Fox Hunting in Wild Apples: Searching for Novel Genes in Malus Sieversii
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
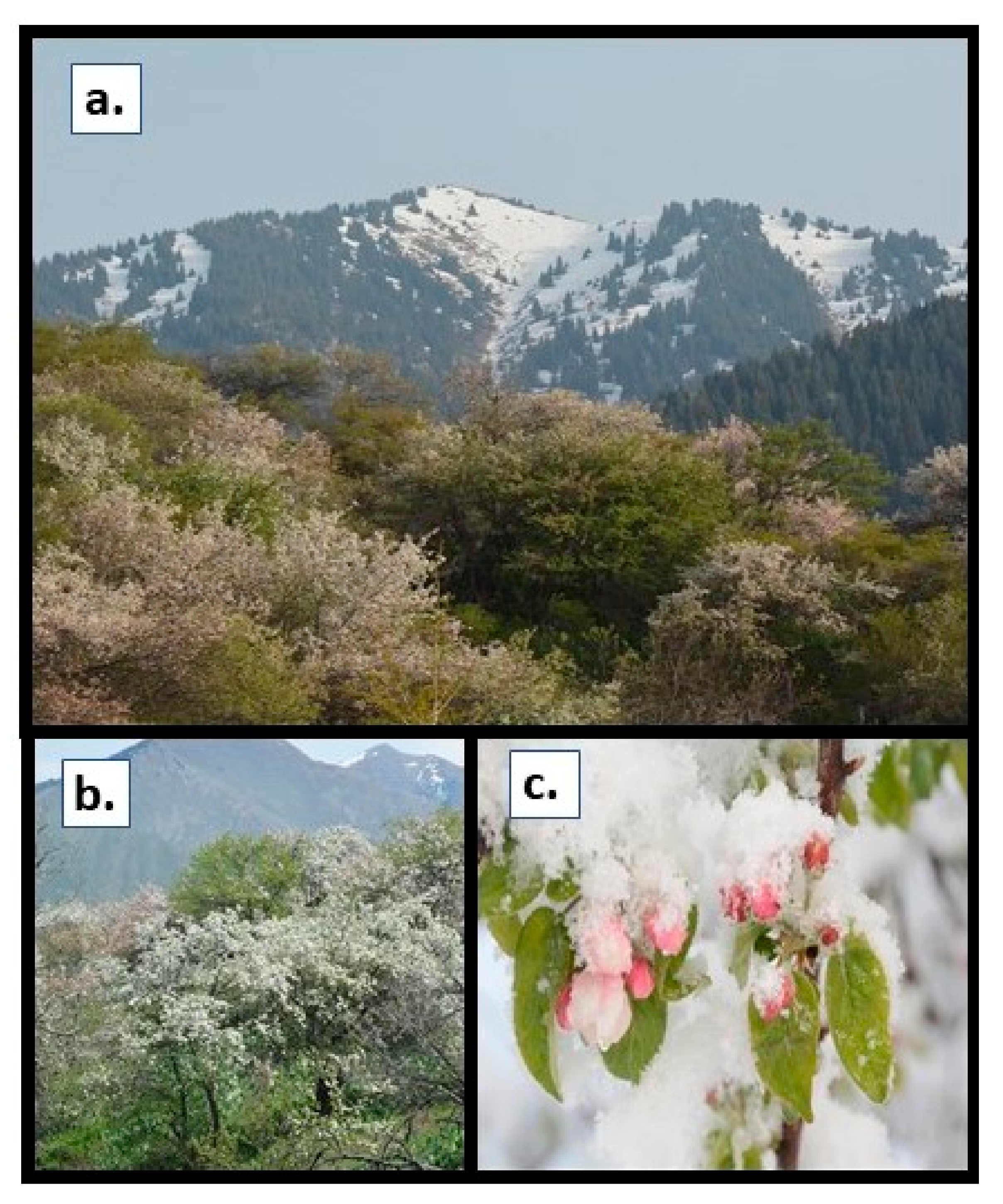
4.1. Selection of Plant Material
4.2. Library Construction
4.3. Arabidopsis Transformation and Screening
4.4. Morphological Data
4.5. Insert Sequence Data and Bioinformatics Analyses
4.6. Freezing Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USApple.org. Available online: http://usapple.org/ (accessed on 17 November 2020).
