Exercise as an Adjuvant to Cartilage Regeneration Therapy
Abstract
:1. Introduction
2. Materials and Methods
3. Definition of Exercise
4. Pathophysiology of Osteoarthritis
5. Exercise and Osteoarthritis
6. Exercise and Mesenchymal Stem Cells
6.1. Exercise Studies in Rodents
6.2. Exercise Studies in Humans
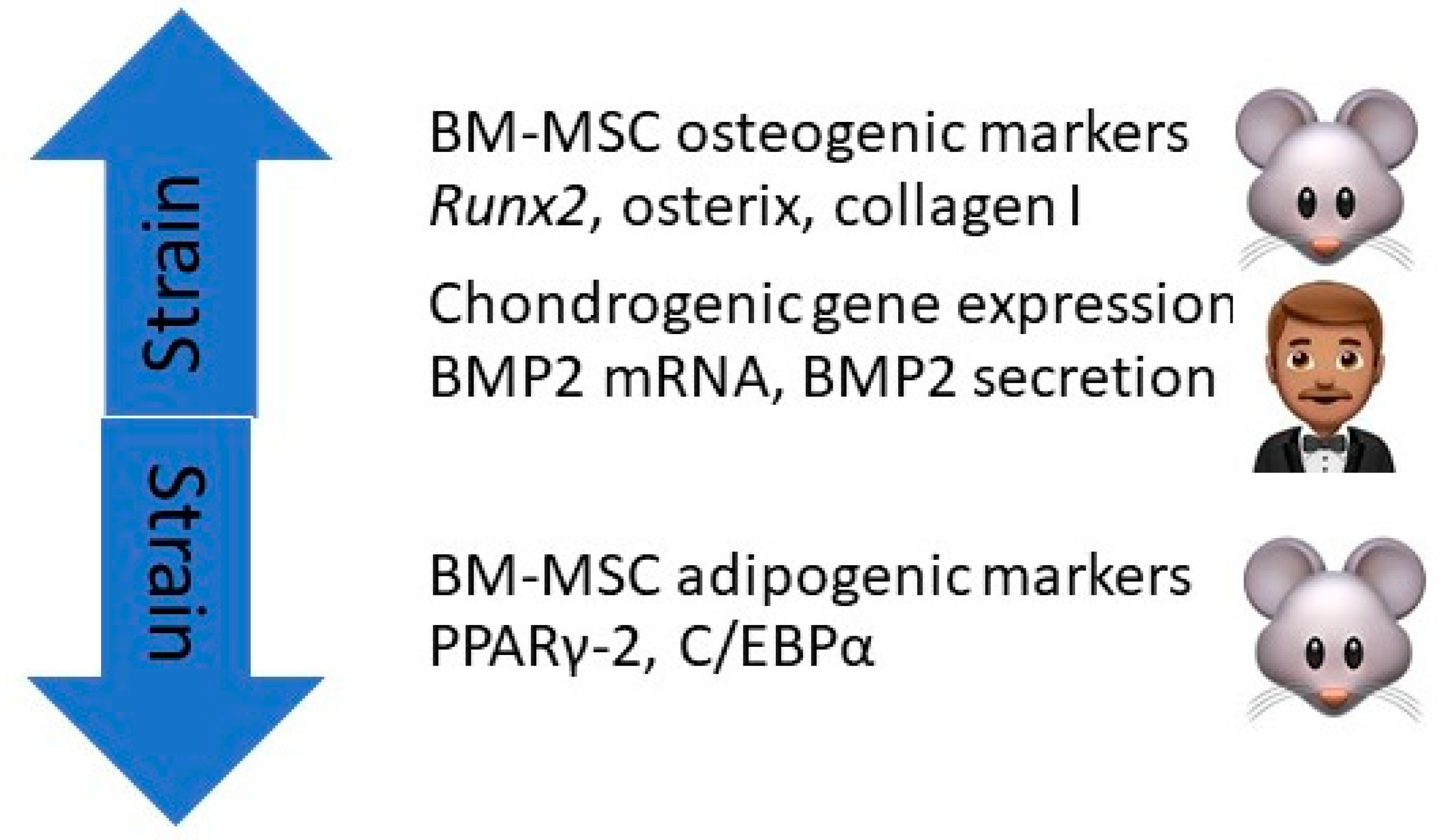
7. Mechanical Strain and Mesenchymal Stem Cells
7.1. In Rodents
7.2. In Humans
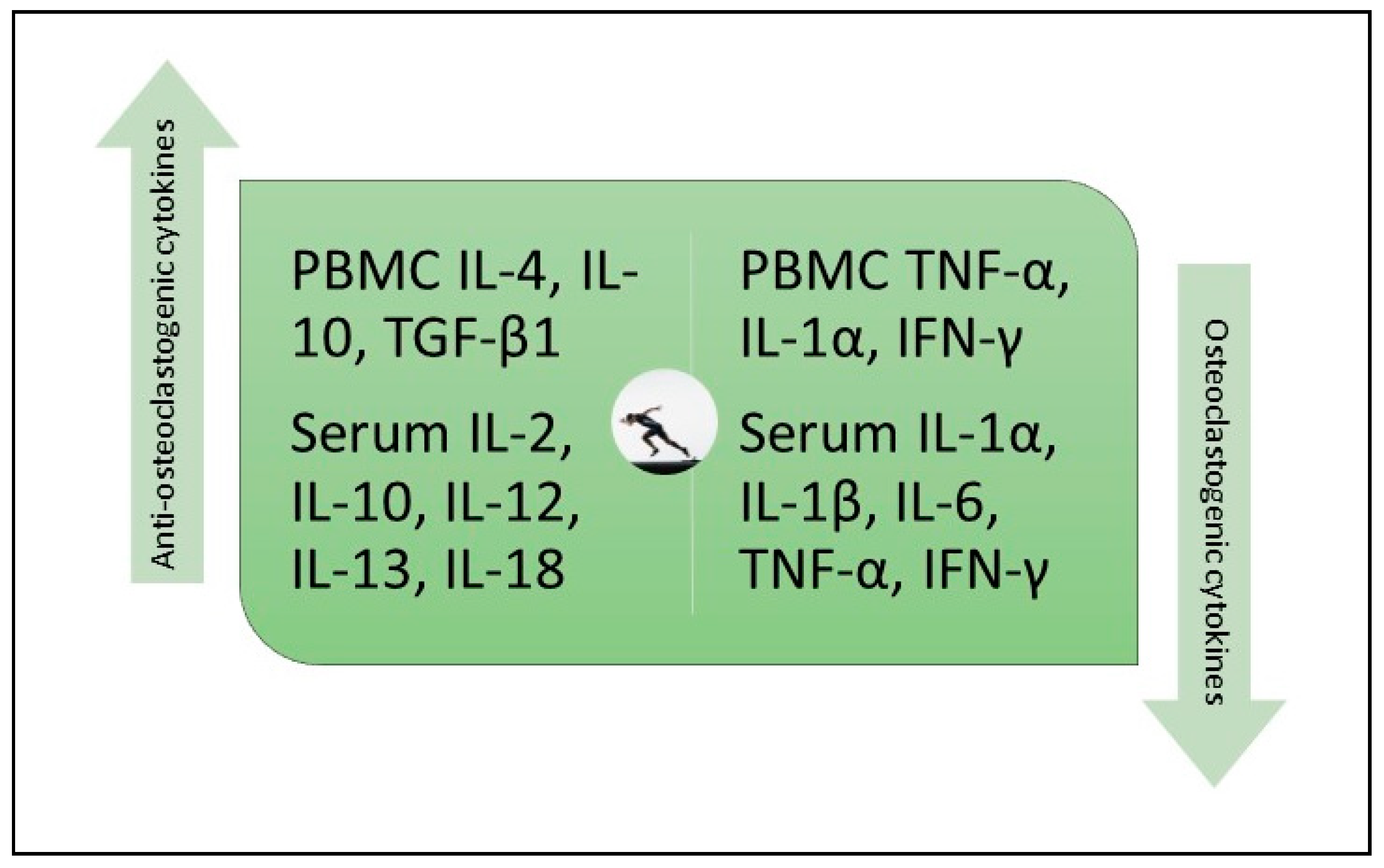
8. Exercise and Osteoclastogenic and Antiosteoclastogenic Cytokines
9. Scaffolds
10. Rehabilitation Protocols
10.1. For Osteoarthritis
10.2. For Post-Transplant Rehabilitation
11. Pre-Implantation Exercises
12. Discussion
13. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise early and often: Effects of physical activity and exercise on women’s bone health. Int. J. Environ. Res. Public Health 2018, 15, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Wellsandt, E.; Golightly, Y. Exercise in the management of knee and hip osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 151–159. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee: A Cochrane systematic review. Br. J. Sports Med. 2015, 49, 1554–1557. [Google Scholar] [CrossRef]
- Cavanaugh, A.R.; Schwartz, G.J.; Blouet, C. Global Health Estimates (2015). Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Freitag, J.; Bates, D.; Boyd, R.; Shah, R.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. Musculoskelet. Disord. 2016, 17, 230. [Google Scholar] [CrossRef] [Green Version]
- Shariatzadeh, M.; Song, J.; Wilson, S.L. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019, 378, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-S.; Hong, K.-T.; Kim, N.-M.; Jung, J.-Y.; Park, H.-S.; Chun, Y.S.; Kim, S.J. Cartilage regeneration in osteoarthritic knees treated with distal femoral osteotomy and intra-lesional implantation of allogenic umbilical cord blood-derived mesenchymal stem cells: A report of two cases. Knee 2019, 26, 1445–1450. [Google Scholar] [CrossRef]
- Richardson, S.M.; Gauthaman, K.; Pushparaj, P.N.; Matta, C.; Richardson, S.M.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Memic, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and interverbal disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Häfner, S.J. The body’s integrated repair kit: Studying mesenchymal stem cells for better ligament repair. Biomed. J. 2019, 42, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Ben, P.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Mitxitorena, I.; Infante, A.; Gener, B.; Rodriguez, C.I. Suitability and limitations of mesenchymal stem cells to elucidate human bone illness. World J. Stem Cells 2019, 11, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.; Minkus, M.; Scheibel, M. Autologous chondrocyte implantation for treatment of focal articular cartilage defects of the humeral head. J. Shoulder Elb. Surg. 2020, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Minas, T.; Ogura, T.; Bryant, T. Autologous chondrocyte implantation. JBJS Essent. Surg. Tech. 2016, 6, e24. [Google Scholar] [CrossRef] [PubMed]
