AmOctα2R: Functional Characterization of a Honeybee Octopamine Receptor Inhibiting Adenylyl Cyclase Activity
Abstract
:1. Introduction
2. Results
2.1. Molecular and Structural Properties of AmOctα2R
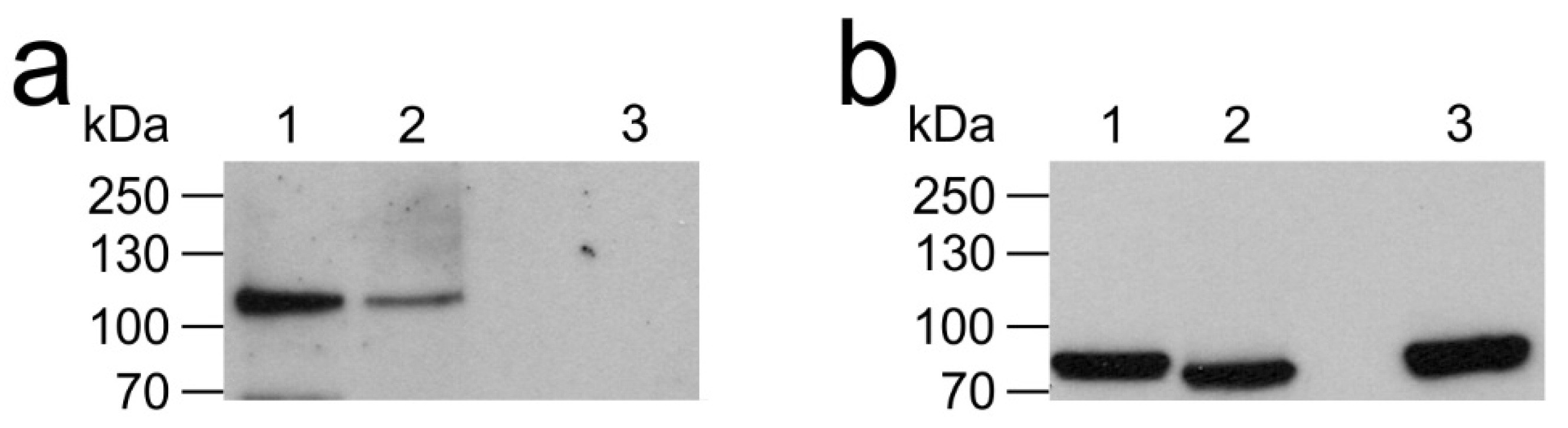
2.2. Expression of AmOctα2R-HA in flpTM Cells
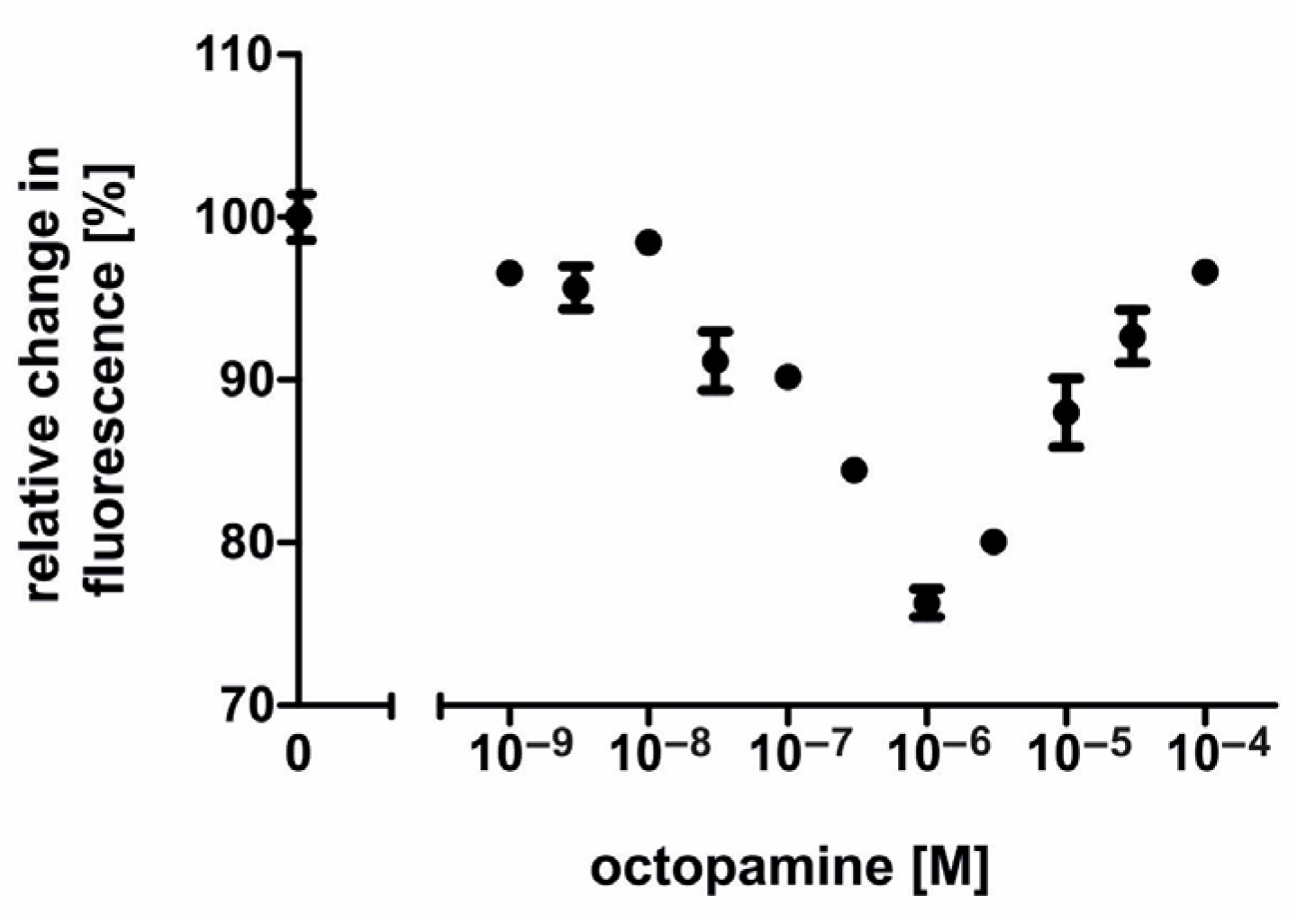
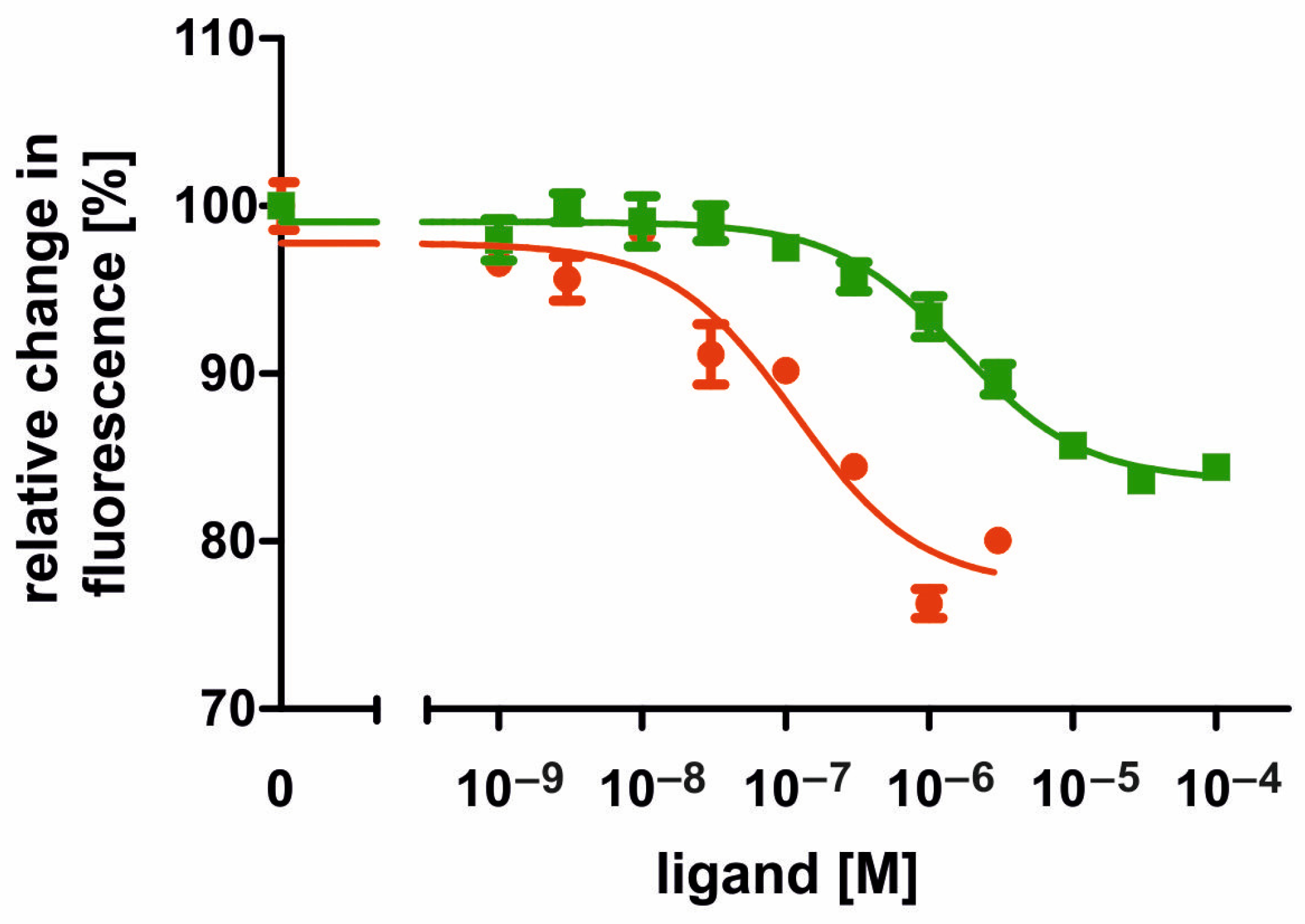
2.3. Ligand Specificity of the AmOctα2R-HA Receptor
2.4. Pharmacological Properties of the AmOctα2R-HA Receptor
3. Discussion
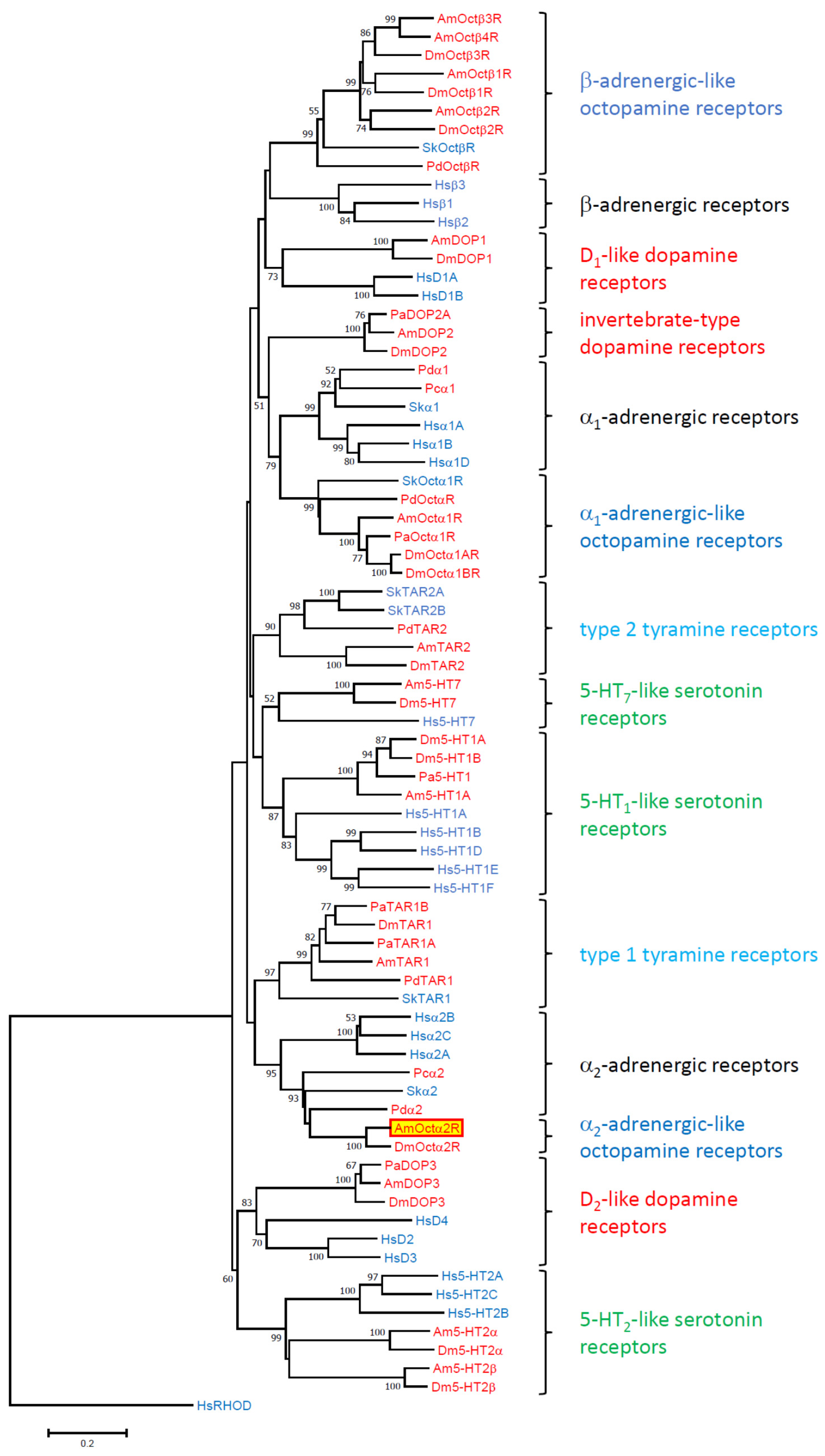
3.1. Gene Structure, Structural Properties of the Protein, and Phylogenetic Classification
3.2. Posttranslational Modification of AmOctα2R
3.3. Pharmacological Properties of the AmOctα2R Protein
4. Materials and Methods
4.1. Amplification of the Honeybee α2-Adrenergic-Like Octopamine Receptor (AmOctα2R) cDNA and Construction of pcAmOctα2R-HA Expression Vector
4.2. Multiple Sequence Alignment and Phylogenetic Analysis
4.3. Functional Expression of the AmOctα2R-HA Receptor
4.4. Functional Analysis of the AmOctα2R-HA Receptor
4.5. Western Blot analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-CT | 5-carboxamidotryptamine |
| 5-MT | 5-methoxytryptamine |
| 5-HT | 5-hydroxytryptamine, serotonin |
| 8-OH-DPAT | 8-Hydroxy-2-(dipropylamino)tetralin |
| GPCR | G protein-coupled receptor |
| HA | hemagglutinin A |
| IBMX | 3-isobutyl-1-methylxanthine |
| NKH477 | water-soluble forskolin analog |
| RFU | relative fluorescence unit |
| TM | transmembrane |
References
- Blenau, W.; Baumann, A. Aminergic signal transduction in invertebrates: Focus on tyramine and octopamine receptors. Recent Res. Dev. Neurochem. 2003, 6, 225–240. [Google Scholar]
- Blenau, W.; Baumann, A. Octopaminergic and tyraminergic signaling in the honeybee (Apis mellifera) brain: Behavioral, pharmacological, and molecular aspects. In Trace Amines and Neurological Disorders, 1st ed.; Farooqui, A., Ed.; Academic Press: Oxford, UK, 2016; pp. 203–220. [Google Scholar]
- Lange, A.B. Tyramine: From octopamine precursor to neuroactive chemical in insects. Gen. Comp. Endocrinol. 2009, 162, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pflüger, H.J.; Blenau, W.; Vanden Broeck, J. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Ozoe, Y. Molecular signalling, pharmacology, and physiology of octopamine and tyramine receptors as potential insect pest control targets. Adv. Insect Physiol. 2014, 46, 73–166. [Google Scholar]
