The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease
Abstract
1. Introduction
2. Review Criteria
3. Alcohol Intake and Its Influence on the Liver
3.1. Alcohol-Related Liver Conditions
3.2. The Liver—Structure, Components and Function
3.3. Toxicity of Alcohol to the Liver
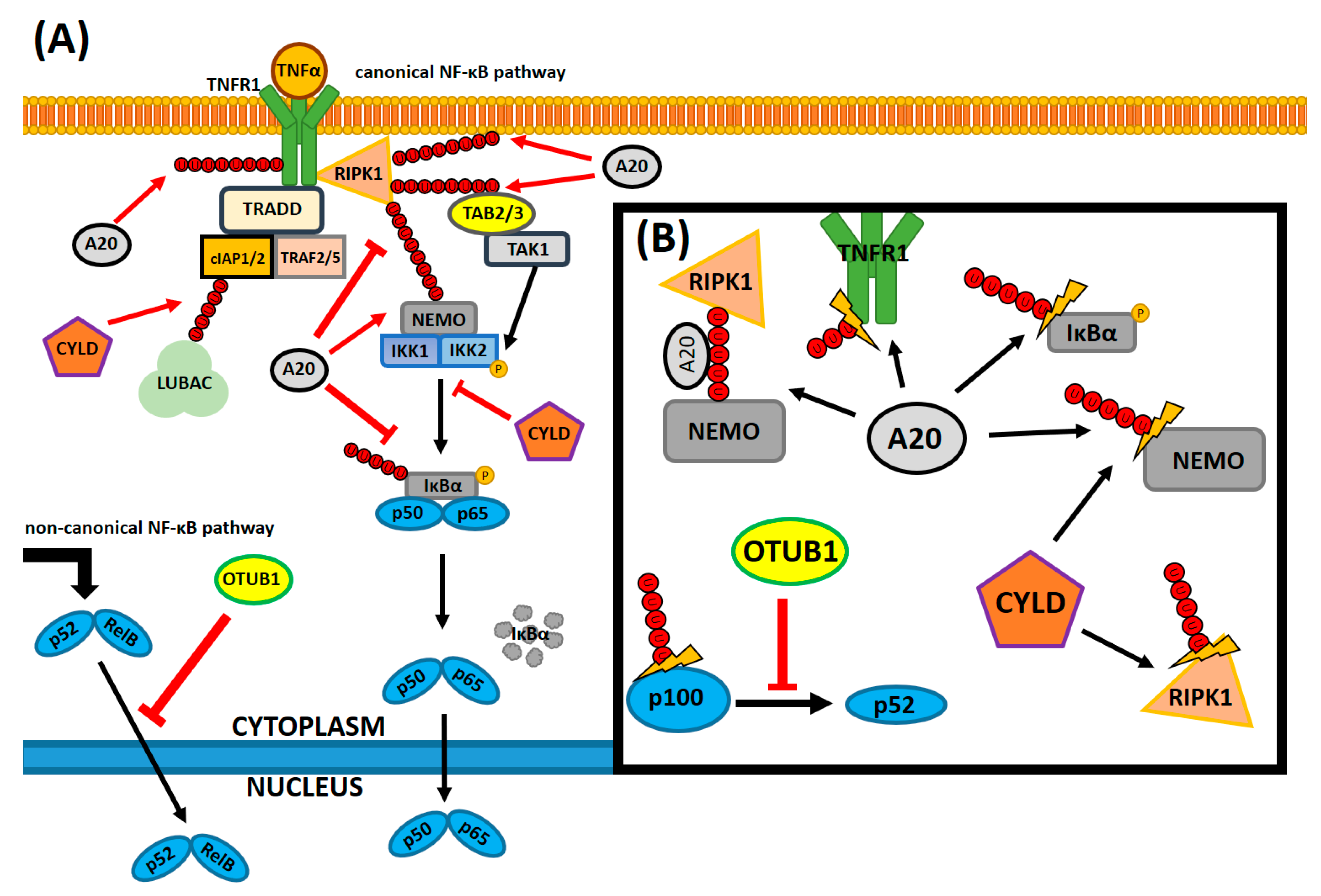
4. The Role of NF-κB in the Innate Immune Response
5. NF-κB-Regulating Molecular Factors
6. NF-κB Activity Research in Alcohol-Related Liver Injury Animal Models
6.1. The Potential Treatment Approach for Alcohol-Induced Liver Inflammation
6.2. Genetic Manipulations
6.3. Further Studies
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Richards, E. Beverages. Science 1890, 396, 127–131. [Google Scholar] [CrossRef][Green Version]
- Le Daré, B.; Gicquel, T. Therapeutic applications of ethanol: A review. J. Pharm. Pharm. Sci. 2019, 22, 525–535. [Google Scholar] [CrossRef]
- Oscar-Berman, M.; Marinković, K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007, 17, 239–257. [Google Scholar] [CrossRef]
- Thompson, T.; Oram, C.; Correll, C.U.; Tsermentseli, S.; Stubbs, B. Analgesic effects of alcohol: A systematic review and meta-analysis of controlled experimental studies in healthy participants. J. Pain. 2017, 5, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013, 35, 155–173. [Google Scholar] [PubMed]
- Rehm, J. The risks associated with alcohol use and alcoholism. Alcohol Res. Health 2011, 34, 135–143. [Google Scholar]
- Frazier, T.H.; Stocker, A.M.; Kershner, N.A.; Marsano, L.S.; McClain, C.J. Treatment of alcoholic liver disease. Ther. Adv. Gastroenterol. 2011, 4, 63–81. [Google Scholar] [CrossRef]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013. [Google Scholar] [CrossRef]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Donohue, T.M., Jr. Alcohol-induced steatosis in liver cells. World J. Gastroenterol. 2007, 13, 4974–4978. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Bataller, R. Trends in the management and burden of alcoholic liver disease. J. Hepatol. 2015, 62, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Rosenberg, D.; Zhao, H.; Lengyel, G.; Nadel, D. Fermented beverage and food storage in 13,000y-old stone mortars at Raqefet Cave, Israel: Investigating Natufian ritual feasting. J. Archaeol. Sci. 2018, 783–793. [Google Scholar] [CrossRef]
- Wadsworth, E.J.K.; Moss, S.C.; Simpson, S.A.; Smith, A.P. Factors associated with recreational drug use. J. Psychopharmacol. 2004, 18, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Researchandmarkets.com. Global Beverage Market—Forecasts from 2019 to 2024—Report ID: 4835375. Available online: https://www.researchandmarkets.com/reports/4835375/ (accessed on 7 August 2020).
- U.S. Substance Abuse and Mental Health Services Administration (SAMHSA). National Survey on Drug Use and Health (NSDUH). Table 2.1B—Tobacco Product and Alcohol Use in Lifetime, Past Year, and Past Month among Persons Aged 12 or Older, by Age Group: Percentages, 2017 and 2018. 2018. Available online: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetTabsSect2pe2018.htm#tab2-1b (accessed on 9 August 2020).