- Marlovits, S.; Striessnig, G.; Kutscha-Lissberg, F.; Resinger, C.; Aldrian, S.M.; Vécsei, V.; Trattnig, S. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg. Sports Traumatol. Arthrosc. 2005, 13, 451–457. [Google Scholar] [CrossRef]
- Ebert, J.R.; Fallon, M.; Ackland, T.R.; Wood, D.J.; Janes, G.C. Arthroscopic matrix-induced autologous chondrocyte implantation: 2-year outcomes. Arthroscopy 2012, 28, 952–964. [Google Scholar] [CrossRef]
- Ebert, J.R.; Fallon, M.; Wood, D.J.; Janes, G.C. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am. J. Sports Med. 2017, 45, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-based treatment strategies for osteoporosis: A literature review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Bernotiene, E.; Bagdonas, E.; Kirdaite, G.; Bernotas, P.; Kalvaityte, U.; Uzieliene, I.; Thudium, C.S.; Hannula, H.; Lorite, G.S.; Dvir-Ginzberg, M.; et al. Emerging technologies and platforms for the immunodetection of multiple biochemical markers in osteoarthritis research and therapy. Front. Med. 2020, 7, 572977. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-Z.; Xie, H.-Q.; Silini, A.; Parolini, O.; Zhang, Y.; Deng, L.; Huang, Y.-C. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: Current status and future prospects. Stem Cell Rev. Rep. 2017, 13, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteo Cart 2009, 17, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar]
- McNulty, A.L.; Guilak, F. Mechanobiology of the meniscus. J. Biomech. 2015, 48, 1469–1478. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, B.M.; Lu, Q.; Rodova, M.; Woodbury, B.G.; Yost, J.G.; Roby, K.F.; Pinson, D.M.; Tawfik, O.; Anderson, H.C. Transcription factor NFAT1 deficiency causes osteoarthritis through dysfunction of adult articular chondrocytes. J. Pathol. 2009, 219, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Rodova, M.; Lu, Q.; Li, Y.; Woodbury, B.G.; Crist, J.D.; Gardner, B.M.; Yost, J.G.; Zhong, X.-B.; Anderson, H.C.; Wang, J. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J. Bone Miner. Res. 2011, 26, 1974–1986. [Google Scholar] [CrossRef] [Green Version]
- Roos, E.M.; Dahlberg, L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage. Arthr. Rheum. 2005, 52, 3507–3514. [Google Scholar] [CrossRef] [Green Version]