- Roeder, T. The control of metabolic traits by octopamine and tyramine in invertebrates. J. Exp. Biol. 2020, 223, jeb194282. [Google Scholar] [CrossRef]
- Hammer, M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 1993, 366, 59–63. [Google Scholar] [CrossRef]
- Farooqui, T.; Robinson, K.; Vaessin, H.; Smith, B.H. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J. Neurosci. 2003, 23, 5370–5380. [Google Scholar] [CrossRef]
- Giurfa, M. Associative learning: The instructive function of biogenic amines. Curr. Biol. 2006, 16, R892–R895. [Google Scholar] [CrossRef] [Green Version]
- Vergoz, V.; Roussel, E.; Sandoz, J.C.; Giurfa, M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE 2007, 2, e288. [Google Scholar] [CrossRef]
- Thamm, M.; Balfanz, S.; Scheiner, R.; Baumann, A.; Blenau, W. Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell. Mol. Life Sci. 2010, 67, 2467–2479. [Google Scholar] [CrossRef]
- Mancini, N.; Giurfa, M.; Sandoz, J.C.; Avarguès-Weber, A. Aminergic neuromodulation of associative visual learning in harnessed honey bees. Neurobiol. Learn. Mem. 2018, 155, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.J.; Robinson, G.E. Octopamine influences division of labor in honey bee colonies. J. Comp. Physiol. A 2001, 187, 53–61. [Google Scholar] [CrossRef]
- Lehman, H.K.; Schulz, D.J.; Barron, A.B.; Wraight, L.; Hardison, C.; Whitney, S.; Takeuchi, H.; Paul, R.K.; Robinson, G.E. Division of labor in the honey bee (Apis mellifera): The role of tyramine β-hydroxylase. J. Exp. Biol. 2006, 209, 2774–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiner, R.; Baumann, A.; Blenau, W. Aminergic control and modulation of honeybee behaviour. Curr. Neuropharmacol. 2006, 4, 259–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, G.; Bicker, G. Habituation of an appetitive reflex in the honeybee. J. Neurophysiol. 1992, 67, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, R.; Plückhahn, S.; Oney, B.; Blenau, W.; Erber, J. Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav. Brain Res. 2002, 136, 545–553. [Google Scholar] [CrossRef]
- Cook, C.N.; Brent, C.S.; Breed, M.D. Octopamine and tyramine modulate the thermoregulatory fanning response in honey bees (Apis mellifera). J. Exp. Biol. 2017, 220, 1925–1930. [Google Scholar] [CrossRef] [Green Version]
- Fussnecker, B.L.; Smith, B.H.; Mustard, J.A. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J. Insect Physiol. 2006, 52, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, R.; Toteva, A.; Reim, T.; Søvik, E.; Barron, A.B. Differences in the phototaxis of pollen and nectar foraging honey bees are related to their octopamine brain titers. Front. Physiol. 2014, 5, 116. [Google Scholar] [CrossRef] [Green Version]