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Research, A.; Staff, C.R.E. Drinking patterns and their definitions. Alcohol Res. 2018, 39, 17–18. [Google Scholar]
- Crabbe, J.C.; Harris, R.A.; Koob, G.F. Preclinical studies of alcohol binge drinking. Ann. N. Y. Acad. Sci. 2011, 1216, 24–40. [Google Scholar] [CrossRef]
- Dguzeh, U.; Haddad, N.C.; Smith, K.T.; Johnson, J.O.; Doye, A.A.; Gwathmey, J.K.; Haddad, G.E. Alcoholism: A multi-systemic cellular insult to organs. Int. J. Environ. Res. Public Health 2018, 15, 1083. [Google Scholar] [CrossRef]
- Pruett, S.; Tan, W.; Howell, G.E.; Nanduri, B. Dosage scaling of alcohol in binge exposure models in mice: An empirical assessment of the relationship between dose, alcohol exposure, and peak blood concentrations in humans and mice. Alcohol 2020, 89, 9–17. [Google Scholar] [CrossRef]
- Ghosh Dastidar, S.; Warner, J.B.; Warner, D.R.; McClain, C.J.; Kirpich, I.A. Rodent models of alcoholic liver disease: Role of binge ethanol administration. Biomolecules 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Coomber, K.; Mayshak, R.; Curtis, A.; Miller, P.G. Awareness and correlates of short-term and long-term consequences of alcohol use among Australian drinkers. Aust. N. Z. J. Public Health 2017, 41, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Moss, H.B. The impact of alcohol on society: A brief overview. Soc. Work Public Health 2013, 28, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J.; King, L.A.; Phillips, L.D. Drug harms in the UK: A multicriteria decision analysis. Lancet 2010, 376, 1558–1565. [Google Scholar] [CrossRef]
- Rao, T.S.; Andrade, C. Alcohol intake, morbidity, and mortality. Indian J. Psychiatry 2016, 58, 1–3. [Google Scholar] [CrossRef]
- Safdar, K.; Schiff, E.R. Alcohol and hepatitis C. Semin. Liver Dis. 2004, 24, 5–15. [Google Scholar] [CrossRef]
- Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef]
- Steiner, J.L.; Lang, C.H. Alcohol, adipose tissue and lipid dysregulation. Biomolecules 2017, 7, 16. [Google Scholar] [CrossRef]
- Bergheim, I.; McClain, C.J.; Arteel, G.E. Treatment of alcoholic liver disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Turhan, N.; Sonsuz, A.; Basaranoglu, G. Mallory-Denk Bodies in chronic hepatitis. World J. Gastroenterol. 2011, 17, 2172–2177. [Google Scholar] [CrossRef]
- Friedman, S.L. Scarring in alcoholic liver disease: New insights and emerging therapies. Alcohol Health Res. World 1997, 21, 310–316. [Google Scholar] [PubMed]
- Moon, D.B.; Lee, S.G. Liver transplantation. Gut Liver 2009, 3, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Wang, M.J.; Chen, F.; Lau, J.T.; Hu, Y.P. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fausto, N.; Campbell, J.S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003, 120, 117–130. [Google Scholar] [CrossRef]
- Messner, D.J.; Murray, K.F.; Kowdley, K.V. Mechanisms of hepatocyte detoxification. Physiol. Gastrointest. Tract 2012, 1507–1527. [Google Scholar] [CrossRef]
- Hautekeete, M.L.; Geerts, A. The hepatic stellate (Ito) cell: Its role in human liver disease. Virchows Arch. 1997, 430, 195–207. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Aydın, M.M.; Akçalı, K.C. Liver fibrosis. Turk. J. Gastroenterol. 2018, 29, 14–21. [Google Scholar] [CrossRef]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, C.L.; Xiao, M.; Yang, R.; Xie, K.Q. Critical roles of kupffer cells in the pathogenesis of alcoholic liver disease: From basic science to clinical trials. Front. Immunol. 2016, 7, 538. [Google Scholar] [CrossRef] [PubMed]
- Hammoutene, A.; Rautou, P.E. Role of liver sinusoidal endothelial cells in nonalcoholic fatty liver disease. J. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Lieber, C.S. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab. Rev. 2004, 34, 511–529. [Google Scholar] [CrossRef]
- Oshino, N.; Oshino, R.; Chance, B. The characteristics of the “peroxidatic” reaction of catalase in ethanol oxidation. Biochem. J. 1973, 131, 555–563. [Google Scholar] [CrossRef]
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health. 2007, 30, 5–13. [Google Scholar]
- Agarwal, D.P.; Goedde, H.W. Human aldehyde dehydrogenase isozymes and alcohol sensitivity. Isozymes Curr. Top. Biol. Med. Res. 1987, 16, 21–48. [Google Scholar]
- Chang, J.S.; Hsiao, J.R.; Chen, C.H. ALDH2 polymorphism and alcohol-related cancers in Asians: A public health perspective. J. Biomed. Sci. 2017, 24, 19. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2010, 5, 121–128. [Google Scholar] [CrossRef]
- Mizumoto, A.; Ohashi, S.; Hirohashi, K.; Amanuma, Y.; Matsuda, T.; Muto, M. Molecular mechanisms of acetaldehyde-mediated carcinogenesis in squamous epithelium. Int. J. Mol. Sci. 2017, 18, 1943. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, M.; Neto, A.C.; Carvalho, G.; Casquillo, N.V.; Carvalho, N.; Okuro, R.; Ribeiro, G.C.M.; Machado, M.; Cardozo, A.; Silva, A.S.E. Does acute exposure to aldehydes impair pulmonary function and structure? Resp. Physiol. Neurobi. 2016, 229, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry. In Ethanol Alters Energy Metabolism in the Liver, 5th ed.; Section 30.5; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Fernandez-Checa, J.C.; Hirano, T.; Tsukamoto, H.; Kaplowitz, N. Mitochondrial glutathione depletion in alcoholic liver disease. Alcohol 1993, 10, 469–475. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Parlesak, A.; Schafer, C.; Schutz, T.; Bode, J.C.; Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000, 32, 742–747. [Google Scholar] [CrossRef]
- Robinson, M.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Mir, H.; Meena, A.S.; Chaudhry, K.K.; Shukla, P.K.; Gangwar, R.; Manda, B.; Padala, M.K.; Shen, L.; Turner, J.R.; Dietrich, P. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim. Biophys. Acta 2016, 1860, 765–774. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Holmes, E.W.; Patel, M.; Iber, F.; Fields, J.Z.; Pethkar, S. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol induced liver damage. Am. J. Gastroenterol. 1999, 94, 200–207. [Google Scholar] [CrossRef]
- Elamin, E.; Jonkers, D.; Juuti-Uusitalo, K.; Jzendoorn, S.v.; Troost, F.; Duimel, H.; Broers, J.; Verheyen, F.; Dekker, J.; Masclee, A. Effects of ethanol and acetaldehyde on tight junction integrity: In vitro study in a three dimensional intestinal epithelial cell culture model. PLoS ONE 2012, 7, e35008. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Seebauer, C.T.; Schnabl, B. Alcoholic liver disease: The gut microbiome and liver cross talk. Alcohol. Clin. Exp. Res. 2015, 39, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.J.; Chaudry, I.H.; Wang, P. Kupffer cells are responsible for producing inflammatory cytokines and hepatocellular dysfunction during early sepsis. J. Surg. Res. 1999, 83, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol 2018, 15, 555–567. [Google Scholar] [CrossRef]
- DeLeve, L.D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 2015, 61, 1740–1746. [Google Scholar] [CrossRef]
- Butterworth, R.F. Hepatic encephalopathy in alcoholic cirrhosis. Alcohol Nerv. Syst. 2014, 589–602. [Google Scholar] [CrossRef]