- Munukka, M.; Waller, B.; Häkkinen, A.; Nieminen, M.T.; Lammentausta, E.; Kujala, U.M.; Paloneva, J.; Kautiainen, H.; Kiviranta, I.; Heinonen, A. Physical activity is related to cartilage quality in women with knee osteoarthritis. Med. Sci. Sports Exerc. 2017, 49, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016, 24, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assis, L.; Milares, L.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.; Medalha, C.; Renno, A.M. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cart. 2016, 24, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (TSCT) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar]
- Liu, S.-Y.; He, Y.-B.; Deng, S.-Y.; Zhu, W.-T.; Xu, S.-Y.; Ni, G.-X. Exercise affects biological characteristics of mesenchymal stromal cells derived from bone marrow and adipose tissue. Int. Orthop. 2017, 41, 1199–1209. [Google Scholar] [CrossRef]
- Emmons, R.; Niemiro, G.M.; Owolabi, O.; De Lisio, M. Acute exercise mobilizes hematopoietic stem and progenitor cells and alters the mesenchymal stromal cell secretome. J. Appl. Physiol. 2016, 120, 624–632. [Google Scholar] [CrossRef]
- Bourzac, C.; Bensidhoum, M.; Pallu, S.; Portier, H. Use of adult mesenchymal stromal cells in tissue repair: Impact of physical exercise. Am. J. Physiol. Cell Physiol. 2019, 317, C642–C654. [Google Scholar] [CrossRef]
- Ocarino, N.M.; Boeloni, J.N.; Goes, A.M.; Silva, J.F.; Marubayashi, U.; Serakides, R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide synthase inhibition. Nitric Oxide 2008, 19, 320–325. [Google Scholar] [CrossRef]
- Hell, R.C.R.; Ocarino, N.M.; Boeloni, J.N.; Silva, J.F.; Goes, A.M.; Santos, R.L.; Serakides, R. Physical activity improves age-related decline in the osteogenic potential of rats’ bone marrow-derived mesenchymal stem cells. Acta Physiol. 2012, 205, 292–301. [Google Scholar] [CrossRef]
- Wallace, I.J.; Pagnotti, G.M.; Rubin-Sigler, J.; Naeher, M.; Copes, L.E.; Judex, S.; Rubin, C.T.; Demes, B. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak exertional forces. J. Exp. Biol. 2015, 218 Pt 19, 3002–3009. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S.; Aoyama, T.; Ito, A.; Nagai, M.; Iijima, H.; Tajino, J.; Zhang, X.; Kiyan, W.; Kuroki, H. The effect of exercise on the early stages of mesenchymal stromal cell-induced cartilage repair in a rat osteochondral defect model. PLoS ONE 2016, 11, e0151580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, A.; Bierwirth, S.; Weber, S.; Platen, P.; Schinköthe, T.; Bloch, W. Short intensive exercise increases the migratory activity of mesenchymal stem cells. Br. J. Sports Med. 2009, 43, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Carbonare, L.D.; Mottes, M.; Cheri, S.; Deiana, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Salvagno, G.L.; Lippi, G.; Valenti, M.T. Increased gene expression of RUNX2 and SOX9 in mesenchymal circulating progenitors is associated with autophagy during physical activity. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Carbonare, L.D.; Mottes, M. Physical exercise modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p expression in progenitor cells promoting osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemiro, G.M.; Parel, J.; Beals, J.; Van Vliet, S.; Paluska, S.A.; Moore, D.R.; Burd, N.A.; De Lisio, M. Kinetics of circulating progenitor cell mobilization during submaximal exercise. J. Appl. Physiol. 2017, 122, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Runguang, L.; Liang, L.; Dou, Y.; Huang, Z.; Mo, H.; Wang, Y.; Yu, B. Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow stem cells. BioMed Res. Int. 2015, 2015, 873251. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, F.; Wu, Y.; Goh, G.S.H.; Ge, Z.; Tan, L.P.; Hui, J.H.P.; Yang, Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials 2015, 38, 72–85. [Google Scholar] [CrossRef]
- Sumanasinghe, R.D.; Bernacki, S.H.; Loboa, E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tension strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006, 12, 3459–3465. [Google Scholar] [CrossRef]
- Smith, J.K.; Dykes, R.; Chi, D.S. The effect of long-term exercise on the production of osteoclastogenic and antiosteoclastogenic cytokines by peripheral blood mononuclear cells and on serum markers of bone metabolism. J. Osteoporos. 2016, 5925380. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.V.T.; Viana, V.A.R.; Boscolo, R.A.; Marques, V.G.; Santana, M.G.; Lira, F.S.; Tufik, S.; de Mello, M.T. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine 2012, 60, 731–735. [Google Scholar] [CrossRef] [Green Version]
- El-Kader, S.M.A.; Al-Shreef, F.M.; Al-Jiffri, O.H. Impact of aerobic exercise versus resisted exercise on endothelial activation markers and inflammatory cytokines among elderly. Afr. Health Sci. 2019, 19, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- El-Kader, S.M.A.; Al-Jiffri, O.H. Aerobic exercise modulates cytokine profile and sleep quality in elderly. Afr. Health Sci. 2019, 19, 2198–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Chen, X.; Zhang, L.; Wu, J.; Guo, J.; Zou, D.; Chen, B.; Sun, Z.; Shen, C.; Zou, J. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog. Biophys. Mol. Biol. 2016, 122, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Helmark, I.C.; Mikkelsen, U.R.; Børglum, J.; Rothe, A.; Petersen, M.C.H.; Andersen, O.; Langberg, H.; Kjaer, M. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: A randomized controlled trial. Arthritis Res. Ther. 2010, 12, R126. Available online: http://arthritis-research.com/content/12/4/R126 (accessed on 5 June 2020). [CrossRef] [Green Version]
