Hepatocyte-Derived Igκ Exerts a Protective Effect against ConA-Induced Acute Liver Injury
Abstract
1. Introduction
2. Results
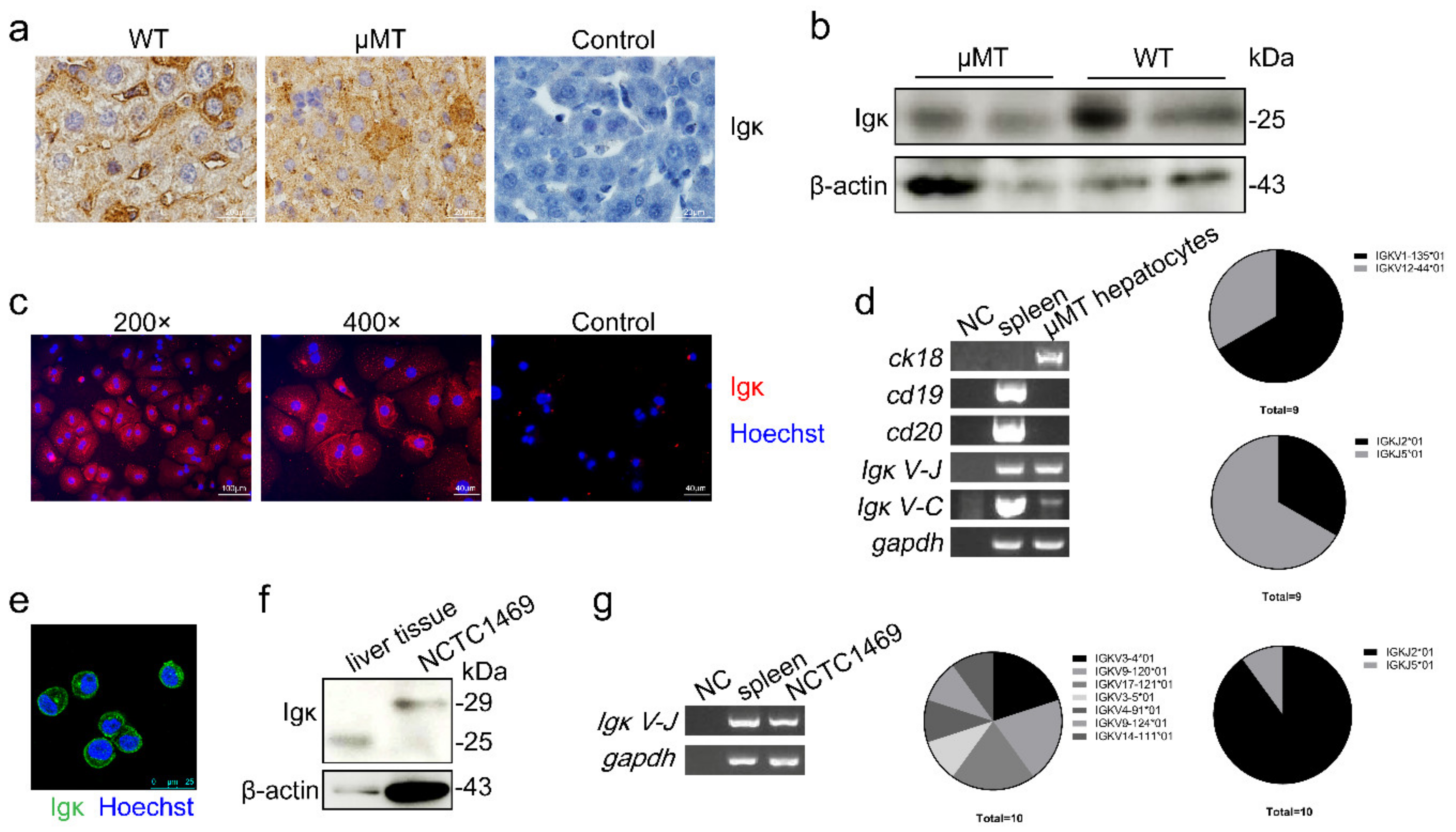
2.1. Igκ Expression in Primary Hepatocytes in μMT Mice and Normal Hepatocyte Cell Line
2.2. Hepatocyte-Derived Igκ Was Elevated and Displayed Unique Localization in ConA-Induced Liver Injury in μMT Mice
2.3. Target Disruption of Igκ Accentuated ConA-Induced Liver Injury In Vivo
2.4. Knockout of Igκ Inhibited Hepatocyte Survival and Promoted Hepatocyte Apoptosis
2.5. Knockout of Igκ Promoted Mitochondria-Mediated Apoptosis in NCTC1469
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. ConA-Induced Liver Injury
4.3. Histology and Immunohistochemistry
4.4. Serum Biochemistry
4.5. Isolation of Primary Mouse Hepatocytes
4.6. Adenovirus Infection of Primary Mouse Hepatocytes
4.7. NCTC1469 Cell Culture and Transient Transfection
4.8. Immunofluorescence Analysis
4.9. Mitochondrial Membrane Potential and Apoptosis Analysis by Flow Cytometry
4.10. Reverse-Transcription PCR and Sequencing Analysis of the Igκ Gene Transcripts
4.11. Western Blot Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, X.; Zhu, X.; Zhang, L.; Mao, Y.; Zhang, J.; Hao, P.; Li, G.; Lv, P.; Li, Z.; Sun, X.; et al. Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003, 63, 6488–6495. [Google Scholar] [PubMed]
- Liao, Q.; Liu, W.; Liu, Y.; Wang, F.; Wang, C.; Zhang, J.; Chu, M.; Jiang, D.; Xiao, L.; Shao, W.; et al. Aberrant high expression of immunoglobulin G in epithelial stem/progenitor-like cells contributes to tumor initiation and metastasis. Oncotarget 2015, 6, 40081–40094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, J.; Sun, X.; Mao, Y.; Zhu, X.; Zhang, P.; Zhang, L.; Du, J.; Qiu, X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int. J. Biochem. Cell Biol. 2008, 40, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, X.P.; Gong, X.T.; He, Z.Q.; Chen, L.; Qiu, X.Y.; Yin, C.C. Rearrangement and expression of the immunoglobulin mu-chain gene in human myeloid cells. Cell Mol. Immunol. 2014, 11, 94–104. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, Y.; Huang, J.; Ma, T.; Zhang, L.; Zhu, X.; Zheng, J.; Wu, L.; Yin, C.C.; Qiu, X. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell. Mol. Life Sci. 2009, 67, 985–994. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, J.; Liao, Q.; Ma, J.; Liu, Y.; Huang, J.; Wang, C.; Xu, W.; Zheng, J.; Shao, W.; et al. IgG and IgA with Potential Microbial-Binding Activity Are Expressed by Normal Human Skin Epidermal Cells. Int. J. Mol. Sci. 2015, 16, 2574–2590. [Google Scholar] [CrossRef]