- Molina, P.E.; Nelson, S. Binge drinking’s effects on the body. Alcohol Res. 2018, 39, 99–109. [Google Scholar]
- Bruha, R.; Dvorak, K.; Petrtyl, J. Alcoholic liver disease. World J. Hepatol. 2012, 4, 81–90. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Keith Roberts, P.W. Molecular biology of the cell. In Innate Immunity, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Dammermann, W.; Wollenberg, L.; Bentzien, F.; Lohse, A.; Lüth, S. Toll like receptor 2 agonists lipoteichoic acid and peptidoglycan are able to enhance antigen specific IFNγ release in whole blood during recall antigen responses. J. Immunol. Methods 2013, 396, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S.; Reddy, M.C.; Prasad, D.V.; Kumar, H. Sweeten PAMPs: Role of sugar complexed PAMPs in innate immunity and vaccine biology. Front. Immunol. 2013, 4, 248. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018. [Google Scholar] [CrossRef] [PubMed]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur. J. Trauma Emerg. Surg. 2020, 46, 751–775. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hinz, M.; Krappmann, D.; Eichten, A.; Heder, A.; Scheidereit, C.; Strauss, M. NF-kappaB function in growth control: Regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol. Cell Biol. 1999, 19, 2690–2698. [Google Scholar] [CrossRef]
- Van Antwerp, D.J.; Martin, S.J.; Verma, I.M.; Green, D.R. Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell Biol. 1998, 8, 107–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W. NFκB signaling regulates embryonic and adult neurogenesis. Front. Biol. 2012, 7, 277–291. [Google Scholar] [CrossRef]
- Huang, H.; Ma, L.; Li, J.; Yu, Y.; Zhang, D.; Jin, J.W.H.; Xu, D.; Gao, J.; Huang, C. NF-κB1 inhibits c-Myc protein degradation through suppression of FBW7 expression. Oncotarget 2014, 5, 493–505. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Huxford, T.; Ghosh, G. A structural guide to proteins of the NF-kappaB signaling module. Cold Spring Harb. Perspect. Biol. 2009, 1, a000075. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Müller, C.W.; Rey, F.A.; Sodeoka, M.; Verdine, G.L.; Harrison, S.C. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature 1995, 373, 311–317. [Google Scholar] [CrossRef]
- Hatada, E.N.; Nieters, A.; Wulczyn, F.G.; Naumann, M.; Meyer, R.; Nucifora, G.; McKeithan, T.W.; Scheidereit, C. The ankyrin repeat domains of the NF-kappa B precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-kappa B DNA binding. Proc. Natl. Acad. Sci. USA 1992, 89, 2489–2493. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin. Ther. Targets 2009, 14, 45–55. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Miyamoto, S. Nuclear initiated NF-κB signaling: NEMO and ATM take center stage. Cell Res. 2011, 21, 116–130. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.F.; Huang, W.; Asagiri, M.; Spann, N.; Hoffmann, A.; Glass, C.; Ghosh, G. The transcriptional specificity of NF-κB dimers is coded within the κB DNA response elements. Cell Rep. 2012, 2, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Hoffmann, A. Crosstalk via the NF-κB signaling system. Cytokine Growth Factor Rev. 2008, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, R.; Melén, K.; Cao, X.; Julkunen, I. NF-κB p52, RelB and c-Rel are transported into the nucleus via a subset of importin α molecules. Cell. Signal. 2008, 20, 1442–1451. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Kim, H.; Kim, K.H. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab. Investig. 2001, 81, 349–360. [Google Scholar] [CrossRef]
- Arias-Salvatierra, D.; Silbergeld, E.K.; Acosta-Saavedra, L.C.; Calderon-Aranda, E.S. Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell. Signal. 2011, 23, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.-F.; Liu, L.P.; Zhong, C.P.; Geng, J.G. NF-κB Activation for Constitutive Expression of VCAM-1 and ICAM-1 on B Lymphocytes and Plasma Cells. Biochem. Biophys. Res. Commun. 2001, 289, 851–856. [Google Scholar] [CrossRef]
- Agrawal, A.; Cha-Molstad, H.; Samols, D.; Kushner, I. Overexpressed nuclear factor-kappaB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPbeta and signal transducer and activator of transcription-3. Immunology 2003, 108, 539–547. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Christman, J.W. The role of nuclear factor- κ B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997, 17, 3–9. [Google Scholar] [CrossRef]
- Jaeschke, H.; Hasegawa, T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006, 26, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Martino, M.; Sutherland, C.L.; Gold, M.R.; Ricciardi-Castagnoli, P. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 1998, 188, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, D.B.; Weeratunga, A.N.; Hu, R.W.; Pandol, S.J.; Hu, R. Alcoholic hepatitis: The pivotal role of Kupffer cells. World J. Gastrointest. Pathophysiol. 2015, 4, 90–98. [Google Scholar] [CrossRef]
- Robertson, M.J.; Caligiuri, M.A.; Manley, T.J.; Levine, H.; Ritz, J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J. Immunol. 1990, 145, 3194–3201. [Google Scholar]
- Ponnuswamy, P.; Ostermeier, E.; Schröttle, A.; Chen, J.; Huang, P.L.; Ertl, G.; Nieswandt, B.; Kuhlencordt, P.J. Oxidative stress and compartment of gene expression determine proatherosclerotic effects of inducible nitric oxide synthase. Am. J. Pathol. 2009, 174, 2400–2410. [Google Scholar] [CrossRef]
- Kickler, K.; Maltby, K.; Ni Choileain, S.; Stephen, J.; Wright, S.; Hafler, D.A.; Jabbour, H.N.; Astier, A.L. Prostaglandin E2 affects T cell responses through modulation of CD46 expression. J. Immunol. 2012, 188, 5303–5310. [Google Scholar] [CrossRef]
- Schmitz, M.; Krappmann, D. Controlling NF-κB activation in T cells by costimulatory receptors. Cell Death Differ. 2006, 13, 834–842. [Google Scholar] [CrossRef]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015, 37, 185–197. [Google Scholar]
- Sun, S.C.; Liu, Z.G. A special issue on NF-kappaB signaling and function. Cell Res 2011, 21, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Van de Pavert, S.; Mebius, R. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 2010, 10, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yamamoto, M.; Taguchi, Y.; Miyauchi, M.; Akiyama, N.; Yamaguchi, N.; Gohda, J.; Akiyama, T.; Inoue, J. Visualization of RelB expression and activation at the single-cell level during dendritic cell maturation in Relb-Venus knock-in mice. J. Biochem. 2015, 158, 485–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kany, S.; Janicova, A.; Relja, B. Innate immunity and alcohol. J. Clin. Med. 2019, 8, 1981. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Pellman, D. Deubiquitinating enzymes: A new class of biological regulators. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 337–352. [Google Scholar] [CrossRef]
- Reyes-Turcu, F.E.; Ventii, K.H.; Wilkinson, K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009, 78, 363–397. [Google Scholar] [CrossRef] [PubMed]
- Amerik, A.Y.; Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1695, 189–207. [Google Scholar] [CrossRef]
