Cellular Basis of Embryonic Hematopoiesis and Its Implications in Prenatal Erythropoiesis
Abstract
:1. Introduction
2. Features of Primitive Erythropoiesis
3. Complicated Nature of Late Embryonic/Early Fetal Hematopoiesis
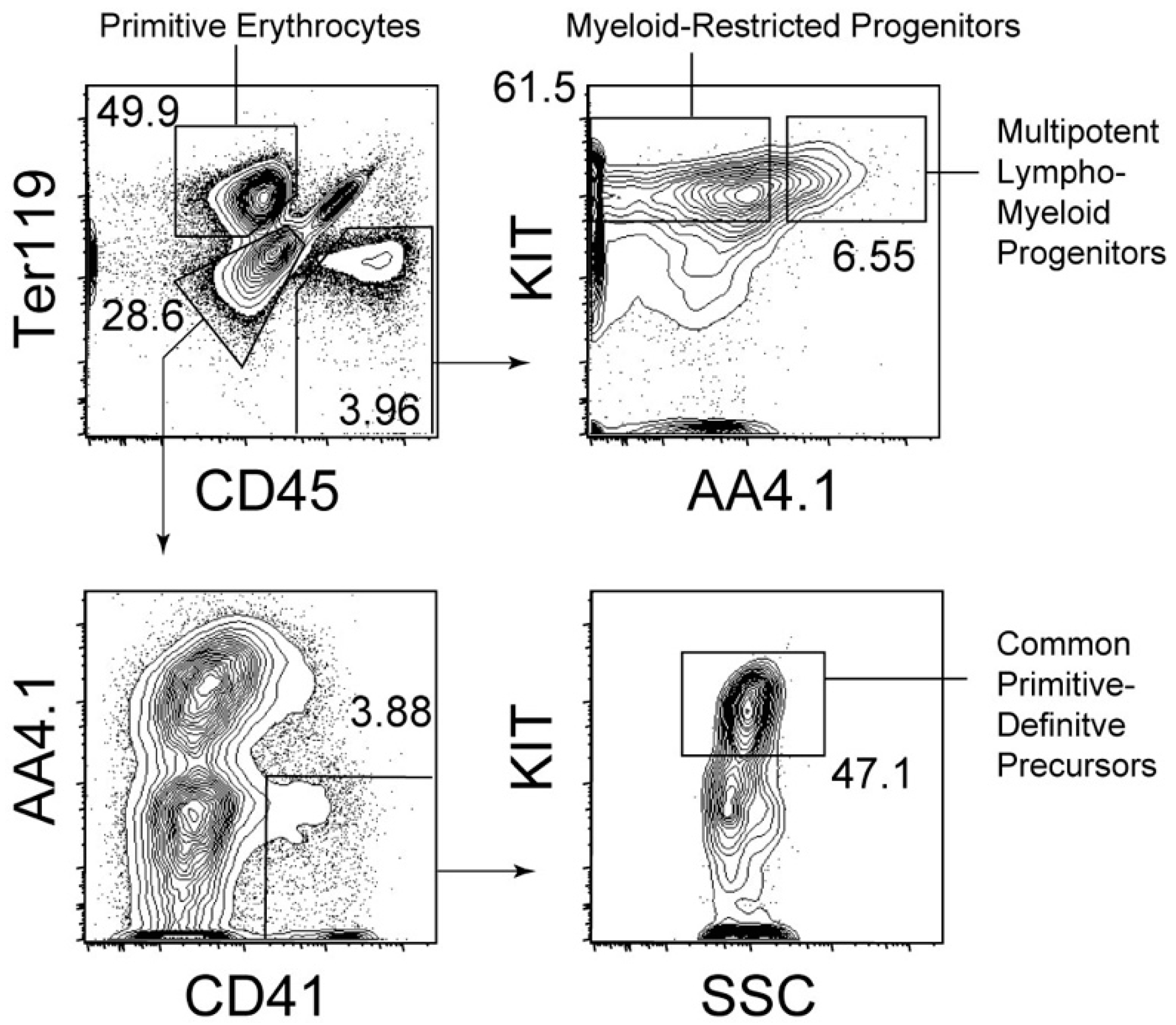
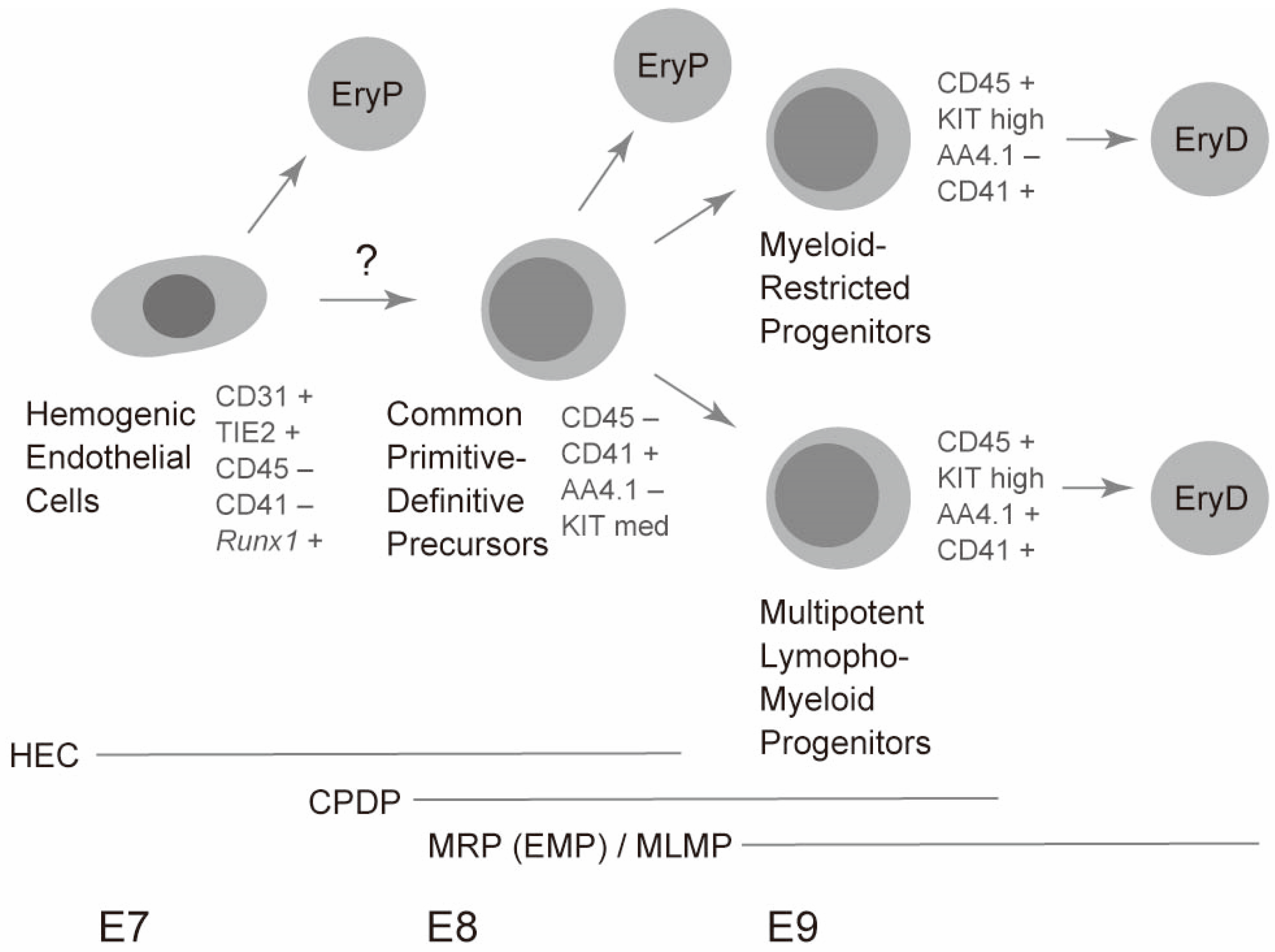
4. Cellular Origin of Primitive Erythrocytes in Mice
5. Erythropoiesis and Hematopoiesis in the Fetal Liver
6. Embryonic and Fetal Erythropoiesis in Humans
7. Conclusions
Funding
Conflicts of Interest
References
- Nikinmaa, M.; Tufts, B.L.; Boutilier, R.G. Volume and pH regulation in agnathan erythrocytes: Comparisons between the hagfish, Myxine glutinosa, and the lampreys, Petromyzon marinus and Lampetra fluviatilis. J. Comp. Physiol. B 1993, 163, 608–613. [Google Scholar] [CrossRef]
- Hoffmann, F.G.; Opazo, J.C.; Storz, J.F. Gene cooption and convergent evolution of oxygen transport hemoglobins in jawed and jawless vertebrates. Proc. Natl. Acad. Sci. USA 2010, 107, 14274–14279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, A.S.; Chandler, S.A.; Liu, Y.; Signore, A.V.; Cortez-Romero, C.R.; Benesch, J.L.P.; Lafanowsky, A.; Storz, J.F.; Hochberg, G.K.A.; Thornton, J.W. Origin of complexity in haemoglobin evolution. Nature 2020, 581, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F. Gene duplication and evolutionary innovations in hemoglobin-oxygen transport. Physiology 2016, 31, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; McColl, B.; Maksimovic, J.; Vadolas, J. Epigenetic interplay at the β-globin locus. Biochim. Biophys. Acta. Gene Regul. Mech. 2017, 1860, 393–404. [Google Scholar] [CrossRef]
- Palis, J.; Malik, J.; McGrath, K.E.; Kingsley, P.D. Primitive erythropoiesis in the mammalian embryo. Int. J. Dev. Biol. 2010, 54, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T. Mouse yolk sac hematopoiesis. Front. Cell Dev. Biol. 2018, 6, 80. [Google Scholar] [CrossRef]
- Wells, R.M.; Brittain, T. Transition to cooperative oxygen-binding by embryonic haemoglobin in mice. J. Exp. Biol. 1981, 90, 351–355. [Google Scholar]
- Sankaran, V.G.; Menne, T.F.; Xu, J.; Akie, T.E.; Lettre, G.; Van Handel, B.; Mikkola, H.K.A.; Hirschhorn, J.N.; Cantor, A.B.; Orkin, S.H. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008, 322, 1839–1842. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, V.G.; Xu, J.; Ragoczy, T.; Ippolito, G.C.; Walkley, C.R.; Maika, S.D.; Fujiwara, Y.; Ito, M.; Groudine, M.; Bender, M.A.; et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature 2009, 460, 1093–1097. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Wang, X.; Maeda, M.; Canver, M.C.; Sher, F.; Funnell, A.P.W.; Fisher, C.; Suciu, M.; Martyn, G.E.; Norton, L.J.; et al. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science 2016, 351, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Martyn, G.E.; Wienert, B.; Yang, L.; Shah, M.; Norton, L.J.; Burdach, J.; Kurita, R.; Nakamura, Y.; Pearson, R.C.M.; Funnell, A.P.W.; et al. Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat. Genet. 2018, 50, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Boontanrart, M.Y.; Schröder, M.S.; Stehli, G.M.; Banović, M.; Wyman, S.K.; Lew, R.J.; Bordi, M.; Gowen, B.G.; DeWirr, M.A.; Corn, J.E. ATF4 Regulates MYB to Increase γ-Globin in Response to Loss of β-Globin. Cell Rep. 2020, 32, 107993. [Google Scholar] [CrossRef] [PubMed]
- Vernimmen, D. Uncovering enhancer functions using the α-globin locus. PLoS Genet. 2014, 10, e1004668. [Google Scholar] [CrossRef] [PubMed]
- Manderson, A.P.; Botto, M.; Walport, M.J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 2004, 22, 431–456. [Google Scholar] [CrossRef]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Delyea, C.; Bozorgmehr, N.; Koleva, P.; Dunsmore, G.; Shahbaz, S.; Huang, V.M.; Elahi, S. CD71+ erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J. Immunol. 2018, 200, 4044–4058. [Google Scholar] [CrossRef] [Green Version]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Wagers, A.J.; Manz, M.G.; Prohaska, S.S.; Scherer, D.C.; Beilhack, G.F.; Shizuru, J.A.; Weissman, I.L. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu. Rev. Immunol. 2003, 21, 759–806. [Google Scholar] [CrossRef]
- Pei, W.; Feyerabend, T.B.; Rössler, J.; Wang, X.; Postrach, D.; Busch, K.; Rode, I.; Klapproth, K.; Dietlein, N.; Quedenau, C.; et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 2017, 548, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Pei, W.; Shang, F.; Wang, X.; Fanti, A.-K.; Greco, A.; Busch, K.; Klapproth, K.; Zhang, Q.; Quedenau, C.; Sauer, S.; et al. Resolving fates and single-cell transcriptomes of hematopoietic stem cell clones by PolyloxExpress barcoding. Cell Stem Cell 2020, 27, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.H.; Bard, J.B. The Anatomical Basis of Mouse Development; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Stefanska, M.; Batta, K.; Patel, R.; Florkowska, M.; Kouskoff, V.; Lacaud, G. Primitive erythrocytes are generated from hemogenic endothelial cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.P.; Phoon, C.K.; Aristizábal, O.; McGrath, K.E.; Palis, J.; Turnbull, D.H. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 2003, 92, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, P.D.; Malik, J.; Fantauzzo, K.A.; Palis, J. Yolk sac–derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 2004, 104, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Kawane, K.; Fukuyama, H.; Kondoh, G.; Takeda, J.; Ohsawa, Y.; Uchiyama, Y.; Nagata, S. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science 2001, 292, 1546–1549. [Google Scholar] [CrossRef]