- USDA Foreign Agriculture Service (fas.usda.gov). Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/statsByCommodity (accessed on 17 November 2020).
- Noiton, D.A.; Alspach, P.A. Founding Clones, Inbreeding, Coancestry, and Status Number of Modern Apple Cultivars. J. Am. Soc. Hortic. Sci. 1996, 121, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Peace, C.; Luby, J.J.; Van De Weg, W.E.; Bink, M.C.A.M.; Iezzoni, A.F. A strategy for developing representative germplasm sets for systematic QTL validation, demonstrated for apple, peach, and sweet cherry. Tree Genet. Genomes 2014, 10, 1679–1694. [Google Scholar] [CrossRef]
- Norelli, J.L.; Jones, A.L.; Aldwinckle, H.S. Fire Blight Management in the Twenty-first Century: Using New Technologies that Enhance Host Resistance in Apple. Plant Dis. 2003, 87, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norelli, J.; Wisniewski, M.; Fazio, G.; Burchard, E.; Gutierrez, B.; Levin, E.; Droby, S. Genotyping-by-sequencing markers facilitate the identification of quantitative trait loci controlling resistance to Penicillium expansum in Malus sieversii. PLoS ONE 2017, 12, e0172949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA Risk Management Agency. Available online: https://www.rma.usda.gov/informationtools/ (accessed on 17 November 2020).
- Richards, C.; Volk, G.M.; Reilley, A.A.; Henk, A.D.; Lockwood, D.R.; Reeves, P.A.; Forsline, P.L. Genetic diversity and population structure in Malus sieversii, a wild progenitor species of domesticated apple. Tree Genet. Genomes 2009, 5, 339–347. [Google Scholar] [CrossRef]
- Volk, G.M.; Henk, A.D.; Richards, C.M.; Forsline, P.L.; Chao, C.T. Malus sieversii: A Diverse Central Asian Apple Species in the USDA-ARS National Plant Germplasm System. HortScience 2013, 48, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- Luby, J.; Forsline, P.; Aldwinckle, H.; Bus, V.; Geibel, M. Silk Road Apples—Collection, Evaluation, and Utilization of Malus sieversii from Central Asia. HortScience 2001, 36, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Forsline, P.L.; Aldwinckle, H.S.; Dickson, E.E.; Hokanson, S.C. Collection, Maintenance, Characterization and Utilization of Wild Apples from Central Asia. In Horticultural Reviews: Wild Apple and Fruit Trees of Central Asia; John Wiley & Sons, Inc.: New York, NY, USA, 2003; Volume 29, pp. 1–6. [Google Scholar]
- Genome Database for Rosaceae. Available online: https://www.rosaceae.org/ (accessed on 17 November 2020).