- Dixit, V.M.; Green, S.; Sarma, V.; Holzman, L.B.; Wolf, F.W.; O’Rourke, K.; Ward, P.A.; Prochownik, E.V.; Marks, R.M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 1990, 265, 2973–2978. [Google Scholar]
- Da Silva, C.G.; Cervantes, J.R.; Studer, P.; Ferran, C. A20-an omnipotent protein in the liver: Prometheus myth resolved? Mult. Ther. Targets A20 2014, 117–139. [Google Scholar] [CrossRef]
- Catrysse, L.; Fukaya, M.; Sze, M.; Meyerovich, K.; Beyaert, R.; Cardozo, A.K.; Van Loo, G. A20 deficiency sensitizes pancreatic beta cells to cytokine-induced apoptosis in vitro but does not influence type 1 diabetes development in vivo. Cell Death Dis. 2015. [Google Scholar] [CrossRef]
- Catrysse, L.; Farhang Ghahremani, M.; Vereecke, L.; Youssef, S.A.; McGuire, C.; Sze MWeber, A.; Heikenwalder, M.; de Bruin, A.; Beyaert, R.; van Loo, G. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell Death Dis. 2016, 7, e2250. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 1, 22–31. [Google Scholar] [CrossRef]
- Wertz, I.; O’Rourke, K.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Dainichi, T.; Rathinam, C.V.; Ghosh, S. The deubiquitinase activity of A20 is dispensable for NF-κB signaling. EMBO Rep. 2014, 15, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Boone, D.L.; Chai, S.; Libby, S.L.; Chien, M.; Lodolce, J.P.; Ma, A. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 2000, 289, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Martens, A.; Priem, D.; Hoste, E.; Vetters, J.; Rennen, S.; Catrysse, L.; Voet, S.; Deelen, L.; Sze, M.; Vikkula, H.; et al. Two distinct ubiquitin-binding motifs in A20 mediate its anti-inflammatory and cell-protective activities. Nat. Immunol. 2020, 21, 381–387. [Google Scholar] [CrossRef]
- Razani, B.; Whang, M.I.; Kim, F.S.; Nakamura, M.C.; Sun, X.; Advincula, R.; Turnbaugh, J.A.; Pendse, M.; Tanbun, P.; Achacoso, P.; et al. Non-catalytic ubiquitin binding by A20 prevents psoriatic arthritis-like disease and inflammation. Nat. Immunol. 2020, 21, 422–433. [Google Scholar] [CrossRef]
- Nakagawa, M.M.; Thummar, K.; Mandelbaum, J.; Pasqualucci, L.; Rathinam, C.V. Lack of the ubiquitin-editing enzyme A20 results in loss of hematopoietic stem cell quiescence. J. Exp. Med. 2015, 212, 203–216. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Cai, Y.; Liu, R.; Lu, M.; Li, T.; Fu, Y.; Guo, M.; Huang, H.; Ou, Y.; et al. A20 targets PFKL and glycolysis to inhibit the progression of hepatocellular carcinoma. Cell Death Dis. 2020, 11, 89. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Luo, Z.; Zhang, J.; Zhang, C.; Qiu, B.; Dong, L.; Tan, Y.; Ding, J.; Tang, S.; et al. A20 suppresses hepatocellular carcinoma proliferation and metastasis through inhibition of Twist1 expression. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef]
- Balakirev, M.Y.; Tcherniuk, S.O.; Jaquinod, M.; Chroboczek, J. Otubains: A new family of cysteine proteases in the ubiquitin pathway. EMBO Rep. 2003, 4, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Wiener, R.; DiBello, A.T.; Lombardi, P.M.; Guzzo, C.M.; Zhang, X.; Matunis, M.J.; Wolberger, C. E2 ubiquitin-conjugating enzymes regulate the deubiquitinating activity of OTUB1. Nat. Struct. Mol. Biol. 2013, 20, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Chroma, K.; Mistrik, M.; Moudry, P.; Gursky, J.; Liptay, M.; Strauss, R.; Skrott, Z.; Vrtel, R.; Bartkova, J.; Kramara, J.; et al. Tumors overexpressing RNF168 show altered DNA repair and responses to genotoxic treatments, genomic instability and resistance to proteotoxic stress. Oncogene 2017, 36, 2405–2422. [Google Scholar] [CrossRef] [PubMed]
- Nakada, S.; Tai, I.; Panier, S.; Al-Hakim, A.; Iemura, S.; Juang, Y.C.; O’Donnell, L.; Kumakubo, A.; Munro, M.; Sicheri, F.; et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 2010, 466, 941–946. [Google Scholar] [CrossRef]
- Wiener, R.; Zhang, X.; Wang, T.; Wolberger, C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 2012, 483, 618–622. [Google Scholar] [CrossRef]
- Mulas, F.; Wang, X.; Song, S.; Nishanth, G.; Yi, W.; Brunn, A.; Larsen, P.K.; Isermann, B.; Kalinke, U.; Barragan, A.; et al. The deubiquitinase OTUB1 augments NF-κB-dependent immune responses in dendritic cells in infection and inflammation by stabilizing UBC13. Cell. Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.Y.; Xie, X.; Jie, Z.; Zhang, L.; Shi, J.; Lin, D.; Gu, M.; Zhou, X.; Li, H.S.; et al. Preventing abnormal NF-κB activation and autoimmunity by Otub1-mediated p100 stabilization. Cell Res. 2019, 29, 474–485. [Google Scholar] [CrossRef]
- Sun, S.C. CYLD: A tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010, 17, 25–34. [Google Scholar] [CrossRef]
- Lork, M.; Verhelst, K.; Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: So similar, yet so different. Cell Death Differ. 2017, 24, 1172–1183. [Google Scholar] [CrossRef]
- Wooten, M.W.; Geetha, T.; Babu, J.R.; Seibenhener, M.L.; Peng, J.; Cox, N.; Diaz-Meco, M.T.; Moscat, J. Essential role of sequestosome 1/p62 in regulating accumulation of Lys63-ubiquitinated proteins. J. Biol. Chem. 2008, 283, 6783–6789. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Dixit, V.M. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2011, 21, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, E.W.; Dixit, V.M. Regulation of NF-κB by deubiquitinases. Immunol. Rev. 2012, 246, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, R.B.; Jolin, H.E.; Allison, M.E.D.; Davies, S.E.; Titheradge, H.L.; McKenzie, A.N.J.; Komander, D. OTULIN protects the liver against cell death, inflammation, fibrosis, and cancer. Cell Death Differ. 2020, 27, 1457–1474. [Google Scholar] [CrossRef]
- Fiil, B.K.; Gyrd-Hansen, M. OTULIN deficiency causes auto-inflammatory syndrome. Cell Res. 2016, 26, 1176–1177. [Google Scholar] [CrossRef][Green Version]
- Martens, A.; van Loo, G. A20 at the Crossroads of cell death, inflammation, and autoimmunity. Cold Spring Harb. Perspect. Biol. 2020, 12. [Google Scholar] [CrossRef]
- Anantharaju, A.; Van Thiel, D.H. Liver transplantation for alcoholic liver disease. Alcohol Res. Health. 2003, 27, 257–268. [Google Scholar]
- Louvet, A.; Naveau, S.; Abdelnour, M.; Ramond, M.J.; Diaz, E.; Fartoux, L.; Dharancy, S.; Texier, F.; Hollebecque, A.; Serfaty, L.; et al. The lille model: A new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007, 45, 1348–1354. [Google Scholar] [CrossRef]
- Saberi, B.; Dadabhai, A.S.; Jang, Y.Y.; Gurakar, A.; Mezey, E. Current management of alcoholic hepatitis and future therapies. J. Clin. Transl. Hepatol. 2016, 4, 113–122. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Shang, H.C.; Gao, X.M.; Zhang, B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother. Res. 2008, 22, 851–858. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, X.; Wang, Q.; Xia, M.; Zhu, Y.; Lian, F.; Ling, W. Cytochrome P4502E1 inhibitor, chlormethiazole, decreases lipopolysaccharide-induced inflammation in rat Kupffer cells with ethanol treatment. Hepatol. Res. 2013. [Google Scholar] [CrossRef]
