Prognostic Significance of COX-2 Overexpression in BRAF-Mutated Middle Eastern Papillary Thyroid Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
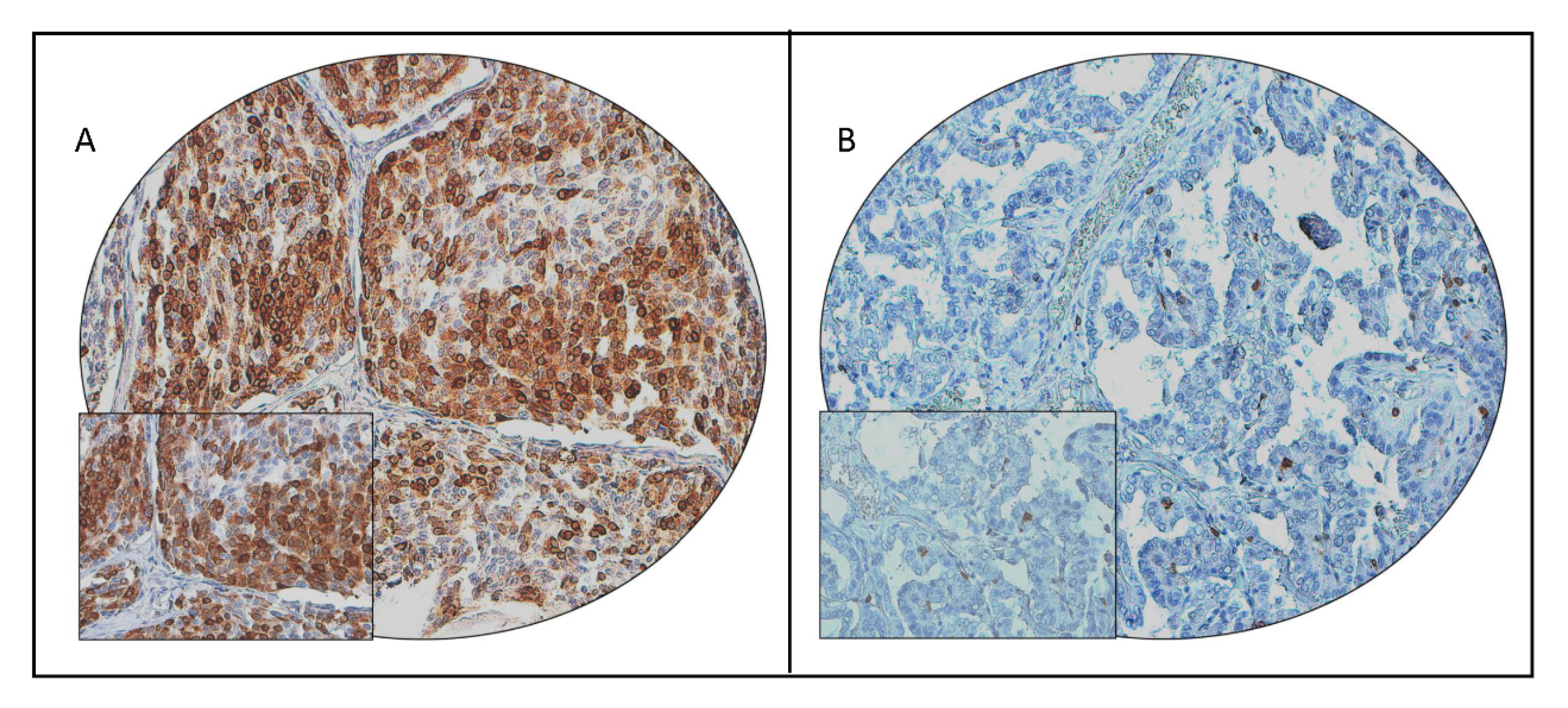
2.2. Clinicopathological Associations of COX-2 Expression in PTC
2.3. COX-2 Expression and BRAF Mutation in PTC
3. Discussion
4. Materials and Methods
4.1. Sample Selection
4.2. DNA Isolation
4.3. Sanger Sequencing Analysis
4.4. Tissue Microarray (TMA) Construction and Immunohistochemistry (IHC) Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandin E2 |
| PTC | Papillary thyroid carcinoma |
| DFS | Disease-free survival |
| FFPE | Formalin-fixed and paraffin-embedded |
| PCR | Polymerase chain reaction |
| TMA | Tissue microarray |
| IHC | Immunohistochemistry |
| SD | Standard deviation |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Vecchia, C.; Malvezzi, M.; Bosetti, C.; Garavello, W.; Bertuccio, P.; Levi, F.; Negri, E. Thyroid cancer mortality and incidence: A global overview. Int. J. Cancer 2015, 136, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Alrawaji, A.; Alshahrani, Z.; Alzahrani, W.; Alomran, F.; Almadouj, A.; Alshehri, S.; Alzahrani, A.; Bazarbashi, S.; Alhashmi, H.; Almutlaq, H.; et al. Cancer Incidence Report Saudi Arabia 2015. In Saudi Cancer Registry; Saudi Health Council: Riyadh, Saudi Arabia, 2018. [Google Scholar]
- Ritter, A.; Mizrachi, A.; Bachar, G.; Vainer, I.; Shimon, I.; Hirsch, D.; Diker-Cohen, T.; Duskin-Bitan, H.; Robenshtok, E. Detecting recurrence following lobectomy for thyroid cancer: Role of thyroglobulin and thyroglobulin antibodies. J. Clin. Endocrinol. Metab. 2020, 105, dgaa152. [Google Scholar] [CrossRef] [PubMed]
- Tumino, D.; Frasca, F.; Newbold, K. Updates on the management of advanced, metastatic, and radioiodine refractory differentiated thyroid cancer. Front. Endocrinol. 2017, 8, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015, 33, 42. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; DuBois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [Green Version]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Petkova, D.; Clelland, C.; Ronan, J.; Pang, L.; Coulson, J.; Lewis, S.; Knox, A. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 2004, 98, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, U.; Kumari, N.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Overexpression of COX2 indicates poor survival in urothelial bladder cancer. Ann. Diagn. Pathol. 2018, 34, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Kirkner, G.J.; Nosho, K.; Irahara, N.; Kure, S.; Shima, K.; Hazra, A.; Chan, A.T.; Dehari, R.; Giovannucci, E.L. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin. Cancer Res. 2008, 14, 8221–8227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Zhang, H.; Chen, Z.; Yang, Z.; Shi, D.; Liu, T.; Chen, W.; Yao, F.; Su, X.; Deng, W. TFAP2B overexpression contributes to tumor growth and progression of thyroid cancer through the COX-2 signaling pathway. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Teng, Y.; Zhang, R.; Luo, L. Antitumor effect of the selective COX-2 inhibitor celecoxib on endometrial adenocarcinoma in vitro and in vivo. Oncol. Lett. 2012, 4, 1219–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci. Transl. Med. 2014, 6, 242ra84. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Y.; Yang, J.; Zhang, Z.; Zhang, Y.; Du, H. Celecoxib induces apoptosis but up-regulates VEGF via endoplasmic reticulum stress in human colorectal cancer in vitro and in vivo. Cancer Chemother. Pharmacol. 2016, 77, 797–806. [Google Scholar] [CrossRef]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef] [Green Version]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk-Rusiecka, K.; Lewiński, A. Cyclooxygenase-2 expression and its association with thyroid lesions. Arch. Med. Sci. 2010, 6, 653. [Google Scholar] [CrossRef]
- Krawczyk-Rusiecka, K.; Wojciechowska-Durczynska, K.; Cyniak-Magierska, A.; Zygmunt, A.; Lewinski, A. Assessment of cyclooxygenase-1 and 2 gene expression levels in chronic autoimmune thyroiditis, papillary thyroid carcinoma and nontoxic nodular goitre. Thyroid Res. 2014, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynik, D.M.; Prayson, R.A. Immunohistochemical expression of cyclooxygenase 2 in follicular carcinomas of the thyroid. Arch. Pathol. Lab. Med. 2005, 129, 736–741. [Google Scholar] [PubMed]
- Perisa, M.M.; Sarcevic, B.; Troselj, K.G.; Grsic, K.; Sitic, S.; Seiwerth, S. Expression of nm23-H1 and COX-2 in thyroid papillary carcinoma and microcarcinoma. Oncol. Lett. 2017, 13, 3547–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B.; Liu, Y.; Zhang, P.; Wang, Y.; Wang, G. COX-2 expression and tumor angiogenesis in thyroid carcinoma patients among northeast Chinese population-result of a single-center study. Int. J. Med. Sci. 2012, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Wang, J.; Wang, H.; Jiang, X.; Liao, Q.; Gong, Q.; Mo, Y.; Li, X.; Li, G.; Xiong, W. The BRAF V600E mutation is a predictor of the effect of radioiodine therapy in papillary thyroid cancer. J. Cancer 2020, 11, 932. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Huang, M.; Li, X.; Wang, T.; Ling, R. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocr. Connect. 2019, 8, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Shen, F.; Deng, X.; Feng, J.; Lu, J.; Cai, W.; Peng, L.; Zheng, W.; Wang, W.; Huang, P. Prognostic implications of the BRAF-V600E mutation in papillary thyroid carcinoma based on a new cut-off age stratification. Oncol. Lett. 2020, 19, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Project, C.G.; Jones, C.M.; Marshall, C.J.; Springer, C.J. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Kosumi, K.; Hamada, T.; Zhang, S.; Liu, L.; da Silva, A.; Koh, H.; Twombly, T.S.; Mima, K.; Morikawa, T.; Song, M. Prognostic association of PTGS2 (COX-2) over-expression according to BRAF mutation status in colorectal cancer: Results from two prospective cohorts and CALGB 89803 (Alliance) trial. Eur. J. Cancer 2019, 111, 82–93. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, Q.; Li, Q.; Lin, S.; Li, J. Immunohistochemical levels of cyclo-oxygenase-2, matrix metalloproteinase-9 and vascular endothelial growth factor in papillary thyroid carcinoma and their clinicopathological correlations. J. Int. Med. Res. 2014, 42, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Erdem, H.; Gündogdu, C.; Şipal, S. Correlation of E-cadherin, VEGF, COX-2 expression to prognostic parameters in papillary thyroid carcinoma. Exp. Mol. Pathol. 2011, 90, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Alexandrou, P.; Delladetsima, I.; Karavokyros, I.; Danas, E.; Giagini, A.; Patsouris, E.; Theocharis, S. Clinical significance of Hu-antigen receptor (HuR) and cyclooxygenase-2 (COX-2) expression in human malignant and benign thyroid lesions. Pathol. Oncol. Res. 2016, 22, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Marcello, M.A.; Nonogaki, S.; Morari, E.C.; Soares, F.A.; Vassallo, J.; Ward, L.S. CD 8+ tumour-infiltrating lymphocytes and COX2 expression may predict relapse in differentiated thyroid cancer. Clin. Endocrinol. 2015, 83, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Si, Y.; Lee, Y.S.; Kim, J.S.; Jeon, H.M.; Park, W.C. The Clinical Significance of Expression of Cyclooxygenase-2 in the Prognosis of the Papillary Thyroid Cancer. Korean J. Endocr. Surg. 2007, 7, 75–79. [Google Scholar] [CrossRef]
- Wang, D.; Fu, L.; Sun, H.; Guo, L.; DuBois, R.N. Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology 2015, 149, 1884–1895.e4. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, H.; Hu, M.; Zhang, Y.; Chen, S.; Tighe, S.; Zhu, Y. The role of cyclooxygenase-2 in colorectal carcinogenesis. Clin. Colorectal Cancer 2017, 16, 165–172. [Google Scholar] [CrossRef]
- Xie, C.; Xu, X.; Wang, X.; Wei, S.; Shao, L.; Chen, J.; Cai, J.; Jia, L. Cyclooxygenase-2 induces angiogenesis in pancreatic cancer mediated by prostaglandin E2. Oncol. Lett. 2018, 16, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Sakai, Y. Prostaglandin E2/EP Signaling in the Tumor Microenvironment of Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 6254. [Google Scholar] [CrossRef] [Green Version]
- Zelenay, S.; Van Der Veen, A.G.; Böttcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef] [Green Version]