- Jung, S.; Lee, T.; Cheng, C.-H.; Buble, K.; Zheng, P.; Yu, J.; Humann, J.; Ficklin, S.P.; Gasic, K.; Scott, K.; et al. 15 years of GDR: New data and functionality in the Genome Database for Rosaceae. Nucleic Acids Res. 2019, 47, D1137–D1145. [Google Scholar] [CrossRef] [Green Version]
- Velasco, R.; Zharkikh, A.; Affourtit, J.P.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van De Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Fladung, M.; Deutsch, F.; Hönicka, H.; Kumar, S. T-DNA and Transposon Tagging in Aspen. Plant Biol. 2004, 6, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Groover, A.; Fontana, J.R.; Dupper, G.; Ma, C.; Martienssen, R.; Strauss, S.; Meilan, R. Gene and Enhancer Trap Tagging of Vascular-Expressed Genes in Poplar Trees. Plant Physiol. 2004, 134, 1742–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borejsza-Wysocka, E.E.; Norelli, J.L.; Aldwinckle, H.S.; Ko, K. Transformation of authentic M.26 apple rootstock for enhanced resistance to fire blight. Acta Hortic. 1999, 489, 259–266. [Google Scholar] [CrossRef]
- Bolar, J.P.; Brown, S.K.; Norelli, J.L.; Aldwinckle, H.S. Factors affecting the transformation of ‘Marshall McIntosh’ apple by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. 1998, 55, 31–38. [Google Scholar] [CrossRef]
- Wilson, F.M.; James, D.J. Regeneration and transformation of the premier UK apple (Malus × pumila Mill.) cultivar Queen Cox. J. Hortic. Sci. Biotechnol. 2003, 78, 656–662. [Google Scholar] [CrossRef]
- Seong, E.S.; Song, K.J. Factors affecting the early gene transfer step in the development of transgenic ‘Fuji’ apple plants. Plant Growth Regul. 2007, 54, 89–95. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.Z.; Yin, H.X.; Liu, X. Efficient Agrobacterium tumefaciens-mediated transformation of Malus zumi (Matsumura) Rehd using leaf explant regeneration system. Electron. J. Biotechnol. 2009, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.A.T.; Gulyás, A.; Magyar-Tábori, K.; Wang, M.-R.; Wang, Q.-C.; Dobránszki, J. In vitro tissue culture of apple and other Malus species: Recent advances and applications. Planta 2019, 249, 975–1006. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, B.S.; Jha, G. Current trends and future prospects of biotechnological interventions through tissue culture in apple. Plant Cell Rep. 2010, 29, 1215–1225. [Google Scholar] [CrossRef]
- Ichikawa, T.; Nakazawa, M.; Kawashima, M.; Iizumi, H.; Kuroda, H.; Kondou, Y.; Tsuhara, Y.; Suzuki, K.; Ishikawa, A.; Seki, M.; et al. The FOX hunting system: An alternative gain-of-function gene hunting technique. Plant J. 2006, 48, 974–985. [Google Scholar] [CrossRef]
- Higuchi, M.; Kondou, Y.; Ichikawa, T.; Matsui, M. Full-length cDNA overexpressor gene hunting system (FOX hunting system). Meth. Mol. Biol. 2011, 678, 77–89. [Google Scholar] [CrossRef]
- Sakurai, T.; Kondou, Y.; Akiyama, K.; Kurotani, A.; Higuchi, M.; Ichikawa, T.; Kuroda, H.; Kusano, M.; Mori, M.; Saitou, T.; et al. RiceFOX: A Database of Arabidopsis Mutant Lines Overexpressing Rice Full-Length cDNA that Contains a Wide Range of Trait Information to Facilitate Analysis of Gene Function. Plant Cell Physiol. 2010, 52, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Mizukado, S.; Fujita, Y.; Ichikawa, T.; Nakazawa, M.; Seki, M.; Matsui, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Identification of stress-tolerance-related transcription-factor genes via mini-scale Full-length cDNA Over-eXpressor (FOX) gene hunting system. Biochem. Biophys. Res. Commun. 