- Madry, H.; Venkatesan, J.K.; Carballo-Pedrares, N.; Rey-Rico, A.; Cucchiarini, M. Scaffold-mediated gene delivery for osteochondral repair. Pharmaceutics 2020, 12, 930. [Google Scholar] [CrossRef]
- Rice, D.; McNair, P.; Huysmans, E.; Letzen, J.; Finan, P. Best evidence rehabilitation for chronic pain Part 5:osteoarthritis. J. Clin. Med. 2019, 8, 1769. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.K.; Ackland, T.; Ebert, J.R. Clinical rehabilitation guidelines for matrix-induced autologous chondrocyte implantation on the tibiofemoral joint. J. Orthop. Sports Phys. Ther. 2014, 44, 102–119. [Google Scholar] [CrossRef] [Green Version]
- Boppart, M.D.; De Lisio, M.; Witkowski, S. Exercise and stem cells. Prog. Mol. Biol. Transl. Sci. 2015, 135, 423–456. [Google Scholar]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Brittberg, M.; Nilsson, A.; Lindahl, A.; Ohlsson, C.; Peterson, L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin. Orthop. Relat. Res. 1996, 362, 270–283. [Google Scholar] [CrossRef]
- Chiang, H.; Kuo, T.-F.; Tsai, C.-C.; Lin, M.-C.; She, B.-R.; Huang, Y.-Y.; Lee, H.-S.; Shieh, C.-S.; Chen, M.-H.; Ramshaw, J.A.; et al. Repair of porcine articular cartilage defect with autologous chondrocyte transplantation. J. Orthop. Res. 2005, 23, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Rahfoth, B.; Weisser, J.; Sternkopf, F.; Aigner, T.; von der Mark, K.; Bräuer, R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthr. Cartil. 1998, 6, 50–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, L.; Minas, T.; Brittberg, M.; Nilsson, A.; Sjögren-Jansson, E.; Lindahl, A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 2000, 374, 212–234. [Google Scholar] [CrossRef] [PubMed]
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesized as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy 2003, 19, 477–484. [Google Scholar] [CrossRef]
- Smith, J.K. Exercise, obesity and CNS regulation of metabolic homeostasis: A review. Front. Physiol. 2018, 9, 574. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.K. Exercise as an Adjuvant to Cartilage Regeneration Therapy. Int. J. Mol. Sci. 2020, 21, 9471. https://doi.org/10.3390/ijms21249471
Smith JK. Exercise as an Adjuvant to Cartilage Regeneration Therapy. International Journal of Molecular Sciences. 2020; 21(24):9471. https://doi.org/10.3390/ijms21249471
Chicago/Turabian StyleSmith, John Kelly. 2020. "Exercise as an Adjuvant to Cartilage Regeneration Therapy" International Journal of Molecular Sciences 21, no. 24: 9471. https://doi.org/10.3390/ijms21249471
APA StyleSmith, J. K. (2020). Exercise as an Adjuvant to Cartilage Regeneration Therapy. International Journal of Molecular Sciences, 21(24), 9471. https://doi.org/10.3390/ijms21249471