- Muller, A.J.; Scherle, P.A. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat. Rev. Cancer 2006, 6, 613–625. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of cancer-promoting inflammation: A new approach for anticancer therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Göbel, C.; Breitenbuecher, F.; Kalkavan, H.; Hähnel, P.; Kasper, S.; Hoffarth, S.; Merches, K.; Schild, H.; Lang, K.; Schuler, M. Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity. Cell Death Dis. 2014, 5, e1568. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.A.; Drake, V.; Huang, H.-S.; Chiu, S.; Zheng, L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology 2015, 4, e1016700. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Matsumura, N.; Mandai, M.; Li, K.; Yagi, H.; Baba, T.; Suzuki, A.; Hamanishi, J.; Fukuhara, K.; Konishi, I. Classification using hierarchical clustering of tumor-infiltrating immune cells identifies poor prognostic ovarian cancers with high levels of COX expression. Mod. Pathol. 2009, 22, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Yang, Y.; Xia, Y.; Okugawa, Y.; Yang, J.; Liang, Y.; Chen, H.; Zhang, P.; Wang, F.; Han, H. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut 2016, 65, 1470–1481. [Google Scholar] [CrossRef]
- Abubaker, J.; Jehan, Z.; Bavi, P.; Sultana, M.; Al-Harbi, S.; Ibrahim, M.; Al-Nuaim, A.; Ahmed, M.; Amin, T.; Al-Fehaily, M. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J. Clin. Endocrinol. Metab. 2008, 93, 611–618. [Google Scholar] [CrossRef] [Green Version]
- Bu, R.; Siraj, A.K.; Al-Obaisi, K.A.; Beg, S.; Al Hazmi, M.; Ajarim, D.; Tulbah, A.; Al-Dayel, F.; Al-Kuraya, K.S. Identification of novel BRCA founder mutations in Middle Eastern breast cancer patients using capture and Sanger sequencing analysis. Int. J. Cancer 2016, 139, 1091–1097. [Google Scholar] [CrossRef] [Green Version]
- Siraj, A.; Bavi, P.; Abubaker, J.; Jehan, Z.; Sultana, M.; Al-Dayel, F.; Al-Nuaim, A.; Alzahrani, A.; Ahmed, M.; Al-Sanea, O. Genome-wide expression analysis of Middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J. Pathol. 2007, 213, 190–199. [Google Scholar] [CrossRef]
- Bavi, P.; Jehan, Z.; Atizado, V.; Al-Dossari, H.; Al-Dayel, F.; Tulbah, A.; Amr, S.S.; Sheikh, S.S.; Ezzat, A.; El-Solh, H. Prevalence of fragile histidine triad expression in tumors from Saudi Arabia: A tissue microarray analysis. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1708–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Hussain, A.R.; Siraj, A.K.; Uddin, S.; Al-Sanea, N.; Al-Dayel, F.; Al-Assiri, M.; Beg, S.; Al-Kuraya, K.S. Co-targeting of Cyclooxygenase-2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Total | BRAF Wild-Type | BRAF Mutant | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Total | 1335 | 584 | 43.7 | 751 | 56.3 | |
| Age at surgery (years) | ||||||
| Mean ± SD | 40.4 ± 16.1 | 36.6 ± 15.5 | 43.3 ± 15.9 | |||
| <55 | 1087 | 81.4 | 509 | 87.2 | 578 | 77.0 |
| ≥55 | 248 | 18.6 | 75 | 12.8 | 173 | 23.0 |
| Sex | ||||||
| Male | 330 | 24.7 | 138 | 23.6 | 192 | 25.6 |