- Ogawa, M.; Yoshinaga, K.; Hayashi, S.; Kunisada, T.; Nakao, J.; Kina, T.; Sudo, T.; Kodama, H.; Nishikawa, S.I. Expression and function of c-Kit in fetal hemopoietic progenitor cells: Transition from the early c-Kit-independent to the late c-Kit-dependent wave of hemopoiesis in the murine embryo. Development 1993, 117, 1089–1098. [Google Scholar]
- Wu, H.; Liu, X.; Jaenisch, R.; Lodish, H.F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 1995, 83, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T.; Washino, A.; Yamazaki, H. Common developmental pathway for primitive erythrocytes and multipotent hematopoietic progenitors in early mouse development. Stem Cell Rep. 2013, 1, 590–603. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T.; Ito, C.; Washino, A.; Isono, K.; Yamazaki, H. Repression of Primitive Erythroid Program Is Critical for the Initiation of Multi-Lineage Hematopoiesis in Mouse Development. J. Cell. Physiol. 2017, 232, 323–330. [Google Scholar] [CrossRef]
- Shalaby, F.; Ho, J.; Stanford, W.L.; Fischer, K.D.; Schuh, A.C.; Schwartz, L.; Bernstein, A.; Rossant, J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 1997, 89, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Taoudi, S.; Gonneau, C.; Moore, K.; Sheridan, J.M.; Blackburn, C.C.; Taylor, E.; Medvinsky, A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+ CD45+ pre-definitive HSCs. Cell Stem Cell 2008, 3, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, T.; Hosen, N.; Yamazaki, H.; Weissman, I.L. Expression of AA4. 1 marks lymphohematopoietic progenitors in early mouse development. Proc. Natl. Acad. Sci. USA 2009, 106, 8953–8958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inlay, M.A.; Serwold, T.; Mosley, A.; Fathman, J.W.; Dimov, I.K.; Seita, J.; Weissman, I.L. Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem Cell Rep. 2014, 2, 457–472. [Google Scholar] [CrossRef] [Green Version]
- Vo, L.T.; Kinney, M.A.; Liu, X.; Zhang, Y.; Barragan, J.; Sousa, P.M.; Jha, D.K.; Han, A.; Cesana, M.; Shao, Z.; et al. Regulation of embryonic haematopoietic multipotency by EZH1. Nature 2018, 553, 506–510. [Google Scholar] [CrossRef]
