TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy
Abstract
:1. Introduction
2. Results
2.1. Study Subjects and Ophthalmic Evaluation
2.2. Clinical Assessment
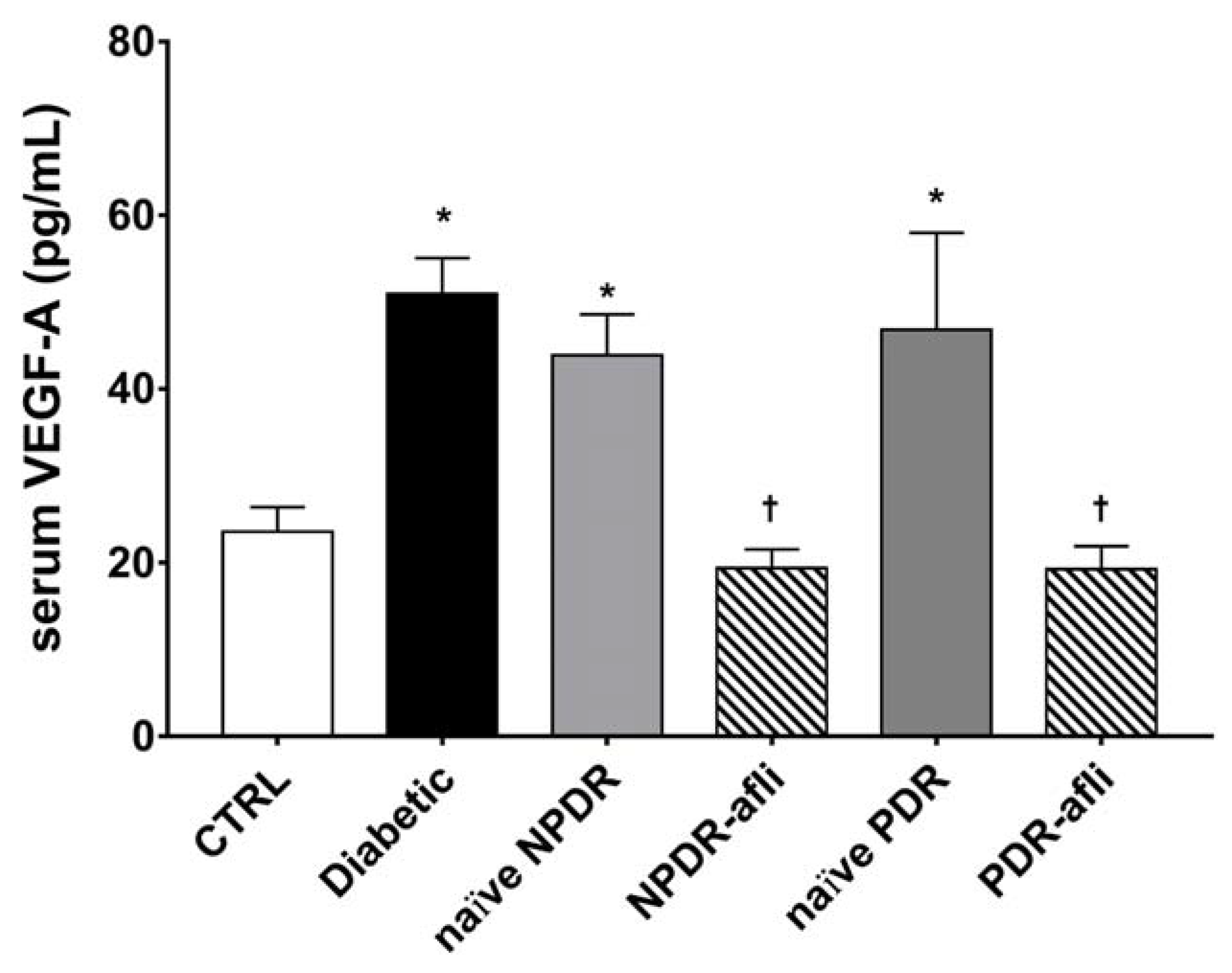
2.3. Serum Growth Factor Levels
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, D.J.; Cascio, M.A.; Rosca, M.G. Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease. Antioxidants 2020, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 2020, 98, 786–806. [CrossRef]
- Parravano, M.; De Geronimo, D.; Scarinci, F.; Querques, L.; Virgili, G.; Simonett, J.M.; Varano, M.; Bandello, F.; Querques, G. Diabetic Microaneurysms Internal Reflectivity on Spectral-Domain Optical Coherence Tomography and Optical Coherence Tomography Angiography Detection. Am. J. Ophthalmol. 2017, 179, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, A.Y.; Baughman, D.; Sim, D.; Akelere, T.; Brand, C.; Crabb, D.P.; Denniston, A.K.; Downey, L.; Fitt, A.; et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group: Report 3: Baseline Retinopathy and Clinical Features Predict Progression of Diabetic Retinopathy. Am. J. Ophthalmol. 2017, 180, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandello, F.; Cicinelli, M.V. 19th EURETINA Congress Keynote Lecture: Diabetic Retinopathy Today. Ophthalmologica 2020, 243, 163–171. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef] [Green Version]
- Bucolo, C.; Gozzo, L.; Longo, L.; Mansueto, S.; Vitale, D.C.; Drago, F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: A systematic review of real-world studies. J. Pharmacol. Sci. 2018, 138, 219–232. [Google Scholar] [CrossRef]
- Moulin, T.A.; Adjei Boakye, E.; Wirth, L.S.; Chen, J.; Burroughs, T.E.; Vollman, D.E. Yearly Treatment Patterns for Patients with Recently Diagnosed Diabetic Macular Edema. Ophthalmol. Retin. 2019, 34, 362–370. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Platania, C.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: A computational approach. Front. Pharmacol. 2015, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Plyukhova, A.A.; Budzinskaya, M.V.; Starostin, K.M.; Rejdak, R.; Bucolo, C.; Reibaldi, M.; Toro, M.D. Comparative Safety of Bevacizumab, Ranibizumab, and Aflibercept for Treatment of Neovascular Age-Related Macular Degeneration (AMD): A Systematic Review and Network Meta-Analysis of Direct Comparative Studies. J. Clin. Med. 2020, 9, 1522. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, T.; Azzolini, C.; Bandello, F.; Boscia, F.; De Falco, S.; Fornasari, D.; Lanzetta, P.; Mastropasqua, L.; Midena, E.; Ricci, F.; et al. Aflibercept in the treatment of diabetic macular edema: A review and consensus paper. Eur. J. Ophthalmol. 2017, 27, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, F.; Fidilio, A.; Platania, C.B.M.; Giurdanella, G.; Salomone, S.; Leggio, G.M.; Tarallo, V.; Cicatiello, V.; De Falco, S.; Eandi, C.M.; et al. Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochem. Pharmacol. 2019, 168, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158, 13–26. [Google Scholar] [CrossRef]
