S.L. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, J.; Cammarota, E.; Wright, J.A.; Cicuta, P.; Gottschalk, R.A.; Li, N.; Fraser, I.D.; Bryant, C.E. Lipopolysaccharide-induced NF-κB nuclear translocation is primarily dependent on MyD88, but TNFα expression requires TRIF and MyD88. Sci. Rep. 2017, 7, 1428. [Google Scholar] [CrossRef]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer’s disease (AD) brain and impairs transcription in human neuronal-glial primary co-cultures. Front. Aging. Neurosci. 2017, 9, 407. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Margolles, A.; Wells, J.M.; Ruas-Madiedo, P. Exopolysaccharides synthesized by Bifidobacterium animalis subsp. lactis interact with TLR4 in intestinal epithelial cells. Anaerobe 2019, 56, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int. J. Cell. Biol. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Goralski, K.B.; Ladda, M.A.; McNeil, J.O. Drug-cytokine interactions. In Drug Interactions in Infectious Diseases: Mechanisms and Models of Drug Interactions; Springer: Berlin, Germany, 2018; pp. 163–204. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Zhang, H.; Baloch, Z. Pathogenetic and therapeutic applications of tumor necrosis factor-α (TNF-α) in major depressive disorder: A systematic review. Int. J. Mol. Sci. 2016, 17, 733. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Chang, Y.-T.; Juan, C.-K.; Shen, J.-L.; Lin, Y.-P.; Shieh, J.-J.; Liu, H.-N.; Chen, Y.-J. Depression and insomnia in patients with psoriasis and psoriatic arthritis taking tumor necrosis factor antagonists. Medicine 2016, 95, e3816. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Cardioimmunology of arrhythmias: The role of autoimmune and inflammatory cardiac channelopathies. Nat. Rev. Immunol. 2019, 19, 63–64. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J.; Kastelein, J.; Koenig, W.; Genest, J.; Lorenzatti, A. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2018, 391, 319–328. [Google Scholar] [CrossRef]
- Baldo, B.A. Other Approved Therapeutic Monoclonal Antibodies; Springer: Berlin, Germany, 2016; pp. 141–215. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B. Transcription factor NRF2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [Green Version]
- Brandes, M.S.; Gray, N.E. NRF2 as a therapeutic target in neurodegenerative diseases. ASN. Neuro. 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Bohush, A.; Niewiadomska, G.; Filipek, A. Role of mitogen activated protein kinase signaling in Parkinson’s disease. Int. J. Mol. Sci. 2018, 19, 2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Karmakar, S.; Babu, S.P.S. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, Y.; Miller, L.C.; Blecha, F. Macrophage polarization in virus-host interactions. J. Clin. Cell. Immunol. 2015, 6, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Sohn, K.M.; Lee, S.-G.; Kim, H.J.; Cheon, S.; Jeong, H.; Lee, J.; Kim, I.S.; Silwal, P.; Kim, Y.J.; Park, C. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 2020, 35. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Strange, D.P.; Wong, T.A.S.; Lehrer, A.T.; Verma, S. Ebola virus glycoprotein induces an innate immune response in vivo via TLR4. Front. Microbiol. 2017, 8, 1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirey, K.A.; Lai, W.; Scott, A.J.; Lipsky, M.; Mistry, P.; Pletneva, L.M.; Karp, C.L.; McAlees, J.; Gioannini, T.L.; Weiss, J. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013, 497, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Huang, W.; Zhao, J.; Yang, Z. Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo. J. Ethnopharmacol. 2020, 252, 112584. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, G.; Li, Y.; Wang, Y.; Liu, Z. Knockdown of TLR4 in the arcuate nucleus improves obesity related metabolic disorders. Sci. Rep. 2017, 7, 7441. [Google Scholar] [CrossRef]
- Bakar, M.H.A.; Azmi, M.N.; Shariff, K.A.; Tan, J.S. Withaferin A protects against high-fat diet–induced obesity via attenuation of oxidative stress, inflammation, and insulin resistance. Appl. Biochem. Biotechnol. 2019, 188, 241–259. [Google Scholar] [CrossRef]
- Ain, Q.U.; Batool, M.; Choi, S. TLR4-targeting therapeutics: Structural basis and computer-aided drug discovery approaches. Molecules 2020, 25, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, V.K.; Majumder, R.; Rather, I.A.; Kim, K. Extraction, isolation and purification of exopolysaccharide from lactic acid bacteria using ethanol precipitation method. Bangladesh J. Pharmacol. 2016, 11, 573–576. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.; Lee, J.; Park, S.; Kwon, O.-H.; Seo, J.; Roh, S. Exopolysaccharide Isolated from Lactobacillus plantarum L-14 Has Anti-Inflammatory Effects via the Toll-Like Receptor 4 Pathway in LPS-Induced RAW 264.7 Cells. Int. J. Mol. Sci. 2020, 21, 9283. https://doi.org/10.3390/ijms21239283
Kwon M, Lee J, Park S, Kwon O-H, Seo J, Roh S. Exopolysaccharide Isolated from Lactobacillus plantarum L-14 Has Anti-Inflammatory Effects via the Toll-Like Receptor 4 Pathway in LPS-Induced RAW 264.7 Cells. International Journal of Molecular Sciences. 2020; 21(23):9283. https://doi.org/10.3390/ijms21239283
Chicago/Turabian StyleKwon, Mijin, Jaehoon Lee, Sangkyu Park, Oh-Hee Kwon, Jeongmin Seo, and Sangho Roh. 2020. "Exopolysaccharide Isolated from Lactobacillus plantarum L-14 Has Anti-Inflammatory Effects via the Toll-Like Receptor 4 Pathway in LPS-Induced RAW 264.7 Cells" International Journal of Molecular Sciences 21, no. 23: 9283. https://doi.org/10.3390/ijms21239283