2007, 364, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Hakata, M.; Amano, K.; Miyao, A.; Toki, N.; Kajikawa, M.; Pang, J.; Higashi, N.; Ando, S.; Toki, S.; et al. A genome-wide gain-of-function analysis of rice genes using the FOX-hunting system. Plant Mol. Biol. 2007, 65, 357–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondou, Y.; Higuchi, M.; Takahashi, S.; Sakurai, T.; Ichikawa, T.; Kuroda, H.; Yoshizumi, T.; Tsumoto, Y.; Horii, Y.; Kawashima, M.; et al. Systematic approaches to using the FOX hunting system to identify useful rice genes. Plant J. 2009, 57, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Maeda, S.; Sugano, S.; Ohtake, M.; Hayashi, N.; Ichikawa, T.; Kondou, Y.; Kuroda, H.; Horii, Y.; Matsui, M.; et al. Screening for resistance against Pseudomonas syringae in rice-FOX Arabidopsis lines identified a putative receptor-like cytoplasmic kinase gene that confers resistance to major bacterial and fungal pathogens in Arabidopsis and rice. Plant Biotechnol. J. 2011, 9, 466–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himuro, Y.; Tanaka, H.; Hashiguchi, M.; Ichikawa, T.; Nakazawa, M.; Seki, M.; Fujita, M.; Shinozaki, K.; Matsui, M.; Akashi, R.; et al. FOX-superroots of Lotus corniculatus, overexpressing Arabidopsis full-length cDNA, show stable variations in morphological traits. J. Plant Physiol. 2011, 168, 181–187. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Zhang, Q.; Liu, Y.; Zhu, B.; Cao, J.; Li, Z.; Han, L.; Jia, J.; Zhao, G.-Y.; et al. Generation of Wheat Transcription Factor FOX Rice Lines and Systematic Screening for Salt and Osmotic Stress Tolerance. PLoS ONE 2015, 10, e0132314. [Google Scholar] [CrossRef]
- Ling, J.; Li, R.; Nwafor, C.C.; Cheng, J.; Li, M.; Xu, Q.; Wu, J.; Gan, L.; Yang, Q.; Liu, C.; et al. Development of iFOX-hunting as a functional genomic tool and demonstration of its use to identify early senescence-related genes in the polyploid Brassica napus. Plant Biotechnol. J. 2018, 16, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, H.; Zheng, J.-X.; Mo, H.; Xia, K.; Jian, S. Functional Identification of Salt-Stress-Related Genes Using the FOX Hunting System from Ipomoea pes-caprae. Int. J. Mol. Sci. 2018, 19, 3446. [Google Scholar] [CrossRef] [Green Version]
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.kegg.jp/ (accessed on 17 November 2020).
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisniewski, M.; Nassuth, A.; Arora, R. Cold Hardiness in Trees: A Mini-Review. Front. Plant Sci. 2018, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Lindow, S.E.; Ashworth, E.N. Observations of Ice Nucleation and Propagation in Plants Using Infrared Video Thermography. Plant Physiol. 1997, 113, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2012, 147, 4–14. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1998, 17, 287–291. [Google Scholar] [CrossRef]
- Cornille, A.; Gladieux, P.; Smulders, M.J.M.; Roldán-Ruiz, I.; Laurens, F.; Le Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties. PLoS Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef] [Green Version]
- Volk, G.M.; Henk, A.D.; Baldo, A.M.; Fazio, G.; Chao, C.T.; Richards, C.M. Chloroplast heterogeneity and historical admixture within the genus Malus. Am. J. Bot. 2015, 102, 1198–1208. [Google Scholar] [CrossRef]
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; McFerson, J.R.; Zhong, G.-Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop. Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Fazio, G.; Aldwinckle, H.S.; Volk, G.M.; Richards, C.M.; Janisiewicz, W.J.; Forsline, P.L. Progress in evaluating Malus sieversii for disease resistance and horticultural traits. Acta Hort. 2009, 814, 59–66. [Google Scholar] [CrossRef]
- Jurick, W.M.; anisiewicz, W.J.; Saftner, R.A.; Vico, I.; Gaskins, V.L.; Park, E.; Forsline, P.L.; Fazio, G.; Conway, W.S. Identification of wild apple germplasm (Malus spp.) accessions with resistance to the postharvest decay pathogens Penicillium expansum and Colletotrichum acutatum. Plant Breed. 2011, 130, 481–486. [Google Scholar] [CrossRef]