| Female | 1005 | 75.3 | 446 | 76.4 | 559 | 74.4 |
| Histologic subtype | ||||||
| Classical variant | 895 | 67.1 | 314 | 53.7 | 581 | 77.4 |
| Follicular variant | 232 | 17.4 | 185 | 31.7 | 47 | 6.3 |
| Tall cell variant | 118 | 8.8 | 21 | 3.6 | 97 | 12.9 |
| Other variants | 90 | 6.7 | 64 | 11.0 | 26 | 3.5 |
| Extrathyroidal extension | ||||||
| Present | 590 | 44.2 | 178 | 30.5 | 412 | 54.9 |
| Absent | 745 | 55.8 | 406 | 69.5 | 339 | 45.1 |
| Multifocality | ||||||
| Yes | 670 | 50.2 | 259 | 44.4 | 411 | 54.7 |
| No | 665 | 49.8 | 325 | 55.6 | 340 | 45.3 |
| pT | ||||||
| T1 | 369 | 28.5 | 176 | 31.3 | 193 | 26.4 |
| T2 | 266 | 20.6 | 138 | 24.6 | 128 | 17.5 |
| T3 | 549 | 42.4 | 212 | 37.7 | 337 | 46.0 |
| T4 | 110 | 8.5 | 36 | 6.4 | 74 | 10.1 |
| pN | ||||||
| N0 | 527 | 39.5 | 280 | 48.0 | 247 | 32.9 |
| N1 | 683 | 51.1 | 245 | 41.9 | 438 | 58.3 |
| Nx | 125 | 9.4 | 59 | 10.1 | 66 | 8.8 |
| pM | ||||||
| M0 | 1205 | 95.4 | 518 | 93.7 | 687 | 96.8 |
| M1 | 58 | 4.6 | 35 | 6.3 | 23 | 3.2 |
| TNM Stage | ||||||
| I | 1084 | 83.4 | 499 | 87.5 | 585 | 80.1 |
| II | 148 | 11.4 | 51 | 8.9 | 97 | 13.3 |
| III | 21 | 1.6 | 4 | 0.7 | 17 | 2.3 |
| IV-A | 17 | 1.3 | 2 | 0.4 | 15 | 2.1 |
| IV-B | 30 | 2.3 | 14 | 2.5 | 16 | 2.2 |
| NRAS mutation | ||||||
| Present | 85 | 6.4 | 83 | 14.3 | 2 | 0.3 |
| Absent | 1244 | 93.6 | 498 | 85.7 | 746 | 99.7 |
| HRAS mutation | ||||||
| Present | 30 | 2.3 | 28 | 4.2 | 2 | 0.3 |
| Absent | 1298 | 97.7 | 553 | 95.2 | 745 | 99.7 |
| Total | Cox-2 Positive | Cox-2 Negative | p Value | ||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total | 1314 | 567 | 43.2 | 747 | 56.8 | ||
| Age at surgery (years) | |||||||
| <55 | 1072 | 81.6 | 418 | 39.0 | 654 | 61.0 | <0.0001 |
| ≥55 | 242 | 18.4 | 149 | 61.6 | 93 | 38.4 | |
| Sex | |||||||
| Male | 320 | 24.4 | 146 | 45.6 | 174 | 54.4 | 0.3049 |
| Female | 994 | 75.6 | 421 | 42.4 | 174 | 57.6 | |
| Histologic subtype | |||||||
| Classical variant | 879 | 66.9 | 394 | 44.8 | 485 | 55.2 | 0.1542 |
| Follicular variant | 229 | 17.4 | 84 | 36.7 | 145 | 63.3 | |
| Tall cell variant | 116 | 8.8 | 52 | 44.8 | 64 | 55.2 | |
| Other variants | 90 | 6.9 | 37 | 41.1 | 53 | 58.9 | |
| Extrathyroidal extension | |||||||
| Present | 579 | 44.1 | 291 | 50.3 | 288 | 49.7 | <0.0001 |
| Absent | 735 | 55.9 | 276 | 37.6 | 459 | 62.4 | |
| Multifocality | |||||||
| Yes | 658 | 50.1 | 295 | 44.8 | 363 | 55.2 | 0.2175 |
| No | 656 | 49.9 | 272 | 41.5 | 384 | 58.5 | |
| pT | |||||||
| T1 | 365 | 28.7 | 140 | 38.4 | 225 | 61.6 | 0.0094 |
| T2 | 265 | 20.8 | 101 | 38.1 | 164 | 61.9 | |
| T3 | 538 | 42.2 | 253 | 47.0 | 285 | 53.0 | |
| T4 | 106 | 8.3 | 53 | 50.0 | 53 | 50.0 | |
| pN | |||||||
| N0 | 516 | 43.3 | 194 | 37.6 | 322 | 62.4 | 0.0003 |
| N1 | 675 | 56.7 | 325 | 48.2 | 350 | 51.8 | |
| pM | |||||||
| M0 | 1186 | 95.4 | 509 | 42.9 | 677 | 57.1 | 0.3575 |