- Shao, W.W.; Zhang, C.; Liu, E.Y.; Zhang, L.; Ma, J.F.; Zhu, Z.; Gong, X.T.; Qin, Z.H.; Qiu, X.Y. Identification of Liver Epithelial Cell-derived Ig Expression in mu chain-deficient mice. Sci. Rep. 2016, 6, 23669. [Google Scholar] [CrossRef]
- Shao, W.; Hu, F.; Ma, J.; Zhang, C.; Liao, Q.; Zhu, Z.; Liu, E.; Qiu, X. Epithelial cells are a source of natural IgM that contribute to innate immune responses. Int. J. Biochem. Cell Biol. 2016, 73, 19–29. [Google Scholar] [CrossRef]
- Kaposi-Novak, P.; Lee, J.-S.; Gòmez-Quiroz, L.; Coulouarn, C.; Factor, V.M.; Thorgeirsson, S.S. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J. Clin. Investig. 2006, 116, 1582–1595. [Google Scholar] [CrossRef]
- Tateno, C.; Miya, F.; Wake, K.; Kataoka, M.; Ishida, Y.; Yamasaki, C.; Yanagi, A.; Kakuni, M.; Wisse, E.E.; Verheyen, F.; et al. Morphological and microarray analyses of human hepatocytes from xenogeneic host livers. Lab. Investig. 2012, 93, 54–71. [Google Scholar] [CrossRef]
- Ravnskjaer, K.; Hogan, M.F.; Lackey, D.; Tora, L.; Dent, S.Y.; Olefsky, J.; Montminy, M. Glucagon regulates gluconeogenesis through KAT2B- and WDR5-mediated epigenetic effects. J. Clin. Investig. 2013, 123, 4318–4328. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.; Ammerpohl, O.; Von Schönfels, W.; Kolarova, J.; Bens, S.; Itzel, T.; Teufel, A.; Herrmann, A.; Brosch, M.; Hinrichsen, H.; et al. DNA Methylation Analysis in Nonalcoholic Fatty Liver Disease Suggests Distinct Disease-Specific and Remodeling Signatures after Bariatric Surgery. Cell Metab. 2013, 18, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Faa, G.; Van Eyken, P.; De Vos, R.; Fevery, J.; Van Damme, B.; De Groote, J.; Desmet, V.J. Light chain deposition disease of the liver associated with AL-type amyloidosis and severe cholestasis. J. Hepatol. 1991, 12, 75–82. [Google Scholar] [CrossRef]
- Michopoulos, S.; Petraki, K.; Petraki, C.; Dimopoulos, M.-A. Light chain deposition disease of the liver without renal involvement in a patient with multiple myeloma related to liver failure and rapid fatal outcome. Dig. Dis. Sci. 2002, 47, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell Death and Cell Death Responses in Liver Disease: Mechanisms and Clinical Relevance. Gastroenterology 2014, 147, 765–783.e4. [Google Scholar] [CrossRef]
- Liu, B.; Min, M.-W.; Bao, J.-K. Induction of apoptosis by Concanavalin A and its molecular mechanisms in cancer cells. Autophagy 2009, 5, 432–433. [Google Scholar] [CrossRef]
- Gantner, F.; Leist, M.; Lohse, A.W.; Germann, P.G.; Tiegs, G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: The role of tumor necrosis factor. Hepatology 1995, 21, 190–198. [Google Scholar]
- Trautwein, C.; Rakemann, T.; Brenner, D.; Streetz, K.; Licato, L.; Manns, M.P.; Tiegs, G. Concanavalin A-induced liver cell damage: Activation of intracellular pathways triggered by tumor necrosis factor in mice. Gastroenterology 1998, 114, 1035–1045. [Google Scholar] [CrossRef]