- Cheng, C.F.; Pan, T.M. Ankaflavin and monascin induce apoptosis in activated hepatic stellate cells through suppression of the Akt/NF-κB/p38 signaling pathway. J. Agric. Food Chem. 2016, 64, 9326–9334. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Qi, S.; Zhang, W.; Mao, J.; Tang, R.; Wang, C.; Liu, J.; Luo, X.M.; Wang, H. Lactobacillus reuteri ZJ617 Culture Supernatant Attenuates Acute Liver Injury Induced in Mice by Lipopolysaccharide. J. Nutr. 2019, 149, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Geng, Y.; Chen, H.; Lu, Z.M.; Shi, J.S.; Xu, Z. Polysaccharide peptides from coriolus versicolor: A multi-targeted approach for the protection or prevention of alcoholic liver disease. J. Funct. Foods 2018, 40, 769–777. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Z.; Yen, H.; Lee, Y.; Yin, M. Reversal of ethanol-induced hepatotoxicity by cinnamic and syringic acids in mice. Food Chem. Toxicol. 2016, 98, 119–126. [Google Scholar] [CrossRef]
- Radic, I.; Mijovic, M.; Hudomal, S.J.; Mitic, M.; Lukic, V.; Joksimovic, B.; Petrovic, Z.; Ristic, S.; Velickovic, S.; Nestorovic, V.; et al. Protective effects of whey on rat liver damage induced by chronic alcohol intake. Hum. Exp. Toxicol. 2019, 38, 632–645. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Han, X.; Yin, L.; Xu, L.; Qi, Y.; Zhao, Y.; Liu, K.; Peng, J. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-κB signaling pathway. Sci. Rep. 2016, 5, 18038. [Google Scholar] [CrossRef]
- Song, J.; Han, X.; Yao, Y.L.; Li, Y.M.; Zhang, J.; Shao, D.Y.; Hou, L.S.; Fan, Y.; Song, S.Z.; Lian, L.H.; et al. Acanthoic acid suppresses lipin1/2 via TLR4 and IRAK4 signalling pathways in EtOH- and lipopolysaccharide-induced hepatic lipogenesis. J. Pharm. Pharmacol. 2018, 70, 393–403. [Google Scholar] [CrossRef]
- Sim, M.O.; Lee, H.I.; Ham, J.R.; Seo, K.I.; Kim, M.J.; Lee, M.K. Anti-inflammatory and antioxidant effects of umbelliferone in chronic alcohol-fed rats. Nutr. Res. Pract. 2015, 9, 364. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar. Drugs. 2019, 17, 552. [Google Scholar] [CrossRef]
- Koneru, M.; Sahu, B.D.; Gudem, S.; Kuncha, M.; Ravuri, H.G.; Kumar, J.M.; Kilari, E.K.; Sistla, R. Polydatin alleviates alcohol-induced acute liver injury in mice: Relevance of matrix metalloproteinases (MMPs) and hepatic antioxidants. Phytomedicine 2017, 27, 23–32. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Chen, X.Y.; Hu, P.Y.; Tan, M.M.; Tang, X.G.; Huang, M.C.; Lou, Z.H. Effects of Linderae radix extracts on a rat model of alcoholic liver injury. Exp. Ther. Med. 2016, 11, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Zhang, S.; Liu, S.; Zhao, L.; Luo, H.; Chen, Y.; Huang, W. Ginsenoside Rg1 inhibits inflammatory responses via modulation of the nuclear factor-κB pathway and inhibition of inflammasome activation in alcoholic hepatitis. Int J. Mol. Med. 2018, 41, 899–907. [Google Scholar] [CrossRef]
- Gao, Y.; Chu, S.; Li, J.; Li, J.; Zhang, Z.; Xia, C.; Heng, Y.; Zhang, M.; Hu, J.; Wei, G.; et al. Anti-inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor-kappa B pathway. J. Ethnopharmacol. 2015, 173, 231–240. [Google Scholar] [CrossRef]
- Qu, L.; Zhu, Y.; Liu, Y.; Yang, H.; Zhu, C.; Ma, P.; Deng, J.; Fan, D. Protective effects of ginsenoside Rk3 against chronic alcohol-induced liver injury in mice through inhibition of inflammation, oxidative stress, and apoptosis. Food Chem. Toxicol. 2019. [Google Scholar] [CrossRef]
- Lavrovsky, Y.; Chatterjee, B.; Clark, R.A.; Roy, A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000, 35, 521–532. [Google Scholar] [CrossRef]
- Shepard, B.D.; Tuma, P.L. Alcohol-induced alterations of the hepatocyte cytoskeleton. World J. Gastroenterol. 2010, 16, 1358–1365. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Song, Y.; Wu, X.; Yang, D.; Fang, F.; Meng, L.; Liu, Y.; Cui, W. Protective effect of andrographolide on alleviating chronic alcoholic liver disease in mice by inhibiting nuclear factor kappa b and tumor necrosis factor alpha activation. J. Med. Food. 2020, 23, 409–415. [Google Scholar] [CrossRef]
- Su, N.W.; Lin, Y.L.; Lee, M.H.; Ho, C.Y. Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J. Agric. Food Chem. 2005, 53, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.H.; Xu, L.N.; Wang, X.N.; Lu, B.N.; Liu, Y.T.; Peng, J.P. An economical method for isolation of dioscin from dioscorea nipponica makino by HSCCC coupled with ELSD and a computer-aided UNIFAC mathematical model. Chromatographia 2010, 71, 15–23. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 2014, 6, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, R.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction, and liver inflammation mediated by the endotoxin-TLR4-NF-κB pathway. J. Agric. Food Chem. 2020, 68, 1237–1247. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Song, S.; Li, S.; Su, N.; Li, J.; Shi, F.; Ye, M. Structural characterization, molecular modification and hepatoprotective effect of melanin from Lachnum YM226 on acute alcohol-induced liver injury in mice. Food Funct. 2016, 7, 3617–3627. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, N.; Yang, D.; Yang, M.; Guo, X.; He, J.; Wu, W.; Ji, B.; Cheng, Q.; Zhou, F. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients 2018, 10, 1754. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Wang, T.; Zhao, G.; Qian, F.; Huang, J.; Wang, H.; Zhang, X.; Wang, Y. Green tea infusion protects against alcoholic liver injury by attenuating inflammation and regulating the PI3K/Akt/eNOS pathway in C57BL/6 mice. Food Funct. 2017, 8, 3165–3177. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice. Nutrients 2017, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, D.; Wang, Y.; Meng, X.; Sun, X.; Tian, J.; Shi, L.; Ma, F. Schisantherin A alleviated alcohol-induced liver injury by the regulation of alcohol metabolism and NF-kB pathway. Exp. Anim. 2018, 67, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Boaru, S.G.; Borkham-Kamphorst, E.; Van de Leur, E.; Lehnen, E.; Liedtke, C.; Weiskirchen, R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2015, 458, 700–706. [Google Scholar] [CrossRef]
- Immenschuh, S.; Baumgart-Vogt, E.; Mueller, S. Heme oxygenase-1 and iron in liver inflammation: A complex alliance. Curr. Drug Targets 2010, 11, 1541–1550. [Google Scholar] [CrossRef]
- He, G.; Karin, M. NF-κB and STAT3—Key players in liver inflammation and cancer. Cell Res. 2010, 21, 159–168. [Google Scholar] [CrossRef]
- Seo, H.Y.; Kim, M.K.; Lee, S.H.; Hwang, J.; Park, K.G.; Jang, B. Kahweol ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 activation in primary kupffer cells and primary hepatocytes. Nutrients 2018, 10, 863. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Remels, A.H.; Langen, R.C.; Gosker, H.R.; Russell, A.P.; Spaapen, F.; Voncken, J.W.; Schrauwen, P.; Schols, A.M. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E174–E183. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Lin, H.H.; Hsu, C.C.; Chen, B.C.; Chen, J.H. Aqueous extract of pepino (solanum muriactum ait) leaves ameliorate lipid accumulation and oxidative stress in alcoholic fatty liver disease. Nutrients 2018, 10, 931. [Google Scholar] [CrossRef]