- McGrath, K.E.; Frame, J.M.; Fegan, K.H.; Bowen, J.R.; Conway, S.J.; Catherman, S.C.; Catherman, S.C.; Kingsley, P.D.; Koniski, A.D.; Palis, J. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015, 11, 1892–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böiers, C.; Carrelha, J.; Lutteropp, M.; Luc, S.; Green, J.C.; Azzoni, E.; Woll, P.S.; Mead, A.J.; Hultquist, A.; Swiers, G.; et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 2013, 13, 535–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, C.; Yamazaki, H.; Yamane, T. Earliest hematopoietic progenitors at embryonic day 9 preferentially generate B-1 B cells rather than follicular B or marginal zone B cells. Biochem. Biophysi. Res. Commun. 2013, 437, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Jorquera, A.; Msallam, R.; Wienert, S.; Bajénoff, M. Epidermal γδ T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. J. Exp. Med. 2018, 215, 2994–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadow, S.; Jux, B.; Zahner, S.P.; Wingerath, B.; Chmill, S.; Clausen, B.E.; Hengstler, J.; Esser, C. Aryl hydrocarbon receptor is critical for homeostasis of invariant γδ T cells in the murine epidermis. J. Immunol. 2011, 187, 3104–3110. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, J.; Luo, G.; He, W. Functions of Vγ4 T cells and dendritic epidermal T cells on skin wound healing. Front. Immunol. 2018, 9, 1099. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.; Firpo, M.; Choi, K.; Wall, C.; Robertson, S.; Kabrun, N.; Keller, G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 1997, 386, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.T.; Kwok, Y.K.; Chui, D.H.; Wong, H.S.; Luo, H.Y.; Tang, M.H. Embryonic and fetal globins are expressed in adult erythroid progenitor cells and in erythroid cell cultures. Prenat. Diagn. 2001, 21, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Wontakal, S.N.; Guo, X.; Smith, C.; MacCarthy, T.; Bresnick, E.H.; Bergman, A.; Snyder, M.P.; Weissman, S.M.; Zheng, D.; Skoultchi, A.I. A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 3832–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacaud, G.; Kouskoff, V. Hemangioblast, hemogenic endothelium, and primitive versus definitive hematopoiesis. Exp. Hematol. 2017, 49, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Tober, J.; Gao, P.; Chen, C.; Tan, K.; Speck, N.A. RUNX1 and the endothelial origin of blood. Exp. Hematol. 2018, 68, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.K.; Ghorbanian, Y.; Wang, W.; Wang, Y.; Kim, Y.J.; Weissman, I.L.; Inlay, M.A.; Mikkola, H.K. LYVE1 marks the divergence of yolk sac definitive hemogenic endothelium from the primitive erythroid lineage. Cell Rep. 2016, 17, 2286–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadland, B.; Yoshimoto, M. Many layers of embryonic hematopoiesis: New insights into B-cell ontogeny and the origin of hematopoietic stem cells. Exp. Hematol. 