- Kutsukake, M.; Komatsu, A.; Yamamoto, D.; Ishiwa-Chigusa, S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene 2000, 245, 31–42. [Google Scholar] [CrossRef]
- Saraswati, S.; Fox, L.E.; Soll, D.R.; Wu, C.F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol. 2004, 58, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, K.G.; Hadden, J.K.; Deady, L.D.; Mercier, A.J.; Krans, J.L. Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J. Neurophysiol. 2013, 110, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Selcho, M.; Pauls, D.; el Jundi, B.; Stocker, R.F.; Thum, A.S. The role of octopamine and tyramine in Drosophila larval locomotion. J. Comp. Neurol. 2012, 520, 3764–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damrau, C.; Toshima, N.; Tanimura, T.; Brembs, B.; Colomb, J. Octopamine and tyramine contribute separately to the counter-regulatory response to sugar deficit in Drosophila. Front. Syst. Neurosci. 2018, 11, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schützler, N.; Girwert, C.; Hügli, I.; Mohana, G.; Roignant, J.Y.; Ryglewski, S.; Duch, C. Tyramine action on motoneuron excitability and adaptable tyramine/octopamine ratios adjust Drosophila locomotion to nutritional state. Proc. Natl. Acad. Sci. USA 2019, 116, 3805–3810. [Google Scholar] [CrossRef] [Green Version]
- Blenau, W.; Balfanz, S.; Baumann, A. Amtyr1: Characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J. Neurochem. 2000, 74, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A.; Kurshan, P.T.; Hamilton, I.S.; Blenau, W.; Mercer, A.R. Developmental expression of a tyramine receptor gene in the brain of the honey bee, Apis mellifera. J. Comp. Neurol. 2005, 483, 66–75. [Google Scholar] [CrossRef]
- Beggs, K.T.; Tyndall, J.D.A.; Mercer, A.R. Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 2011, 6, e26809. [Google Scholar] [CrossRef]
- Reim, T.; Balfanz, S.; Baumann, A.; Blenau, W.; Thamm, M.; Scheiner, R. AmTAR2: Functional characterization of a honeybee tyramine receptor stimulating adenylyl cyclase activity. Insect Biochem. Mol. Biol. 2017, 80, 91–100. [Google Scholar] [CrossRef]