- Moslehi, A.; Hamidi-Zad, Z. Role of SREBPs in liver diseases: A mini-review. J. Clin. Transl. Hepatol. 2018, 6, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Reue, K.; Dwyer, J.R. Lipin proteins and metabolic homeostasis. J. Lipid Res. 2009, 50, S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Hull, V.V.; Math, A.A.; Vernekar, S.N.; Kulkarni, S.S. A review on laboratory liver function tests. Pan. Afr. Med. J. 2009, 3, 17. [Google Scholar] [PubMed]
- Matthaei, K.I. Genetically manipulated mice: A powerful tool with unsuspected caveats. J. Physiol. 2007, 582 Pt 2, 481–488. [Google Scholar] [CrossRef]
- Michailidis, E.; Vercauteren, K.; Mancio-Silva, L.; Andrus, L.; Jahan, C.; Ricardo-Lax, I.; Zou, C.; Kabbani, M.; Park, P.; Quirk, C.; et al. Expansion, in vivo-ex vivo cycling, and genetic manipulation of primary human hepatocytes. Proc. Natl. Acad. Sci. USA 2020, 117, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Maraslioglu, M.; Oppermann, E.; Blattner, C.; Weber, R.; Henrich, D.; Jobin, C.; Schleucher, E.; Marzi, I.; Lehnert, M. Chronic ethanol feeding modulates inflammatory mediators, activation of nuclear factor-κb, and responsiveness to endotoxin in murine kupffer cells and circulating leukocytes. Mediators Inflamm. 2014, 1–16. [Google Scholar] [CrossRef]
- Wang, J.; Kainrad, N.; Shen, H.; You, M.; Zhou, Z.; Rote, P.; Zhang, Y.; Nagy, L.E.; Wu, J. Hepatic knockdown of splicing regulator Slu7 ameliorates inflammation and attenuates liver injury in ethanol-fed mice. Am. J. Pathol. 2018, 188, 1807–1819. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell. Commun. Signal. 2011, 9, 11. [Google Scholar] [CrossRef]
- Wang, J.; Kim, C.; Jogasuria, A.; Han, Y.; Hu, X.; Wu, J.; Shen, H.; Chrast, R.; Finck, B.N.; You, M. Myeloid cell-specific lipin-1 deficiency stimulates endocrine adiponectin-FGF15 axis and ameliorates ethanol-induced liver injury in mice. Sci. Rep. 2016, 6, 34117. [Google Scholar] [CrossRef]
- Hu, X.; Jogasuria, A.; Wang, J.; Kim, C.; Han, Y.; Shen, H.; Wu, J.; You, M. MitoNEET deficiency alleviates experimental alcoholic steatohepatitis in mice by stimulating endocrine adiponectin-Fgf15 axis. J. Biol. Chem. 2016, 291, 22482–22495. [Google Scholar] [CrossRef]
- Wiley, S.E.; Paddock, M.L.; Abresch, E.C.; Gross, L.; van der Geer, P.; Nechushtai, R.; Murphy, A.N.; Jennings, P.A.; Dixon, J.E. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J. Biol. Chem. 2007, 282, 23745–23749. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kaczanowska, S.; Davila, E. IL-1 receptor-associated kinase signaling and its role in inflammation, cancer progression, and therapy resistance. Front. Immunol. 2014, 5, 553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, M.; Zhao, J.; Martin, B.N.; Roychowdhury, S.; McMullen, M.R.; Wang, E.; Fox, P.L.; Yamasaki, S.; Nagy, L.E.; et al. IRAKM-mincle axis links cell death to inflammation: Pathophysiological implications for chronic alcoholic liver disease. Hepatology 2016, 64, 1978–1993. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Hara, H.; Ogata, K.; Saito, T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008, 9, 1179–1188. [Google Scholar] [CrossRef]
- Zmijewski, E.; Lu, S.; Harrison-Findik, D.D. TLR4 signaling and the inhibition of liver hepcidin expression by alcohol. World J. Gastroenterol. 2014, 20, 12161–12170. [Google Scholar] [CrossRef]
- Bárcena, C.; Stefanovic, M.; Tutusaus, A.; Morales, A. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J. Hepatol. 2015, 63, 670–678. [Google Scholar] [CrossRef]
- Sloot, Y.J.E.; Smit, J.W.; Joosten, L.A.B.; Netea-Maier, R.T. Insights into the role of IL-32 in cancer. Semin. Immunol. 2018, 38, 24–32. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, D.H.; Hwang, C.J.; Song, S.; Han, S.B.; Kim, Y.; Yoo, H.S.; Jung, Y.S.; Kim, S.H.; Yoon, D.Y.; et al. Interleukin-32γ attenuates ethanol-induced liver injury by the inhibition of cytochrome P450 2E1 expression and inflammatory responses. Clin. Sci. 2015, 128, 695–706. [Google Scholar] [CrossRef]
- Chang, B.; Xu, M.J.; Zhou, Z.; Cai, Y.; Li, M.; Wang, W.; Feng, D.; Bertola, A.; Wang, H.; Kunos, G.; et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology 2015, 62, 1070–1085. [Google Scholar] [CrossRef]
- Chen, P.J.; Cai, S.P.; Yang, Y.; Li, W.X.; Huang, C.; Meng, X.M.; Li, J. PTP1B confers liver fibrosis by regulating the activation of hepatic stellate cells. Toxicol. Appl. Pharmacol. 2016, 292, 8–18. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Y.Y.; Liu, Y.R.; Yin, N.N.; Bu, F.T.; Yu, H.X.; Du, X.S.; Li, J.; Huang, C. PTP1B promotes macrophage activation by regulating the NF-κB pathway in alcoholic liver injury. Toxicol. Lett. 2020, 319, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Wang, S.; Zhao, K.; Li, Y.; Williams, J.A.; Li, T.; Chavan, H.; Krishnamurthy, P.; He, X.C.; Li, L.; et al. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology 2018, 155, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M. Transcription factor EB: From master coordinator of lysosomal pathways to candidate therapeutic target in degenerative storage diseases. Ann. N. Y. Acad. Sci. 2016, 1371, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, L.; Ding, M.; Luo, Z.; Yuan, S.; Bansal, M.B.; Gilkeson, G.; Lang, R.; Jiang, W. Estrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin. Immunol. 2017, 183, 174–180. [Google Scholar] [CrossRef]
- Kanuri, G.; Wagnerberger, S.; Landmann, M.; Prigl, E.; Hellerbrand, C.; Bischoff, S.C.; Bergheim, I. Effect of acute beer ingestion on the liver: Studies in female mice. Eur. J. Nutr. 2015, 54, 465–474. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Torres, J.L.; Novo-Veleiro, I.; Manzanedo, L.; Alvela-Suárez, L.; Macías, R.; Laso, F.J.; Marcos, M. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 4104–4118. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Dippold, R.P.; Vadigepalli, R.; Gonye, G.E.; Patra, B.; Hoek, J.B. Chronic ethanol feeding alters miRNA expression dynamics during liver regeneration. Alcohol. Clin. Exp. Res. 2012, 37, E59–E69. [Google Scholar] [CrossRef]
- Eguchi, A.; Franz, N.; Kobayashi, Y.; Iwasa, M.; Wagner, N.; Hildebrand, F.; Takei, Y.; Marzi, I.; Relja, B. Circulating extracellular vesicles and their miR “Barcode” differentiate alcohol drinkers with liver injury and those without liver injury in severe trauma patients. Front. Med. (Lausanne) 2019, 6, 30. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, Y.; Chang, B.; Wang, B. Nod-like receptor protein 3 inflammasome activation by Escherichia coli RNA induces transforming growth factor beta 1 secretion in hepatic stellate cells. Bosn. J. Basic Med. Sci. 2016, 16, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Xu, Z.M.; Zhang, C.M.; Dai, H.Y.; Ji, X.Q.; Wang, X.F.; Li, C. Pyrrolidine dithiocarbamate inhibits nuclear factor-κB pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stromal cells. Mol. Hum. Reprod. 2011, 17, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Yang, Y.; Li, W.X.; Cheng, Y.H.; Li, X.F.; Huang, C.; Meng, X.M.; Wu, B.M.; Liu, X.H.; Zhang, L. Telomerase reverse transcriptase acts in a feedback loop with NF-κB pathway to regulate macrophage polarization in alcoholic liver disease. Sci. Rep. 2016, 6, 18685. [Google Scholar] [CrossRef] [PubMed]