- Gucciardo, E.; Loukovaara, S.; Korhonen, A.; Repo, P.; Martins, B.; Vihinen, H.; Jokitalo, E.; Lehti, K. The microenvironment of proliferative diabetic retinopathy supports lymphatic neovascularization. J. Pathol. 2018, 245, 172–185. [Google Scholar] [CrossRef]
- Klaassen, I.; van Geest, R.J.; Kuiper, E.J.; van Noorden, C.J.F.; Schlingemann, R.O. The role of CTGF in diabetic retinopathy. Exp. Eye Res. 2015, 133, 37–48. [Google Scholar] [CrossRef]
- Fan, J.; Shen, W.; Lee, S.R.; Mathai, A.E.; Zhang, R.; Xu, G.; Gillies, M.C. Targeting the Notch and TGF-β signaling pathways to prevent retinal fibrosis in vitro and in vivo. Theranostics 2020, 10, 7956. [Google Scholar] [CrossRef]
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFβ1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013, 91, 531–539. [Google Scholar] [CrossRef]
- Lazzara, F.; Trotta, M.C.; Platania, C.B.M.; D’Amico, M.; Petrillo, F.; Galdiero, M.; Gesualdo, C.; Rossi, S.; Drago, F.; Bucolo, C. Stabilization of HIF-1α in Human Retinal Endothelial Cells Modulates Expression of miRNAs and Proangiogenic Growth Factors. Front. Pharmacol. 2020, 11, 1063. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Samuel, W.; Nagineni, S.; Pardhasaradhi, K.; Wiggert, B.; Detrick, B.; Hooks, J.J. Transforming Growth Factor-β Induces Expression of Vascular Endothelial Growth Factor in Human Retinal Pigment Epithelial Cells: Involvement of Mitogen-Activated Protein Kinases. J. Cell. Physiol. 2003, 197, 453–462. [Google Scholar] [CrossRef]
- Ferrari, G.; Pintucci, G.; Seghezzi, G.; Hyman, K.; Galloway, A.C.; Mignatti, P. VEGF, a prosurvival factor, acts in concert with TGF-β1 to induce endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2006, 103, 17260–17265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigsby, J.; Betts, B.; Vidro-Kotchan, E.; Culbert, R.; Tsin, A. A possible role of acrolein in diabetic retinopathy: Involvement of a VEGF/TGFβ signaling pathway of the retinal pigment epithelium in hyperglycemia. Curr. Eye Res. 2012, 37, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, E.A.; Liu, L.; Steinle, J.J. miR-15a/16 inhibits TGF-beta3/VEGF signaling and increases retinal endothelial cell barrier proteins. Vis. Res. 2017, 139, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Silverman, S.M.; Zhao, L.; Villasmil, R.; Campos, M.M.; Amaral, J.; Wong, W.T. Absence of TGFβ signaling in retinal microglia induces retinal degeneration and exacerbates choroidal neovascularization. eLife 2019, 8, e42049. [Google Scholar] [CrossRef]
- Fisichella, V.; Giurdanella, G.; Platania, C.B.M.; Romano, G.L.; Leggio, G.M.; Salomone, S.; Drago, F.; Caraci, F.; Bucolo, C. TGF-β1 prevents rat retinal insult induced by amyloid-β (1–42) oligomers. Eur. J. Pharmacol. 2016, 787, 72–77. [Google Scholar] [CrossRef]