- Liu, B.-H.; Cheng, L.; Ma, F.-W.; Liang, D.; Zou, Y.-J. Influence of rootstock on drought response in young ‘Gale Gala’ apple (Malus domestica Borkh.) trees. J. Sci. Food Agric. 2012, 92, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.L.; Glenn, D.M.; Forsline, P.L.; Wisniewski, M.E.; Farrell, R.E. Characterizing Water Use Efficiency and Water Deficit Responses in Apple (Malus × domestica Borkh. and Malus sieversii Ledeb.) M. Roem. HortScience 2011, 46, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Bassett, C.L. Water Use and Drought Response in Cultivated and Wild Apples. In Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Norelli, J.L.; Howard, N.P.; Wisniewski, M.; Flachowsky, H.; Hanke, M.-V.; Peace, C. Introgressing blue mold resistance into elite apple germplasm by rapid cycle breeding and foreground and background DNA-informed selection. Tree Genet. Genomes 2020, 16, 28. [Google Scholar] [CrossRef]
- Tatum, T.C.; Stepanovic, S.; Biradar, D.P.; Rayburn, A.L.; Korban, S.S. Variation in nuclear DNA content in Malus species and cultivated apples. Genome 2005, 48, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Korban, S.; Wannarat, W.; Rayburn, C.M.; Tatum, T.C.; Rayburn, A.L. Genome size and nucleotypic variation in Malus germplasm. Genome 2009, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.L.; Wisniewski, M.E.; Artlip, T.S.; Norelli, J.L.; Renaut, J.; Farrell, R.E. Global Analysis of Genes Regulated by Low Temperature and Photoperiod in Peach Bark. J. Am. Soc. Hortic. Sci. 2006, 131, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, M.; Bassett, C.L.; Norelli, J.; Macarisin, D.; Artlip, T.S.; Gasic, K.; Korban, S. Expressed sequence tag analysis of the response of apple (Malus × domestica ‘Royal Gala’) to low temperature and water deficit. Physiol. Plant. 2008, 133, 298–317. [Google Scholar] [CrossRef]
- Tuteja, N. Cold, Salinity and Drought Stress. In Plant Stress Biology—From Genomics to System Biology; Hirt, H., Ed.; Wiley-Blackwell: Weinheim, Germany, 2009; pp. 137–159. [Google Scholar]
- Park, S.; Keathley, D.E.; Han, K.-H. Transcriptional profiles of the annual growth cycle in Populus deltoides. Tree Physiol. 2008, 28, 321–329. [Google Scholar] [CrossRef]
- Artlip, T.; McDermaid, A.; Ma, Q.; Wisniewski, M. Differential gene expression in non-transgenic and transgenic “M.26” apple overexpressing a peach CBF gene during the transition from eco-dormancy to bud break. Hortic. Res. 2019, 6, 86. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The Enigmatic LEA Proteins and Other Hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierling, E. The Roles of Heat Shock Proteins in Plants. Annu. Rev. Plant Biol. 1991, 42, 579–620. [Google Scholar] [CrossRef]
- Artlip, T.S.; Wisniewski, M.E. Induction of Proteins in Response to Biotic and Abiotic Stresses. In Handbook of Plant and Crop Physiology, 2nd ed.; Pessarakli, M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 657–679. [Google Scholar]
- Wisniewski, M.; Enorelli, J.; Eartlip, T. Overexpression of a peach CBF gene in apple: A model for understanding the integration of growth, dormancy, and cold hardiness in woody plants. Front. Plant Sci. 2015, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, M.; Asquini, E.; Miolli, G.V.; Weigl, K.; Hanke, M.-V.; Flachowsky, H.; Si-Ammour, A. The MADS-Box Gene MdDAM1 Controls Growth Cessation and Bud Dormancy in Apple. Front. Plant Sci. 2020, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Puhakainen, T.; Hess, M.W.; Mäkelä, P.; Svensson, J.; Heino, P.; Palva, E.T. Overexpression of Multiple Dehydrin Genes Enhances Tolerance to Freezing Stress in Arabidopsis. Plant Mol. Biol. 2004, 54, 743–753. [Google Scholar] [CrossRef] [PubMed]
- USDA-ARS. Plant Materials Introduced in 2000 (Nos. 612387-615161); Plant Inventory No. 209; USDA-ARS: Washington, DC, USA, 2000; pp. 223–224. Available online: http://www.ars.usda.gov/SP2UserFiles/ad_hoc/12751500PIBooks/pibooks/plantinv209.pdf (accessed on 17 November 2020).