- Braselmann, S.; Taylor, V.; Zhao, H.; Wang, S.; Sylvain, C.; Baluom, M.; Qu, K.; Herlaar, E.; Lau, A.; Young, C.; et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharm. Exp. 2006, 319, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Bukong, T.N.; Iracheta-Vellve, A.; Saha, B.; Ambade, A.; Satishchandran, A.; Gyongyosi, B.; Lowe, P.; Catalano, D.; Kodys, K.; Szabo, G. Inhibition of spleen tyrosine kinase activation ameliorates inflammation, cell death, and steatosis in alcoholic liver disease. Hepatology 2016, 64, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N.; Sarkar, D.K. Effects of alcohol on the endocrine system. Endocrinol. Metab. Clin. N. Am. 2013, 42, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Klein, M.; Xie, W.; Zanger, U.M.; Mohammad, M.K.; Cave, M.C.; Gaikwad, N.W.; Dias, N.J.; Selcer, K.W.; Guo, Y.; et al. Inflammatory regulation of steroid sulfatase: A novel mechanism to control estrogen homeostasis and inflammation in chronic liver disease. J. Hepatol. 2016, 64, 44–52. [Google Scholar] [CrossRef]
- Lee, K.J.; Jang, Y.O.; Cha, S.K.; Kim, M.Y.; Park, K.S.; Eom, Y.W.; Baik, S.K. Expression of fibroblast growth factor 21 and β-klotho regulates hepatic fibrosis through the nuclear factor-κB and c-Jun N-terminal kinase pathways. Gut Liver 2018, 12, 449–456. [Google Scholar] [CrossRef]
- Stärkel, P.; Schnabl, B.; Leclercq, I.; Leclercq, S.; Komuta, M.; Bataller, R.; Argemi, J.; Palma, E.; Chokshi, S.; Hellerbrand, C.; et al. Deficient IL-6/stat3 signaling, high TLR7, and type I interferons in early human alcoholic liver disease: A triad for liver damage and fibrosis. Hepatol. Commun. 2019, 3, 867–882. [Google Scholar] [CrossRef]
- Purohit, V. Moderate alcohol consumption and estrogen levels in postmenopausal women: A review. Alcohol. Clin. Exp. Res. 1998, 22, 994–997. [Google Scholar] [CrossRef]
- Cai, X.; Bao, L.; Wang, N.; Ren, J.; Chen, Q.; Xu, M.; Li, D.; Mao, R.; Li, Y. Dietary nucleotides protect against alcoholic liver injury by attenuating inflammation and regulating gut microbiota in rats. Food Funct. 2016, 6, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Lazaro, R.G.; Wang, J.; Kim, J.; Povero, D.; Willliams, B.; Ho, S.B.; Stärkel, P.; Schnabl, B.; Ohno-Machado, L.; et al. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology 2017, 65, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, A.; Nakhaee, N. A review of alcohol-related harms: A recent update. Addict. Health 2019, 11, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, M.; Groothuis, G.M.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Y.; Chen, Y.C.; Liu, S.P. Chronic ingestion of alcohol modulates expression of ubiquitin editing enzyme A20 in lung macrophages. Multidiscip. Respir. Med. 2011, 6, 364–370. [Google Scholar] [CrossRef] [PubMed]

| Type of Study | Model Organism/Isolation Source | Cell Type/Cell Line/Tissue | Tested Substance(S), Derivative(S), Compound(s) | Compound(s) Source(s) | Conditions | Primary Findings/Results | Ref. |
|---|---|---|---|---|---|---|---|
| In vivo | SD rats | Kupffer cells | CMZ | semi-synthetic | ALD, LPS stimulation | ↓ levels of CYP2E1, ↓ accumulation of NF-κB p65 subunit and TNF-α | [154] |
| In vitro | SD rats | HSCs/ HSC-T6, primary hepatocytes | ankaflavin, monascin | Monascus purpureus, fermentation | ALD | ↑ levels of p53, ↑ caspase 3 activity, ↓ levels of NF-κB expression, ↑ levels of IκB expression | [155] |
| In vivo | C57BL/6 mice | blood, liver tissue | culture supernatant | Lactobacillus reuteri ZJ617, Lactobacillus rhamnosus GG | ALD, LPS stimulation | ↓ levels of serum ALT and AST, ↑ levels of claudin 3, ZO-1 and occludin, ↓ IL-6 and TNFα, ↑ expression of IL-10, ↓ expression of TLR4, ↓ NF-κB, ZJ617s suppress TLR4/MAPK/NF-κB activation | [156] |
| In vivo | C57BL/6J mice | blood, liver tissue | polysaccharide peptide (PSP) | Coriolus versicolor JNPF-CV05 strain | ALD, chronic and binge models | ↓ ALT, AST and MDA, ↑ activity of AMPK and PPARα, ↓ levels of TLR2, TLR4 and NF-κB | [157] |
| In vivo | BALB/cA mice | liver tissue | cinnamic acid, syringic acid | - | ALD, ALI | ↓ levels of CYP2E1, COX-2 and NF-κB, ↑ Nrf2 expression, ↓ levels of IL-6 and TNFα | [158] |
| In vivo | Wistar rats | liver tissue | whey | - | ALD, ALI | ↑ SOD and NF-kB protein levels, lower inflammation after whey consumption | [159] |
| In vivo | ICR mice | liver tissue | fucoxanthin | marine seaweed | ALD, ALI | ↑ expression of Nrf2-mediated signaling pathway, ↓ TLR4 and NF-κB | [163] |
| In vivo | C57BL/6 mice | blood, liver tissue | polydatin (piceid) | Picea sitchensis | ALD, ALI | ↓ levels of serum ALT and AST, ↓ expression of CYP2E1, ↓ of NF-κB | [164] |
| In vivo | SPF-Wistar rats | blood, liver samples | quercetin | - | ALD, AH, acute ALI model | ↑ HO-1, ↓ NLRP3, ↓ activity of NF-κB, ↑ promotion of IL-10 | [166] |
| In vivo | SD rats | blood, liver tissue | linderae radix | Lindera aggregata | ALD, ALI | ↓ levels of serum ALT, AST, MDA, ↓ level of CYP2E1, ↓ NF-κB, TNF-α and IL-1β | [167] |
| In vivo | SD rats | blood, liver tissue | ginsenoside Rk3 | Panax ginseng | ALD, chronic drinking | ↓ levels of caspase-3 and caspase-8, ↓ levels of CYP2E1 expression, ↓ levels of NF-κB | [168] |
| In vivo | C57BL/6 mice | blood, liver tissue | ginsenoside Rg1 | Panax ginseng C.A. Mayer | ALD, ALI, binge drinking | ↓ levels of hepatic TNF-α, IL-1β and IL-6, ↓ levels of NF-κB activity, ↑ levels of glucocorticoid receptor, ↓ levels of ALT and AST | [169] |
| In vivo | C57BL/6 mice | blood, liver tissue | ginsenoside Rg1 | Panax ginseng | ALD, chronic drinking | ↓ levels of NF-κB, ↓ production of TNF-α, IL-6 and IL-1β, ↑ expression levels of SOD and GSH | [170] |
| In vivo | C57BL/6J | blood, liver tissue | andrographolide | Andrographis paniculata | ALD, ALI | ↓ the hepatic levels of NF-κB and TNFα, ↓ levels of serum ALT, AST, | [174] |
| In vivo | C57BL/6 mice | liver tissue | RBPE | Oryza sativa | ALD, ALI | ↓ expression of ZO-1, claudin-1 and claudin-4, ↓ microbiota dysbiosis, attenuated activation of LPS/TLR4/NF-κB pathway | [179] |
| In vivo, in vitro | Kunming mice | liver tissue, HepG2 | melanin | Lachnum YM226 | ALD, ALI | ↓ hepatic levels of NF-κB, IL-6 and TNFα, ↓ hepatic activities of iNOS and COX-2 | [181] |
| In vivo | ICR mice | liver tissue | apigenin, quercetin, naringenin, (−)-epigallocatechin gallate, genistein | flavonoids | ALD, ALI | genistein mitigates fibrosis and naringenin mitigates apoptosis, ↓ levels of NF-κB p65, COX-2 and IL-6, ↓ serum levels of AST, ALT | [182] |
| In vivo | C57BL/6 mice | blood, liver tissue | green tea infusion | Camellia sinensis | ALD, ALI | ↓ levels of serum ALT, AST, MDA, ↓ expression of TLR4 and NF-κB, ↓ expression of iNOS | [183] |
| In vivo | ICR mice | blood, liver tissue | artichoke extract | Cynara scolymus L. | ALD, ALI | ↓ levels of serum ALT, AST, MDA, ↓ expression of TLR4 and NF-κB | [186] |
| In vivo | C57BL/6 | blood, liver tissue | schisantherin A | Schisandra chinensis | ALD, ALI | ↓ levels of serum ALT and AST, ↓ CYP2E1 and CYP1A2 expression, ↓ NF-κB, ↓ ADH, ↑ ALDH | [187] |
| In vivo | C57BL/6 mice | blood, liver tissue | aqueous extract | Pepino (Solanum muriactum) | ALD, Lieber–DeCarli diet | ↓ serum levels of AST and ALT, ↑ AMPK and PPAR-α, ↓ SREBP-1c, ↓ TNFα and IL-6, ↓ activity of NF-κB | [194] |