- Dagher, Z.; Gerhardinger, C.; Vaz, J.; Goodridge, M.; Tecilazich, F.; Lorenzi, M. The Increased Transforming Growth Factor-β Signaling Induced by Diabetes Protects Retinal Vessels. Am. J. Pathol. 2017, 187, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Tosi, G.M.; Orlandini, M.; Galvagni, F. The Controversial Role of TGF-β in Neovascular Age-Related Macular Degeneration Pathogenesis. Int. J. Mol. Sci. 2018, 19, 3363. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Lee, S.R.; Yam, M.; Zhu, L.; Zhang, T.; Pye, V.; Mathai, A.E.; Shibagaki, K.; Zhang, J.Z.; Matsugi, T.; et al. A combination therapy targeting endoglin and VEGF-A prevents subretinal fibro-neovascularization caused by induced müller cell disruption. Investig. Ophthalmol. Vis. Sci. 2018, 59, 6075–6088. [Google Scholar] [CrossRef] [Green Version]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A.; et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: An in silico and in vivo approach. Br. J. Pharmacol. 2019, 176, 2179–2194. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs as biomarkers of diabetic retinopathy and disease progression. Neural Regen. Res. 2019, 41, 1858. [Google Scholar]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in diabetic retinopathy. Rev. Diabet. Stud. 2015, 12, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in Diabetes. Diabetes Care 2004, 27 (Suppl. S1), s84–s87. [Google Scholar] [CrossRef] [Green Version]
- Bandello, F.; Toni, D.; Porta, M.; Varano, M. Diabetic retinopathy, diabetic macular edema, and cardiovascular risk: The importance of a long-term perspective and a multidisciplinary approach to optimal intravitreal therapy. Acta Diabetol. 2020, 57, 513–526. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. Jama-J. Am. Med Assoc. 2015, 314, 2137–2146. [Google Scholar] [CrossRef] [Green Version]
- Maggio, E.; Sartore, M.; Attanasio, M.; Maraone, G.; Guerriero, M.; Polito, A.; Pertile, G. Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema in a Real-World Clinical Setting. Am. J. Ophthalmol. 2018, 195, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lien, R.; Wang, N.K.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C.; Wu, W.C. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 479–487. [Google Scholar] [CrossRef]
- Hirano, T.; Toriyama, Y.; Iesato, Y.; Imai, A.; Murata, T. Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina 2018, 38, 1801. [Google Scholar] [CrossRef]
- Ang, W.J.; Zunaina, E.; Norfadzillah, A.J.; Raja-Norliza, R.O.; Julieana, M.; Ab-Hamid, S.A.; Mahaneem, M. Evaluation of vascular endothelial growth factor levels in tears and serum among diabetic patients. PLoS ONE 2019, 14, e0221481. [Google Scholar] [CrossRef]
- Rini, B.I.; Michaelson, M.D.; Rosenberg, J.E.; Bukowski, R.M.; Sosman, J.A.; Stadler, W.M.; Hutson, T.E.; Margolin, K.; Harmon, C.S.; DePrimo, S.E.; et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3743–3748. [Google Scholar] [CrossRef]
- Willett, C.G.; Boucher, Y.; Duda, D.G.; Di Tomaso, E.; Munn, L.L.; Tong, R.T.; Kozin, S.V.; Petit, L.; Jain, R.K.; Chung, D.C.; et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: Continued experience of a phase I trial in rectal cancer patients. J. Clin. Oncol. 2005, 23, 8136–8139. [Google Scholar] [CrossRef]