2018, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Hemmati, H.D.; Wandycz, A.M.; Weissman, I.L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1995, 92, 10302–10306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Kim, I.; Lim, M.S.; Morrison, S.J. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Gene Dev. 2011, 25, 1613–1627. [Google Scholar] [CrossRef] [Green Version]
- Säwen, P.; Eldeeb, M.; Erlandsson, E.; Kristiansen, T.A.; Laterza, C.; Kokaia, Z.; Karlsson, G.; Yuan, J.; Soneji, S.; Mandel, P.K.; et al. Murine HSCs contribute actively to native hematopoiesis but with reduced differentiation capacity upon aging. Elife 2018, 7, e41258. [Google Scholar] [CrossRef]
- Suzuki, N.; Hirano, I. The neural crest as the first production site of the erythroid growth factor erythropoietin. Front. Cell Dev. Biol. 2019, 7, 105. [Google Scholar]
- Ustianenko, D.; Chiu, H.S.; Treiber, T.; Weyn-Vanhentenryck, S.M.; Treiber, N.; Meister, G.; Sumazin, P.; Zhang, C. LIN28 selectively modulates a subclass of let-7 microRNAs. Mol. Cell 2018, 71, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, R.G.; Wang, L.D.; Coma, S.; Han, A.; Mathieu, R.; Pearson, D.S.; Ross, S.; Sousa, P.; Nguyen, P.T.; Rodriguez, A.; et al. Developmental regulation of myeloerythroid progenitor function by the Lin28b–let-7–Hmga2 axis. J. Exp. Med. 2016, 213, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chim, B.; Su, Y.; Khil, P.; Wong, M.; Wang, X.; Foroushani, A.; Smith, P.T.; Liu, X.; Ganesan, S.; et al. Enhancement of LIN28B-induced hematopoietic reprogramming by IGF2BP3. Gen. Dev. 2019, 33, 1048–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, M.; Hasegawa, N.; Mochizuki-Kashio, M.; Muto, T.; Miyagi, S.; Koide, S.; Yabata, S.; Wendt, G.R.; Saraya, A.; Wang, C.; et al. Ezh2 regulates the Lin28/let-7 pathway to restrict activation of fetal gene signature in adult hematopoietic stem cells. Exp. Hematol. 2016, 44, 282–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereda, J.; Niimi, G. Embryonic erythropoiesis in human yolk sac: Two different compartments for two different processes. Microsc. Res. Tech. 2008, 71, 856–862. [Google Scholar] [CrossRef]
- Ivanovs, A.; Rybtsov, S.; Ng, E.S.; Stanley, E.G.; Elefanty, A.G.; Medvinsky, A. Human haematopoietic stem cell development: From the embryo to the dish. Development 2017, 144, 2323–2337. [Google Scholar] [CrossRef] [Green Version]