| M1 | 57 | 4.6 | 28 | 49.1 | 29 | 50.9 | |
| TNM Stage | |||||||
| I | 1069 | 83.4 | 415 | 38.8 | 654 | 61.2 | <0.0001 |
| II | 146 | 11.4 | 86 | 58.9 | 60 | 41.1 | |
| III | 20 | 1.6 | 17 | 85.0 | 3 | 15.0 | |
| IV-A | 17 | 1.3 | 10 | 58.8 | 7 | 41.2 | |
| IV-B | 29 | 2.3 | 21 | 72.4 | 8 | 27.6 | |
| BRAF mutation | |||||||
| Present | 740 | 56.3 | 344 | 46.5 | 396 | 53.5 | 0.0055 |
| Absent | 574 | 43.7 | 223 | 38.9 | 351 | 61.1 | |
| 5-year disease-free survival | 69.5 | 81.7 | <0.0001 | ||||
| Clinical Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age | ||||
| ≥55 years (vs. <55 years) | 2.82 (2.21–3.57) | <0.0001 | 2.04 (1.51–2.76) | <0.0001 |
| Sex | ||||
| Male (vs. Female) | 0.59 (0.47–0.74) | <0.0001 | 0.66 (0.51–0.85) | 0.0016 |
| Extrathyroidal extension | ||||
| Present (vs. Absent) | 2.89 (2.24–3.77) | <0.0001 | 2.26 (1.72–2.99) | <0.0001 |
| pM | ||||
| M1 (vs. M0) | 4.52 (3.12–6.34) | <0.0001 | 2.84 (1.74–4.63) | <0.0001 |
| Stage | ||||
| IV (vs. I–III) | 4.40 (2.84–6.52) | <0.0001 | 0.83 (0.45–1.54) | 0.5560 |
| COX-2 IHC | ||||
| Overexpression (vs. low expression) | 1.66 (1.32–2.08) | <0.0001 | 1.57 (1.22–2.03) | 0.0004 |
| Clinical Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) | p-Value | Risk Ratio (95% CI) | p-Value | |
| Age | ||||
| ≥55 years (vs. <55 years) | 2.45 (1.84–3.25) | <0.0001 | 1.75 (1.22–2.48) | 0.0020 |
| Sex | ||||
| Male (vs. Female) | 0.55 (0.42–0.74) | <0.0001 | 0.52 (0.39–0.72) | <0.0001 |
| Extrathyroidal extension | ||||
| Present (vs. Absent) | 2.77 (1.96–4.01) | <0.0001 | 2.11 (1.46–3.10) | <0.0001 |
| pM | ||||
| M1 (vs. M0) | 4.69 (2.64–7.72) | <0.0001 | 4.44 (1.61–11.71) | 0.0036 |
| Stage | ||||
| IV (vs. I–III) | 3.15 (1.77–5.16) | <0.0001 | 0.49 (0.17–1.25) | 0.1656 |
| COX-2 IHC | ||||
| Overexpression (vs. low expression) | 2.10 (1.59–2.79) | <0.0001 | 2.10 (1.52–2.92) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvathareddy, S.K.; Siraj, A.K.; Annaiyappanaidu, P.; Al-Sobhi, S.S.; Al-Dayel, F.; Al-Kuraya, K.S. Prognostic Significance of COX-2 Overexpression in BRAF-Mutated Middle Eastern Papillary Thyroid Carcinoma. Int. J. Mol. Sci. 2020, 21, 9498. https://doi.org/10.3390/ijms21249498
Parvathareddy SK, Siraj AK, Annaiyappanaidu P, Al-Sobhi SS, Al-Dayel F, Al-Kuraya KS. Prognostic Significance of COX-2 Overexpression in BRAF-Mutated Middle Eastern Papillary Thyroid Carcinoma. International Journal of Molecular Sciences. 2020; 21(24):9498. https://doi.org/10.3390/ijms21249498
Chicago/Turabian StyleParvathareddy, Sandeep Kumar, Abdul K. Siraj, Padmanaban Annaiyappanaidu, Saif S. Al-Sobhi, Fouad Al-Dayel, and Khawla S. Al-Kuraya. 2020. "Prognostic Significance of COX-2 Overexpression in BRAF-Mutated Middle Eastern Papillary Thyroid Carcinoma" International Journal of Molecular Sciences 21, no. 24: 9498. https://doi.org/10.3390/ijms21249498