- Cubero, F.J.; Singh, A.; Borkham-Kamphorst, E.; Nevzorova, Y.A.; Al Masaoudi, M.; Haas, U.; Boekschoten, M.V.; Gassler, N.; Weiskirchen, R.; Muller, M.; et al. TNFR1 determines progression of chronic liver injury in the IKKgamma/Nemo genetic model. Cell Death Differ. 2013, 20, 1580–1592. [Google Scholar] [CrossRef]
- Deng, H.; Ma, J.; Jing, Z.; Deng, Z.; Liang, Y.; Lata, A.; Liu, Y.; Qiu, X.; Wang, Y. Expression of immunoglobulin A in human mesangial cells and its effects on cell apoptosis and adhesion. Mol. Med. Rep. 2018, 17, 5272–5282. [Google Scholar] [CrossRef]
- Jing, Z.; Deng, H.; Ma, J.; Guo, Y.; Liang, Y.; Wu, R.; Lata, A.; Geng, Z.; Qiu, X.; Wang, Y. Expression of immunoglobulin G in human podocytes, and its role in cell viability and adhesion. Int. J. Mol. Med. 2018, 41, 3296–3306. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, J.; Liu, Y.; Liao, Q.; Huang, J.; Geng, Z.; Xu, W.; Sheng, Z.; Lee, G.; Zhang, Y.; et al. Lung squamous cell carcinoma cells express non-canonically glycosylated IgG that activates integrin-FAK signaling. Cancer Lett. 2018, 430, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Jiang, D.; Gong, X.; Shao, W.; Zhu, Z.; Xu, W.; Qiu, X. Free immunoglobulin light chain (FLC) promotes murine colitis and colitis-associated colon carcinogenesis by activating the inflammasome. Sci. Rep. 2017, 7, 5165. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Knolle, P.A.; Gerken, G.; Loser, E.; Dienes, H.P.; Gantner, F.; Tiegs, G.; Meyer zum Buschenfelde, K.H.; Lohse, A.W. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology 1996, 24, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Suen, Y.K.; Fung, K.P.; Choy, Y.M.; Lee, C.Y.; Chan, C.W.; Kong, S.K. Concanavalin A induced apoptosis in murine macrophage PU5-1.8 cells through clustering of mitochondria and release of cytochrome c. Apoptosis 2000, 5, 369–377. [Google Scholar] [CrossRef]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Chaisson, M.L.; Brooling, J.T.; Ladiges, W.; Tsai, S.; Fausto, N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J. Clin. Investig. 2002, 110, 193–202. [Google Scholar] [CrossRef]
- Liu, H.; Lo, C.R.; Czaja, M.J. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 2002, 35, 772–778. [Google Scholar] [CrossRef]
- Maeda, S.; Chang, L.; Li, Z.W.; Luo, J.L.; Leffert, H.; Karin, M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity 2003, 19, 725–737. [Google Scholar] [CrossRef]