- Grohmann, L.; Blenau, W.; Erber, J.; Ebert, P.R.; Strünker, T.; Baumann, A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J. Neurochem. 2003, 86, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Balfanz, S.; Jordan, N.; Langenstück, T.; Breuer, J.; Bergmeier, V.; Baumann, A. Molecular, pharmacological, and signaling properties of octopamine receptors from honeybee (Apis mellifera) brain. J. Neurochem. 2014, 129, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Xu, G.; Qi, Y.X.; Xia, R.Y.; Huang, J.; Ye, G.Y. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J. Neurochem. 2014, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.X.; Xu, G.; Gu, G.X.; Mao, F.; Ye, G.Y.; Liu, W.; Huang, J. A new Drosophila octopamine receptor responds to serotonin. Insect Biochem. Mol. Biol. 2017, 90, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Cazzamali, G.; Williamson, M.; Blenau, W.; Grimmelikhuijzen, C.J. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog. Neurobiol. 2006, 80, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Kroeze, W.K.; Sheffler, D.J.; Roth, B.L. G-protein-coupled receptors at a glance. J. Cell Sci. 2003, 116, 4867–4869. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995, 25, 366–428. [Google Scholar]
- Eilers, M.; Hornak, V.; Smith, S.O.; Konopka, J.B. Comparison of class a and dg protein-coupled receptors: Common features in structure and activation. Biochemistry 2005, 44, 8959–8975. [Google Scholar] [CrossRef] [Green Version]
- Bauknecht, P.; Jékely, G. Ancient coexistence of norepinephrine, tyramine, and octopamine signaling in bilaterians. BMC Biol. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustard, J.A.; Blenau, W.; Hamilton, I.S.; Ward, V.K.; Ebert, P.R.; Mercer, A.R. Analysis of two D1-like dopamine receptors from the honey bee Apis mellifera reveals agonist-independent activity. Brain Res. Mol. Brain Res. 2003, 113, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Troppmann, B.; Balfanz, S.; Krach, C.; Baumann, A.; Blenau, W. Characterization of an invertebrate-type dopamine receptor of the American cockroach, Periplaneta americana. Int. J. Mol. Sci. 2014, 15, 629–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, G.; Hannan, F.; Reale, V.; Hon, Y.Y.; Kousky, C.T.; Evans, P.D.; Hall, L.M. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J. Neurosci. 1996, 16, 3925–3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Higgins, D.G.; and Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotte, C.; Krach, C.; Balfanz, S.; Baumann, A.; Walz, B.; Blenau, W. Molecular characterization and localization of the first tyramine receptor of the American cockroach (Periplaneta americana). Neuroscience 2009, 162, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Wachten, S.; Schlenstedt, J.; Gauss, R.; Baumann, A. Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J. Neurochem. 2006, 96, 1580–1590. [Google Scholar] [CrossRef]