- Easterbrook, J.; Rybtsov, S.; Gordon-Keylock, S.; Ivanovs, A.; Taoudi, S.; Anderson, R.A.; Medvinsky, A. Analysis of the spatiotemporal development of hematopoietic stem and progenitor cells in the early human embryo. Stem Cell Rep. 2019, 12, 1056–1068. [Google Scholar] [CrossRef] [Green Version]
- Wienert, B.; Martyn, G.E.; Funnell, A.P.; Quinlan, K.G.; Crossley, M. Wake-up sleepy gene: Reactivating fetal globin for β-hemoglobinopathies. Trends Genet. 2018, 34, 927–940. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.; Tan, Y.; Beyer, A.I.; Xie, F.; Muench, M.O.; Kan, Y.W. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc. Natl. Acad. Sci. USA 2016, 113, 10661–10665. [Google Scholar] [CrossRef] [Green Version]
- Basak, A.; Munschauer, M.; Lareau, C.A.; Montbleau, K.E.; Ulirsch, J.C.; Hartigan, C.R.; Schenone, M.; Lian, J.; Wang, Y.; Huang, Y.; et al. Control of human hemoglobin switching by LIN28B-mediated regulation of BCL11A translation. Nat. Genet. 2020, 52, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alegria, E.; Menegatti, S.; Fadlullah, M.Z.; Menendez, P.; Lacaud, G.; Kouskoff, V. Early human hemogenic endothelium generates primitive and definitive hematopoiesis in vitro. Stem Cell Rep. 2018, 11, 1061–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruveris, F.F.; Ng, E.S.; Leitoguinho, A.R.; Motazedian, A.; Vlahos, K.; Sourris, K.; Mayberry, R.; McDonald, P.; Azzola, L.; Davidson, N.D.; et al. Human yolk sac-like haematopoiesis generates RUNX1-, GFI1-and/or GFI1B-dependent blood and SOX17-positive endothelium. Development 2020, 147, dev193037. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Perdiguero, E.G.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Bian, Z.; Gong, Y.; Huang, T.; Lee, C.Z.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering human macrophage development at single-cell resolution. Nature 2020, 582, 571–576. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamane, T. Cellular Basis of Embryonic Hematopoiesis and Its Implications in Prenatal Erythropoiesis. Int. J. Mol. Sci. 2020, 21, 9346. https://doi.org/10.3390/ijms21249346
Yamane T. Cellular Basis of Embryonic Hematopoiesis and Its Implications in Prenatal Erythropoiesis. International Journal of Molecular Sciences. 2020; 21(24):9346. https://doi.org/10.3390/ijms21249346
Chicago/Turabian StyleYamane, Toshiyuki. 2020. "Cellular Basis of Embryonic Hematopoiesis and Its Implications in Prenatal Erythropoiesis" International Journal of Molecular Sciences 21, no. 24: 9346. https://doi.org/10.3390/ijms21249346
APA StyleYamane, T. (2020). Cellular Basis of Embryonic Hematopoiesis and Its Implications in Prenatal Erythropoiesis. International Journal of Molecular Sciences, 21(24), 9346. https://doi.org/10.3390/ijms21249346