- van Cutsem, E.; Paccard, C.; Chiron, M.; Tabernero, J. Impact of prior bevacizumab treatment on VEGF-A and PLGF levels and outcome following second-line aflibercept treatment: Biomarker post hoc analysis of the VELOUR trial. Clin. Cancer Res. 2020, 26, 717–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehetner, C.; Bechrakis, N.E.; Stattin, M.; Kirchmair, R.; Ulmer, H.; Kralinger, M.T.; Kieselbach, G.F. Systemic counterregulatory response of placental growth factor levels to intravitreal aflibercept therapy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3279–3286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Pang, L.; Yang, W.; Liu, X.; Su, G.; Dong, Y. Long non-coding RNA of myocardial infarction associated transcript (LncRNA-MIAT) promotes diabetic retinopathy by upregulating transforming growth factor-β1 (TGF-β1) signaling. Med Sci. Monit. 2018, 24, 9497. [Google Scholar] [CrossRef]
- Kaneko, H.; Takayama, K.; Asami, T.; Ito, Y.; Tsunekawa, T.; Iwase, T.; Funahashi, Y.; Ueno, S.; Nonobe, N.; Yasuda, S.; et al. Cytokine profiling in the sub-silicone oil fluid after vitrectomy surgeries for refractory retinal diseases. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khuu, L.A.; Tayyari, F.; Sivak, J.M.; Flanagan, J.G.; Singer, S.; Brent, M.H.; Huang, D.; Tan, O.; Hudson, C. Aqueous humour concentrations of TGF-β, PLGF and FGF-1 and total retinal blood flow in patients with early non-proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, e206–e211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziller, N.; Kotolloshi, R.; Esmaeili, M.; Liebisch, M.; Mrowka, R.; Baniahmad, A.; Liehr, T.; Wolf, G.; Loeffler, I. Sex Differences in Diabetes- and TGF-β1-Induced Renal Damage. Cells 2020, 9, 2236. [Google Scholar] [CrossRef]
- Lane, P.H.; Snelling, D.M.; Babushkina-Patz, N.; Langer, W.J. Sex differences in the renal transforming growth factor-β1 system after puberty. Pediatric Nephrol. 2001, 16, 61–68. [Google Scholar] [CrossRef]
- Chatziralli, I.P. The Role of Glycemic Control and Variability in Diabetic Retinopathy. Diabetes Ther. 2018, 9, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Fullerton, B.; Jeitler, K.; Seitz, M.; Horvath, K.; Berghold, A.; Siebenhofer, A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2014, 2, CD009122. [Google Scholar] [CrossRef]





| Gender (F; M) | HbA1c (%) | Diabetes Duration (Years) | Insulin Treatment (Y; N) | Subjects with Glycemic Control (Y; N) | Age (Years) | |
|---|---|---|---|---|---|---|
| CTRL (N = 7) | (4; 3) | 3.9 ± 0.9 | NA | NA | NA | 66 ± 14 |
| Diabetic (N = 6) | (4; 2) | 6.6 ± 0.3 | 5 ± 5 | (0; 6) | (6; 0) | 75 ± 10 |
| NPDR naïve (N = 6) | (2; 4) | 7 ± 1 | 19 ± 8 | (4; 2) | (4; 2) | 74 ± 6 |
| NPDR aflibercept (N = 6) | (2; 4) | 6.9 ± 0.5 | 20 ± 8 | (5; 1) | (6; 0) | 70 ± 7 |
| PDR naïve (N = 7) | (4; 3) | 7.3 ± 0.6 | 21 ± 6 | (7; 0) | (2; 5) | 70 ± 7 |
| PDR aflibercept (N = 6) | (3; 3) | 7 ± 1 | 18 ± 9 | (3; 3) | (4; 2) | 67 ± 8 |
| Kolmogorov–Smirnov | Shapiro–Wilk | |||||
|---|---|---|---|---|---|---|
| Statistics | gf | Sign. | Statistics | gf | Sign. | |
| Diabetes duration | 0.163 | 38 | 0.014 | 0.926 | 38 | 0.017 |
| HbA1c | 0.190 | 38 | 0.002 | 0.914 | 38 | 0.007 |
| TGFβ1 | 0.151 | 38 | 0.033 | 0.937 | 38 | 0.036 |
| VEGF-A | 0.154 | 38 | 0.028 | 0.794 | 38 | 0.000 |
| PlGF | 0.245 | 38 | 0.000 | 0.642 | 38 | 0.000 |
| Duration | HbA1c | TGFβ1 | VEGF-A | PlGF | ||
|---|---|---|---|---|---|---|
| Diabetes duration | Pearson coefficient | 1 | 0.595 ** | 0.335 * | 0.102 | 0.084 |