- Bogdanova, E.A.; Shagina, I.; Barsova, E.V.; Kelmanson, I.; Shagin, D.A.; Lukyanov, S.A. Normalizing cDNA Libraries. Curr. Protoc. Mol. Biol. 2010, 90, 5.12.1–5.12.27. [Google Scholar] [CrossRef]
- Rivero-Lepinka, L.; Crist, D.; Scholl, R. Growth, Plants and Preservation of Seeds. In Arabidopsis Protocols, 2nd ed.; Salina, J., Sanchez-Serrano, J.J., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 3–12. [Google Scholar]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279671/ (accessed on 17 November 2020).
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 17 November 2020).
- The Arabidopsis Information Resource. Available online: https://www.arabidopsis.org/ (accessed on 17 November 2020).





| FOX ID | GDDH Accession | GDDH Description |
|---|---|---|
| 145 | MD01G1079300 | P-loop_containing NTP_hydrolases_superfamily_ |
| 167 | MD09G1209600 | RING-H2_finger_C1A |
| 186 | MD15G1163400 | RING/U-box_superfamily_protein |
| 216 | MD10G1078600 | mediator_of_RNA_polymerase_II_transcription_subunit_32 |
| 236 | MD00G1140600 | HIT_zinc_finger |
| 263 | MD11G1241900 | Aluminium_induced_protein_with_YGL_and_LRDR_motifs |
| FOX ID | % Control | % Line | GDDH Accession | GDDH Description |
|---|---|---|---|---|
| 124 | 30 | 44.7 | MD07G1138200 | beta-amylase_7 |
| 159 | 26 | 36 | MD13G1087000 | Putative_thiol-disulphide_oxidoreductase_DCC |
| 185 | 6.66 | 5.33 | MD17G1034100 | DegP_protease_1 |
| 697 | 0 | 9.33 | MD17G1116400 | unknown |
| 797 | 0 | 0 | MD11G1120700 | Protein_kinase_superfamily_protein |
| 133 | 3.33 | 12 | MD15G1293600 | casein_kinase_alpha_1 |
| 134 | 13.33 | 26.76 | MD15G1293600 | casein_kinase_alpha_1 |
| 1847 | 0 | 1.3 | MD15G1003900 | cold-regulated_47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisniewski, M.; Artlip, T.; Liu, J.; Ma, J.; Burchard, E.; Norelli, J.; Dardick, C. Fox Hunting in Wild Apples: Searching for Novel Genes in Malus Sieversii. Int. J. Mol. Sci. 2020, 21, 9516. https://doi.org/10.3390/ijms21249516
Wisniewski M, Artlip T, Liu J, Ma J, Burchard E, Norelli J, Dardick C. Fox Hunting in Wild Apples: Searching for Novel Genes in Malus Sieversii. International Journal of Molecular Sciences. 2020; 21(24):9516. https://doi.org/10.3390/ijms21249516
Chicago/Turabian StyleWisniewski, Michael, Timothy Artlip, Jia Liu, Jing Ma, Erik Burchard, John Norelli, and Christopher Dardick. 2020. "Fox Hunting in Wild Apples: Searching for Novel Genes in Malus Sieversii" International Journal of Molecular Sciences 21, no. 24: 9516. https://doi.org/10.3390/ijms21249516