- Li, Y.; Hoffmann, J.; Li, Y.; Stephano, F.; Bruchhaus, I.; Fink, C.; Roeder, T. Octopamine controls starvation resistance, life span and metabolic traits in Drosophila. Sci. Rep. 2016, 6, 35359. [Google Scholar] [CrossRef] [Green Version]
- Sujkowski, A.; Ramesh, D.; Brockmann, A.; Wessells, R. Octopamine drives endurance exercise adaptations in Drosophila. Cell Rep. 2017, 21, 1809–1823. [Google Scholar] [CrossRef] [Green Version]
- Selcho, M.; Pauls, D. Linking physiological processes and feeding behaviors by octopamine. Curr. Opin. Insect Sci. 2019, 36, 125–130. [Google Scholar] [CrossRef]
- Ohta, H.; Utsumi, T.; Ozoe, Y. Amino acid residues involved in interaction with tyramine in the Bombyx mori tyramine receptor. Insect Mol. Biol. 2004, 13, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Congreve, M.; Langmead, C.; Marshall, F.H. The use of GPCR structures in drug design. Adv. Pharmacol. 2011, 62, 1–36. [Google Scholar] [PubMed]
- Probst, W.C.; Snyder, L.A.; Schuster, D.I.; Brosius, J.; Sealfon, S.C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kobilka, B.K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta 2007, 1768, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Karageorgos, V.; Venihaki, M.; Sakellaris, S.; Pardalos, M.; Kontakis, G.; Matsoukas, M.T.; Gravanis, A.; Margioris, A.; Liapakis, G. Current understanding of the structure and function of family B GPCRs to design novel drugs. Hormones 2018, 17, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jékely, G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 8702–8707. [Google Scholar] [CrossRef] [Green Version]
- Elphick, M.R.; Mirabeau, O.; Larhammar, D. Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018, 221, jeb151092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfanz, S.; Strünker, T.; Frings, S.; Baumann, A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Han, K.A.; Millar, N.S.; Davis, R.L. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 1998, 18, 3650–3658. [Google Scholar] [CrossRef] [Green Version]
- Hoff, M.; Balfanz, S.; Ehling, P.; Gensch, T.; Baumann, A. A single amino acid residue controls Ca2+ signaling by an octopamine receptor from Drosophila melanogaster. FASEB J. 2011, 25, 2484–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Dowd, B.F.; Hnatowich, M.; Caron, M.G.; Lefkowitz, R.J.; Bouvier, M. Palmitoylation of the human β2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J. Biol. Chem. 1989, 264, 7564–7569. [Google Scholar] [PubMed]