| Sign. (two tails) | 0.000 | 0.043 | 0.547 | 0.622 | ||
| N | 38 | 38 | 38 | 38 | 38 | |
| HbA1c | Pearson coefficient | 0.595 ** | 1 | 0.592 ** | 0.271 | 0.163 |
| Sign. (two tails) | 0.000 | 0.000 | 0.104 | 0.334 | ||
| N | 38 | 38 | 38 | 38 | 38 | |
| TGFβ1 | Pearson coefficient | 0.335 * | 0.592 ** | 1 | 0.003 | −0.132 |
| Sign. (two tails) | 0.043 | 0.000 | 0.984 | 0.436 | ||
| N | 38 | 38 | 38 | 38 | 38 | |
| VEGF-A | Pearson coefficient | 0.102 | 0.271 | 0.003 | 1 | −0.156 |
| Sign. (two tails) | 0.547 | 0.104 | 0.984 | 0.358 | ||
| N | 38 | 38 | 38 | 38 | 38 | |
| PlGF | Pearson coefficient | 0.084 | 0.163 | −0.132 | −0.156 | 1 |
| Sign. (two tails) | 0.622 | 0.334 | 0.436 | 0.358 | ||
| N | 38 | 38 | 38 | 38 | 38 | |
| Effects | Wilks λ | F | p-Value |
|---|---|---|---|
| group | 0.037 | 6.836 | 0.0001 |
| insulin treatment | 0.953 | 0.266 | 0.849 |
| glycemic control | 0.358 | 0.573 | 0.001 |
| gender | 0.869 | 0.805b | 0.509 |
| Group * glycemic control | 0.627 | 3.167 | 0.05 |
| Group * gender | 0.39 | 2.049 | 0.05 |
| glycemic control * gender | 0.464 | 6.152 | 0.006 |
| Source of Variation | Dependent Variable | F | p-Value |
|---|---|---|---|
| group | HbA1c | 9.624 | 0.0001 |
| TGFβ1 | 12.708 | 0.0001 | |
| Diabetes duration | 2.077 | 0.116 | |
| Insulin treatment | HbA1c | 0.35 | 0.562 |
| TGFβ1 | 0.272 | 0.608 | |
| Diabetes duration | 0.095 | 0.762 | |
| Glycemic control | HbA1c | 13.579 | 0.002 |
| TGFβ1 | 6.873 | 0.017 | |
| Diabetes duration | 4.582 | 0.046 | |
| gender | HbA1c | 0.486 | 0.494 |
| tgfbeta1 | 1.998 | 0.175 | |
| Diabetes duration | 0.062 | 0.806 | |
| group * glycemic control | HbA1c | 6.217 | 0.023 |
| TGFβ1 | 0.112 | 0.742 | |
| Diabetes duration | 1.523 | 0.233 | |
| group * gender | HbA1c | 0.337 | 0.799 |
| TGFβ1 | 6.253 | 0.004 | |
| Diabetes duration | 1.123 | 0.366 | |
| Glycemic control * gender | HbA1c | 0.068 | 0.798 |
| TGFβ1 | 15,571 | 0.001 | |
| Diabetes duration | 2.478 | 0.133 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F.; et al. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int. J. Mol. Sci. 2020, 21, 9558. https://doi.org/10.3390/ijms21249558
Bonfiglio V, Platania CBM, Lazzara F, Conti F, Pizzo C, Reibaldi M, Russo A, Fallico M, Ortisi E, Pignatelli F, et al. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. International Journal of Molecular Sciences. 2020; 21(24):9558. https://doi.org/10.3390/ijms21249558
Chicago/Turabian StyleBonfiglio, Vincenza, Chiara Bianca Maria Platania, Francesca Lazzara, Federica Conti, Corrado Pizzo, Michele Reibaldi, Andrea Russo, Matteo Fallico, Elina Ortisi, Francesco Pignatelli, and et al. 2020. "TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy" International Journal of Molecular Sciences 21, no. 24: 9558. https://doi.org/10.3390/ijms21249558
APA StyleBonfiglio, V., Platania, C. B. M., Lazzara, F., Conti, F., Pizzo, C., Reibaldi, M., Russo, A., Fallico, M., Ortisi, E., Pignatelli, F., Longo, A., Avitabile, T., Drago, F., & Bucolo, C. (2020). TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. International Journal of Molecular Sciences, 21(24), 9558. https://doi.org/10.3390/ijms21249558