- Reuther-Madrid, J.Y.; Kashatus, D.; Chen, S.; Li, X.; Westwick, J.; Davis, R.J.; Earp, H.S.; Wang, C.Y.; Baldwin, A.S., Jr. The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol. Cell Biol. 2002, 22, 8175–8183. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.; Algül, H.; Paxian, S.; Schmidt, G. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology 2007, 132, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Ku, N.-O.; Strnad, P.; Hanada, S. Toward unraveling the complexity of simple epithelial keratins in human disease. J. Clin. Investig. 2009, 119, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.R. Faculty Opinions recommendation of Keratins modulate the shape and function of hepatocyte mitochondria: A mechanism for protection from apoptosis. J. Cell Sci. 2009, 122, 3851–3855. [Google Scholar] [CrossRef]
- Yoon, H.-N.; Yoon, S.-Y.; Hong, J.-H.; Ku, N. A mutation in keratin 18 that causes caspase-digestion resistance protects homozygous transgenic mice from hepatic apoptosis and injury. J. Cell Sci. 2017, 130, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Shen, Y.; Amir, M.; Farris, A.B.; Czaja, M.J. Stathmin 1 Induces Murine Hepatocyte Proliferation and Increased Liver Mass. Hepatol. Commun. 2019, 4, 38–49. [Google Scholar] [CrossRef]




| Dataset * | Title | Organism | Igκ |
|---|---|---|---|
| GDS5673 | Glucagon effect on hepatocytes deficient in lysine acetyltransferase 2B or WD repeat-containing protein 5 | Mus musculus | Igκv4–9 Igκv10–96 |
| GDS3148 | Hepatocyte growth factor effect on Met receptor-knockout primary hepatocytes: time course | Mus musculus | Igκv1–117 |
| GDS1648 | Hypoxia effect on HIF-1 alpha null hepatocytes | Mus musculus | Igκv6–23 Igκv16–104 |
| GDS4327 | Human hepatocytes from xenogeneic host livers | Mus musculus Homo sapiens | Igκ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Shi, Q.; Shao, W.; Zhang, C.; Zhang, Y.; Qiu, X.; Huang, J. Hepatocyte-Derived Igκ Exerts a Protective Effect against ConA-Induced Acute Liver Injury. Int. J. Mol. Sci. 2020, 21, 9379. https://doi.org/10.3390/ijms21249379
Yin S, Shi Q, Shao W, Zhang C, Zhang Y, Qiu X, Huang J. Hepatocyte-Derived Igκ Exerts a Protective Effect against ConA-Induced Acute Liver Injury. International Journal of Molecular Sciences. 2020; 21(24):9379. https://doi.org/10.3390/ijms21249379
Chicago/Turabian StyleYin, Sha, Qianwen Shi, Wenwei Shao, Chi Zhang, Yixiao Zhang, Xiaoyan Qiu, and Jing Huang. 2020. "Hepatocyte-Derived Igκ Exerts a Protective Effect against ConA-Induced Acute Liver Injury" International Journal of Molecular Sciences 21, no. 24: 9379. https://doi.org/10.3390/ijms21249379
APA StyleYin, S., Shi, Q., Shao, W., Zhang, C., Zhang, Y., Qiu, X., & Huang, J. (2020). Hepatocyte-Derived Igκ Exerts a Protective Effect against ConA-Induced Acute Liver Injury. International Journal of Molecular Sciences, 21(24), 9379. https://doi.org/10.3390/ijms21249379