- Docherty, J.R. Subtypes of functional α1- and α2-adrenoceptors. Eur. J. Pharmacol. 1998, 361, 1–15. [Google Scholar] [CrossRef]
- Goldstein, I. Oral phentolamine: An alpha-1, alpha-2 adrenergic antagonist for the treatment of erectile dysfunction. Int. J. Impot. Res. 2000, 12, S75–S80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, J.; Sugimoto, Y.; Noma, T.; Yoshikawa, T. Effects of the non-selective 5-HT receptor agonist, 5-carboxamidotryptamine, on plasma glucose levels in rats. Eur. J. Pharmacol. 1998, 359, 81–86. [Google Scholar] [CrossRef]
- Schlenstedt, J.; Balfanz, S.; Baumann, A.; Blenau, W. Am5-HT7: Molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera). J. Neurochem. 2006, 98, 1985–1998. [Google Scholar] [CrossRef]
- Troppmann, B.; Balfanz, S.; Baumann, A.; Blenau, W. Inverse agonist and neutral antagonist actions of synthetic compounds at an insect 5-HT1 receptor. Br. J. Pharmacol. 2010, 159, 1450–1562. [Google Scholar] [CrossRef] [Green Version]
- Minhas, N.; Gole, J.W.D.; Orr, G.L.; Downer, R.G.H. Pharmacology of [3H]mianserin binding in the nerve cord of the American cockroach, Periplaneta americana. Arch. Insect Biochem. Physiol. 1987, 6, 191–201. [Google Scholar] [CrossRef]
- Roeder, T. High-affinity antagonists of the locust neuronal octopamine receptor. Eur. J. Pharmacol. 1990, 191, 221–224. [Google Scholar] [CrossRef]
- Blenau, W.; Balfanz, S.; Baumann, A. PeaTAR1B: Characterization of a second type 1 tyramine receptor of the American cockroach, Periplaneta americana. Int. J. Mol. Sci. 2017, 18, 2279. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Saudou, F.; Amlaiky, N.; Plassat, J.L.; Borrelli, E.; Hen, R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990, 9, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Vanden Broeck, J.; Vulsteke, V.; Huybrechts, R.; De Loof, A. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. J. Neurochem. 1995, 64, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Utsumi, T.; Ozoe, Y. B96Bom encodes a Bombyx mori tyramine receptor negatively coupled to adenylate cyclase. Insect Mol. Biol. 2003, 12, 217–223. [Google Scholar] [CrossRef]
- Hildebrandt, H.; Müller, U. Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honeybees. J. Neurobiol. 1995, 27, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Uzzan, A.; Dudai, Y. Aminergic receptors in Drosophila melanogaster: Responsiveness of adenylate cyclase to putative neurotransmitters. J. Neurochem. 1982, 38, 1542–1550. [Google Scholar] [CrossRef]