| In vitro | mice | HSCs/HSC-T6, primary hepatocytes | dioscin | - | ALD, LPS stimulation | ↓ levels of MyD88, NF-κB, IL-1, IL-6, TNFα, TLR4, expression | [160] |
| In vivo, in vitro | C57BL/6 | HSC-T6, liver tissue | acanthoic acid | Annona amazonica | lipogenesis model, LPS stimulation | ↓ expression of SREBP-1, and lipin1/2, ↓ fat droplets caused by EtOH/LPS. ↓ expression of TLR4 and NF-κB | [164] |
| In vivo | C57BL/6 mice | Kupffer cells, hepatocytes | kahweol | coffee beans | ALD, LPS stimulation | ↓ levels of IL-1α, IL-1β, IL-6 and TNFα, ↓ STAT3 and MAPK, ↓ activation of NF-κB | [191] |
| In vivo | SD rats | liver tissue | umbelliferone (7-hydroxycoumarin) | Umbelliferae plant family | ALD, fibrosis, Lieber–DeCarli | ↓ levels of TNF-α and IL-6, ↑ levels of IL-10, ↓ levels of TLR4 and NF-κB, improved mild hepatic fibrosis | [162] |
| Type of Study | Model Organism, Isolation Source | Cell Type/Cell Line | Target, Method | Condition | Primary Findings/Results | Ref. |
|---|---|---|---|---|---|---|
| In vivo | mice | HSCs | Gas6/Axl, siRNA silencing | ALD, ALI, fibrosis | ↑ serum levels of Gas6 and Axl with chronic disease progression, Gas6/Axl compulsory for HSCs activation | [210] |
| In vivo | mice | HCs | TFEB, deletion and OE | ALD, ALI | overexpression of TFEB led to ↓ of lysosomal biogenesis and mitochondrial activities, KO mice developed more severe ALI syndromes | [216] |
| In vivo, in vitro | mice | HCs from mice, HepG2 and Huh7 lines | IL-32γ, OE and transfection | ALD, ALI | ↓ levels of COX-2 and IL-6, ↓ level of NF-κB activity | [212] |
| In vivo | mice | Kupffer cells | NF-κB with EGFP, ER | ALD, ALI, LPS stimulation | LPS and chronic EtOH ↑ levels of NF-κB activity, ↑ expression levels of IL-6 and TNF-𝛼 | [200] |
| In vivo | mice | myeloid cells | lipin-1, deletion | ALD, ALI | ↑ levels of adiponectin and FGF-15 expression, ↓ NF-κB activity | [203] |
| In vivo | mice | HCs | SLU7, KO | ALD, ALI | ↑ expression levels of SIRT1 and lipin-1, thus ↓ NF-κB activity | [201] |
| In vivo | mice | HCs | mNT, KO | ALD, ALI, SH, Lieber–DeCarli | ↓ levels of AST and ALT, ↑ levels of adiponectin and FGF-15 expression, ↑ of SIRT1 and ↓ NF-κB activity | [204] |
| In vivo, ex vivo | mice | HCs, bone marrow macrophages | IRAKM, KO | ALD, ALI, LPS stimulation | Mincle ligand SAP130 activates inflammatory response, LPS activates the formation of IRAKM Myddosome, IRAKM or Mincle deficiency protects from ALI, ↓ NF-κB activity | [207] |
| In vivo | mice | HCs | TLR4, KO | ALD, ALI, Lieber–DeCarli | ↓ translocation of NF-κB p65, ↓ binding of NF-κB p50 to hepcidin, ↓ NF-κB activity | [209] |
| In vivo, in vitro | mice | liver cells | cxcl1, deletion | HFD, binge drinking, ALD | Cxcl11 deletion caused ↓ level of HFD + EtOH-related inflammatory response, overexpression of cxcl11 caused ↑ of SH syndrome, | [210] |
| In vivo, in vitro | mice | liver cells, macrophages | PTP1B, siRNA silencing, OE | ALD, ALI, LPS stimulation | silencing of PTP1B resulted in ↓ levels of IL-6 and TNF-α, while PTP1B overexpression led to ↑ inflammation, PTP1B can regulate NF-κB activity | [214] |
| Type of Study | Model Organism, Isolation Source | Cell Type/Organ | Approach | Condition | Primary Findings/Results | Ref. |
|---|---|---|---|---|---|---|
| In vivo | Wistar rats | blood, liver cells | nucleotide-supplemented AIN-93G rodent diet | ALD, ALI | ↓ serum levels of AST and ALT, ↓ plasma LPS and inflammatory cytokine levels, ↓ levels of TLR4 and CD14, ↓ phosphorylation of IκBα and NF-κB p65 in the liver | [235] |
| In vivo | SD rats | liver cells | EtOH-induced hepatic miRNA expression before/after partial hepatectomy | ALD, ALI | hepatic miRNAs expression pattern changes in chronic drinking rather than acute binging, after hepatectomy the miRNA expression changed in chronically alcohol-exposed liver | [223] |
| In vivo | ASH mice | blood, liver cells, HCs | miRNA (barcodes) in extracellular vesicles (EV) measurement | ALD, ASH, ALI | ↑ expressed blood EV in early ASH, 9 ↑ and 4 ↓ miRNAs expression, 121 potential target genes incl. NF-κB, EGF, Wnt, and bcl2; let7f, miR-29a, and miR-340 were expressed in EVs from ASH mice | [236] |
| Human studies, in vitro | human | blood, liver cells | STS expression levels analysis | ALD, ASH, cirrhosis | ↑ levels of circulating estrogens in patients’ serum, activation of NF-κB leads to STS expression | [231] |
| In vivo, in vitro | C57BL/6J female mice | blood, liver cells | Female mice beer (stout and pilsner) feeding | ALD, acute beer consumption, LPS stimulation | mRNA expression of SREBP1c stays the same between groups, ↑ levels of expression of MyD88, iNOS, 4-HNE adducts, NF-κB and PAI-1 in EtOH groups, not in the beer groups | [219] |
| Human studies, in vitro | human | blood, liver cells, Huh-7 | FGF19, FGF21 and β-Klotho levels evaluation | ALD, ALI, ASH | in human samples ↑ expression levels of IL-1β, IL-6 and TNFα, ↓ levels of β-Klotho, in cell cultures ↑ levels of FGF21 and ↓ levels of β-Klotho levels | [232] |
| Human studies, in vitro | human | blood, liver cells, HCs | qRT-PCR in early ALD in human patients, in vitro TLR7-IFN pathway stimulation | ALD, ALI | ↑ levels of IL-1β, TNFα and NF-κB in early ALD, ↓ levels of IL-6/STAT3 and cyclin D lead to ↓ proliferation and HCs apoptosis, ↑ activation of TLR7–IFN axis in HCs | [233] |
| In vitro | rat | HSCs (HSC-T6) | HSCs stimulation by E. coli RNA | ALD, ALI, fibrosis | ↑ levels of IL-1β and TGF-β1 secretion by HSC-T6 after E. coli RNA stimulation, as well as ↑ expression of caspase-1, while ↓ procaspase-1, (TGF-β1) overproduction in HSC-T6, E. coli RNA can stimulate NLRP3 inflammasome activation | [225] |
| In vivo, in vitro | C57BL/6 mice | liver cells, KCs | The role of TERT in macrophage activation in ALD | ALD, ALI, LPS stimulation | ↑ levels of TERT expression and TA in vivo, ↑ levels of TERT in vitro, an NF-κB inhibitor PDTC ↓ levels of TERT in macrophage polarization | [227] |
| In vivo, in vitro | mice | liver cells | inhibition of SYK to evaluate its role in inflammation | ALD, ALI, steatosis | EtOH ↑ levels of SYK in HCs and mononuclear cells, inhibition of SYK ↓ levels of neutrophil infiltration, immune cell activation and kinase 1- and kinase 2-mediated NF-κB activity | [229] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.J.; Relja, B. The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. Int. J. Mol. Sci. 2020, 21, 9407. https://doi.org/10.3390/ijms21249407
Nowak AJ, Relja B. The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. International Journal of Molecular Sciences. 2020; 21(24):9407. https://doi.org/10.3390/ijms21249407
Chicago/Turabian StyleNowak, Aleksander J., and Borna Relja. 2020. "The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease" International Journal of Molecular Sciences 21, no. 24: 9407. https://doi.org/10.3390/ijms21249407
APA StyleNowak, A. J., & Relja, B. (2020). The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. International Journal of Molecular Sciences, 21(24), 9407. https://doi.org/10.3390/ijms21249407