- Gole, J.W.; Orr, G.L.; Downer, R.G.H. Interaction of formamidines with octopamine-sensitive adenylate cyclase receptor in the nerve cord of Periplaneta americana L. Life Sci. 1983, 32, 2939–2947. [Google Scholar] [CrossRef]
- Orr, G.L.; Gole, J.W.D.; Downer, R.G.H. Characterisation of an octopamine-sensitive adenylate cyclase in haemocyte membrane fragments of the American cockroach, Periplaneta americana L. Insect Biochem. 1985, 15, 695–701. [Google Scholar] [CrossRef]
- Aoyama, M.; Nakane, T.; Ono, T.; Khan, M.A.; Ohta, H.; Ozoe, Y. Substituent-dependent, positive and negative modulation of Bombyx mori adenylate cyclase by synthetic octopamine/tyramine analogues. Arch. Insect Biochem. Physiol. 2001, 47, 1–7. [Google Scholar] [CrossRef]
- Orr, N.; Orr, G.L.; Hollingworth, R.M. The Sf9 cell line as a model for studying insect octopamine-receptors. Insect Biochem. Mol. Biol. 1992, 22, 591–597. [Google Scholar] [CrossRef]
- Van Poyer, W.; Torfs, H.; Poels, J.; Swinnen, E.; De Loof, A.; Akerman, K.; Vanden Broeck, J. Phenolamine-dependent adenylyl cyclase activation in Drosophila Schneider 2 cells. Insect Biochem. Mol. Biol. 2001, 31, 333–338. [Google Scholar] [CrossRef]
- Näsman, J.; Kukkonen, J.P.; Akerman, K.E. Dual signalling by different octopamine receptors converges on adenylate cyclase in Sf9 cells. Insect Biochem. Mol. Biol. 2002, 32, 285–293. [Google Scholar] [CrossRef]
- Röser, C.; Jordan, N.; Balfanz, S.; Baumann, A.; Walz, B.; Baumann, O.; Blenau, W. Molecular and pharmacological characterization of serotonin 5-HT2α and 5-HT7 receptors in the salivary glands of the blowfly Calliphora vicina. PLoS ONE 2012, 7, e49459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984, 12, 857–872. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Bio. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Ludwig, J.; Margalit, T.; Eismann, E.; Lancet, D.; Kaupp, U.B. Primary structure of cAMP-gated channel from bovine olfactory epithelium. FEBS Lett. 1990, 270, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Okayama, H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 1987, 7, 2745–2752. [Google Scholar] [CrossRef]

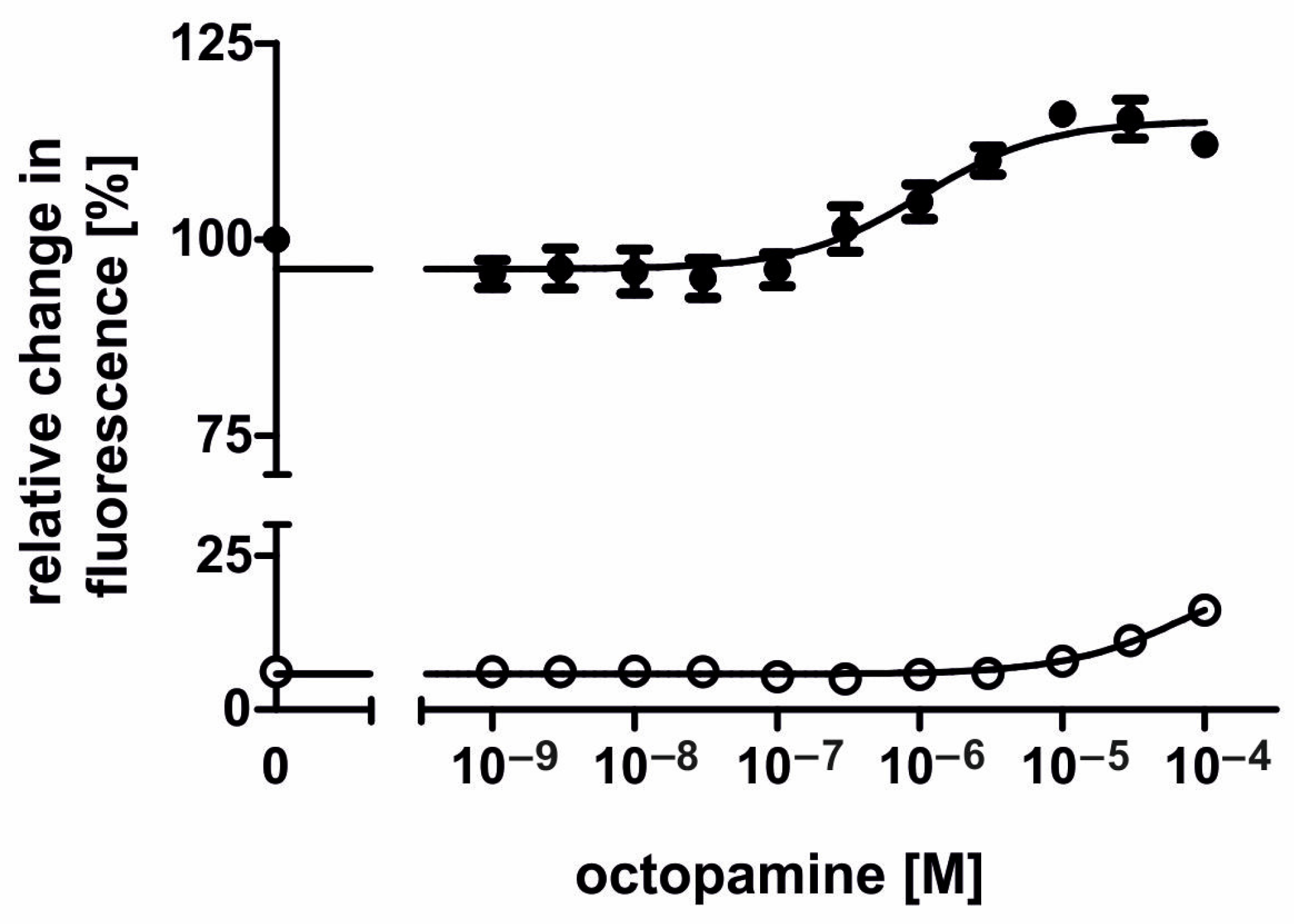

| Octopamine (n = 7) | Tyramine (n = 11) | |
|---|---|---|
| EC50 [M] | 5.87 × 10−8 | 1.85 × 10−6 |
| logEC50 | −7.43 ± 0.24 | −5.78 ± 0.17 |
| Substance | Specificity in Humans 1 | IC50 [M] | logIC50 | Maximal Inhibition [%] | n |
|---|---|---|---|---|---|
| 5-CT | agonist at 5-HT1A, 5-HT1B, 5-HT1D, 5-HT5, and 5-HT7 receptors | 4.16 × 10−9 | −8.48 ± 0.20 | 10.8 | 3 |
| phentolamine | nonselective α-adrenergic antagonist | 5.63 × 10−9 | −8.30 ± 0.20 | 16.1 | 3 |
| epinastine | nonsedating histamine H1 receptor antagonist | 1.98 × 10−8 | −7.75 ± 0.29 | 17.31 | 6 |
| 5-MT | agonist at 5-HT1, 5-HT2, 5-HT4, 5-HT6, and 5-HT7 receptors | 2.06 × 10−8 | −7.72 ± 0.20 | 20.3 | 4 |
| mianserin | antagonist at the histamine H1, 5-HT1D, 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, 5-HT7, α1-adrenergic and α2-adrenergic receptors | 2.95 × 10−8 | −7.71 ± 0.31 | 21.5 | 5 |
| yohimbine | high affinity for the α2-adrenergic receptor, moderate affinity for the α1-adrenergic, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2B, and D2 receptors, and weak affinity for the 5-HT1E, 5-HT2A, 5-HT5A, 5-HT7, and D3 receptors; behaves as an antagonist at α1-adrenergic, α2-adrenergic, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B and D2, and as a partial agonist at 5-HT1A | 8.14 × 10−8 | −7.12 ± 0.32 | 14.3 | 4 |
| ketanserin | affinity for multiple GPCR; antagonist at 5-HT2A and 5-HT2C receptors; high affinity for α1-adrenergic receptors and very high affinity for histamine H1 receptors; moderate affinity for α2-adrenergic and 5-HT6 receptors as well as weak affinity for dopamine D1 and D2 receptors | 5.14 × 10−7 | −6.29 ± 0.29 | 17.11 | 3 |
| 8-OH-DPAT | standard selective 5-HT1A agonist; also has moderate affinity for 5-HT7 receptors | 1.09 × 10−6 | −6.15 ± 0.36 | 7.21 | 3 |
| AS-19 | agonist at the 5-HT7 receptor | no effect | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blenau, W.; Wilms, J.A.; Balfanz, S.; Baumann, A. AmOctα2R: Functional Characterization of a Honeybee Octopamine Receptor Inhibiting Adenylyl Cyclase Activity. Int. J. Mol. Sci. 2020, 21, 9334. https://doi.org/10.3390/ijms21249334
Blenau W, Wilms JA, Balfanz S, Baumann A. AmOctα2R: Functional Characterization of a Honeybee Octopamine Receptor Inhibiting Adenylyl Cyclase Activity. International Journal of Molecular Sciences. 2020; 21(24):9334. https://doi.org/10.3390/ijms21249334
Chicago/Turabian StyleBlenau, Wolfgang, Joana Alessandra Wilms, Sabine Balfanz, and Arnd Baumann. 2020. "AmOctα2R: Functional Characterization of a Honeybee Octopamine Receptor Inhibiting Adenylyl Cyclase Activity" International Journal of Molecular Sciences 21, no. 24: 9334. https://doi.org/10.3390/ijms21249334






